ABSTRACT
Objective: Most patients with type 2 diabetes eventually require exogenous insulin therapy to achieve good glycemic control due to the progressive nature of the disease. Insulin aspart is a rapid-acting insulin analog developed for prandial use. This study aimed to illustrate the implications on healthcare costs of adding insulin aspart to basal therapy in a real-world setting.
Methods: Patients with type 2 diabetes who intensified previous basal therapy with insulin aspart were identified from a large commercial US healthcare data source between April 2007 and September 2008. Patients were required to have received basal insulin treatment with or without concomitant oral antidiabetic (OAD) therapy for at least 90 days pre- and post-initiation of insulin aspart. Wilcoxon signed-rank test and McNemar's test were used for continuous and categorical variables, respectively, to analyze the difference of self-comparison between pre- and post insulin aspart add-on.
Results: In total, 1,739 patients with an average age of 56 years were identified, of whom 55% were male. After initiation of insulin aspart, a significant improvement in glycemic control was observed (change in HbA1c: –0.5%, p=0.0013). Similarly, a reduction of 0.4% in HbA1c was observed for the subpopulation of 151 patients, who had both pre-and post-index HbA1c data (p=0.0085). Also, significantly fewer patients used OADs after insulin aspart initiation (56 vs. 64%, p< 0.0001). Overall and diabetes-related healthcare costs also significantly decreased by $2,283 and $2,028, respectively (p≤0.0001). Diabetes-related inpatient visits appear to be the main contributor to total cost (46%); however, after initiation of insulin aspart the number of inpatient visits decreased by 0.50 visits/patient/year (p< 0.05). This decrease was reflected in a large reduction in cost related to inpatient visits ($3,019/patient).
Limitations: A regression to the mean effect may be associated with this pre-post comparison. The ability to make conclusions regarding cause and effect may be limited due to the retrospective design of this study.
Conclusions: Patients with type 2 diabetes achieved better glycemic control and needed less OAD treatment after adding insulin aspart to previous basal therapy. Furthermore, patients experienced on average reduced healthcare utilization after initiation of insulin aspart, which resulted in significant cost savings.
Introduction
The prevalence of diabetes in the Unites States (US) is continually rising. In 1990 an estimated 6.6 million individuals had a diagnosis of diabetes; however, by 2007 this estimate had increased to 17.9 millionCitation1. The increase in the number of individuals with diabetes in the US has been estimated to be ∼1 million/yearCitation2, and it has been estimated that a staggering 48.3 million individuals will be diagnosed with diabetes in the US by 2050Citation3. Hence, in recent years diabetes has become an epidemic associated with a continually increasing cost burden for the US healthcare system. Indeed, the total expenditure attributed to diabetes has increased from an estimated $132 billion in 2002Citation4 to $174 billion in 2007Citation2. Excess medical expenditure accounted for $116 billion of the total cost, which includes direct medical expenditure for diabetes care ($27 billion) and the treatment of diabetes-related chronic complications ($58 billion)Citation2. Indirect costs, such as increased absenteeism and reduced productivity, also contribute to the total estimated cost of diabetesCitation2.
It is well-known that type 2 diabetes is a progressive disease characterized by beta-cell failure and deteriorating glycemic control. When patients with type 2 diabetes are first diagnosed, they are often advised to make lifestyle changes and prescribed oral antidiabetic therapy (OADs)Citation5. Maintaining glycemic control has been shown to have significant beneficial effects and to reduce the risk of developing long-term diabetes- related complicationsCitation6,7. However, due to the progressive nature of the disease, most patients with type 2 diabetes will eventually require exogenous insulin to maintain good glycemic controlCitation5. Hence, patients with type 2 diabetes are often initiated on basal insulin in combination with previous oral therapy (‘basal-oral therapy’)Citation5. When glycemic control is inadequately maintained with basal-oral therapy, treatment is often intensified by adding a rapid-acting bolus insulinCitation5.
Insulin analogs are synthetic insulin molecules that were developed to more closely mimic the pharmacokinetic and pharmacodynamic characteristics of endogenous insulin. Insulin analogs have consistently been shown to improve the balance between glycemic control and tolerability. However, insulin analogs are often associated with higher acquisition costs compared to traditional human insulinsCitation8. Nevertheless, pharmacoeconomic models have shown that over an extended period of time the higher prescription costs of insulin analogs are offset by a lower incidence of hypoglycemia and diabetes-related complications, which implies that insulin analogs are a cost-effective treatment optionCitation8. Moreover, it is important to keep in mind that drug cost accounts for only part of diabetes-related costs. Medical costs, such as inpatient visits, physician office visits, and other services, all contribute to the overall diabetes-related costs.
Insulin aspart, a rapid-acting insulin analog developed for meal time use, has been shown to be well-tolerated and to improve glycemic control in patients with type 2 diabetesCitation9–12. This may reduce the incidence of diabetes-related complications, which in turn may influence overall healthcare costs. The present study was carried out within the context of a large US managed-care population and aimed to illustrate the cost implications of intensifying basal insulin therapy with insulin aspart in patients with type 2 diabetes in a real-world setting. Insulin aspart is one of three available rapid-acting insulin analogs on the US market. The other two are insulin lispro (Eli Lily, Indianapolis, IN, USA) and insulin glulisine (Sanofi-Aventis, Paris, France).
Patients and methods
This was a retrospective analysis of healthcare claims data and laboratory results for patients with type 2 diabetes who were enrolled in a large US managed-care organization (i3 Innovus division of Ingenix Pharmaceutical Services, Inc; ∼14 million covered lives/year) between April 2007 and September 2008. Patient information was made anonymous to protect patient privacy in accordance with the Health Insurance Portability and Accountability Act.
Patient population
This retrospective study included fully insured adults (age ≥18 years) who had a prescription fill for insulin aspart between April 1, 2007 and September 30, 2008, with the first fill defined as the index date. Included patients were required to be enrolled in a health plan for at least 180 days prior to and 180 days after the index date. Furthermore, patients were required to have been on basal insulin treatment for at least 90 days with or without OADs before and after the index date. Only patients diagnosed with type 2 diabetes who continued previous basal insulin therapy were included. Patients who received bolus therapy other than insulin aspart were excluded.
Patient characteristics
Patient characteristics were identified from administrative enrollment and claims data. The patient characteristics analyzed included gender, age at index date, and geographic region. Pharmacy claims were also examined to identify insulin treatment regimens pre- and post-initiation.
Daily insulin use
The daily average consumption (DACON) of insulin was analyzed from all prescription fills of the index insulin (insulin aspart) and basal insulins (neutral protamine Hagedorn (NPH) insulin, insulin detemir and insulin glargine) during both the pre-index period and post-index period. The mean number of units used per day (U/day) was calculated as the total quantity prescribed excluding the last fill in the study periods divided by the total number of days between refills.
Glycemic control
Laboratory test results were evaluated to identify glycosylated hemoglobin (HbA1c) values. The pre-index date HbA1c value was defined as the mean of the HbA1c values measured for each patient during the pre-index period. Similarly, the post-index date HbA1c value was the mean HbA1c value measured for each patient during the post-index period.
Healthcare costs
Both total healthcare costs and diabetes-related healthcare costs were calculated during the pre- and post-index date periods. Total healthcare costs were defined as the sum of total medical and pharmacy costs, including laboratory costs. Total medical costs were defined as the total paid amounts for all medical services, including inpatient stays, emergency room visits, outpatient visits, and physician office visits during the study period. Total pharmacy costs were defined as the total paid amounts for all prescription fills during the study period. Similarly, diabetes-related healthcare costs were defined as the sum of diabetes-related pharmacy and medical costs. Diabetes-related pharmacy costs were defined as the amounts paid for all diabetes medication, insulin (including devices such as syringes and glucose monitoring meters) and non-insulin (OADs) during the study period. Diabetes-related medical costs were defined as the amounts paid for all diabetes-related medical services during the study period. Healthcare costs were annualized.
Statistical methods
Statistical analyses were carried out using SAS version 9.1.3 (SAS Institute Inc., Cary, NC, USA). All patient characteristics and study measures were compared between patients pre- and post-index date. Only descriptive analyses were performed. Wilcoxon signed rank tests were carried out for the self-comparison of continuous variables pre- and post-index date. McNemar's tests were performed for the self-comparison of categorical variables pre- and post-index date.
Results
Patient flow
A total of 14,020 patients were identified who had more than one prescription fill of insulin aspart during the specified period. Of these patients, 7,039 patients were enrolled in a healthcare plan for more than 180 days before and after the index period. The majority (n=6,889) were diagnosed with diabetes mellitus; among these patients 4,150 patients received basal insulin treatment with or without concomitant OAD therapy for at least 90 days pre- and post-initiation of insulin aspart, of which 2,596 patients had more than one prescription fill of insulin aspart and were not prescribed any other bolus insulin. Most of these patients were diagnosed with type 2 diabetes (n=1,960). Among these patients, 1,739 continued a pre-index basal insulin treatment regimen; treatment was intensified from basal ±OADs and did not change their basal insulin after intensification. Records were analyzed for a mean period of 211 days prior to initiation of insulin aspart, and 339 days post-initiation.
Patient characteristics
Patient characteristics are summarized in . The mean age was 56 years, the majority of patients were male (55%), and the mean pre-index HbA1c value was 8.8%. Most patients (81.7%) were treated with insulin glargine prior to initiation of insulin aspart treatment. The study population is geographically diverse, with the majority of patients situated in the South and Midwest. The most commonly used OAD was metformin, with 40% of patients using metformin pre-index date. A large proportion of patients also used thiazolidinediones (23%) and sulfonylureas (25%).
Table 1. Patient characteristics at the index date.
Daily insulin use
The mean (SD) DACON for NPH insulin, insulin detemir and insulin glargine were 50.84 (29.53) U/day, 45.92 (27.93) U/day, and 43.46 (25.09) U/day, respectively, during the pre-index period compared with 51.06 (28.07) U/day, 47.55 (27.35) U/day, and 44.25 (26.68) U/day, respectively during the post-index period. The mean (SD) DACON for insulin aspart during the post-index period was 31.54 (24.13) U/day.
Glycemic control and hypoglycemic events
The mean (SD) HbA1c value was 8.8% (2.0) during the pre-index period, compared with 8.3% (1.7) during the post-index period. This reflects a statistically significant reduction in mean HbA1c of 0.5% after initiation of insulin aspart (p=0.0013). In total, 151 patients had both pre-and post-index HbA1c data. For this subpopulation mean (SD) pre- and post-index date HbA1c values were 8.6% (2.0) and 8.2% (1.7), respectively, which in turn reflects a reduction in mean HbA1c of 0.4% after treatment intensification with insulin aspart (p=0.0085).
Also, a reduction in the mean (SD) incidence of hypoglycemic events was seen after insulin aspart initiation from 0.87 (5.74) events/patient-year to 0.53 (3.54) events/patient-year (not significant [NS]). Claims databases are not well suited to analyze incidence of hypoglycemic events. Only hypoglycemic events that result in contact with a healthcare practitioner (hospital or other) are correctly recorded in claims databases. Hence, only a fraction of hypoglycemic events are recorded as a claim, and therefore the incidence of events is greatly under-reported when using claims databases.
Healthcare costs
Overall healthcare costs and total diabetes-related healthcare costs were significantly lower for the post-index period compared with the pre-index period (). The mean reduction in overall healthcare costs and diabetes-related costs per patient per year was $2,283 (p=0.0001) and $2,028 (p< 0.001), respectively. Furthermore, a decrease in medical costs and diabetes-related medical costs was observed ($3,670 and $2,511, respectively, NS for both). In contrast, a statistically significant increase in overall costs of prescription drugs and diabetes-related prescription drugs was seen post initiation of insulin aspart ($1,387 and $483, respectively, p< 0.0001 for both); however, non-insulin diabetes-related pharmacy costs decreased by $294 (p< 0.0001).
Table 2. Overall and diabetes-related healthcare costs during the pre- and post-index date periods (costs are per patient per year).
Concomitant oral therapy
The proportion of patients using any OADs significantly decreased from 64% prior to initiation of insulin aspart to 56% post-index date (p< 0.0001) (). The proportion of patients on metformin significantly decreased after initiation of insulin aspart (40 vs. 35%, p< 0.0001). Furthermore, the proportion of patients using a thiazolidinedione or a sulfonylurea significantly decreased post-initiation (p< 0.0001 for both). In total, 9% of patients received an OAD combination regimen (OADs consisting of more than one active substance) prior to initiation of insulin aspart; the proportion of patients on OAD combination therapy decreased to 5% post-index date (p< 0.0001).
Table 3. Number and proportion of patients on concomitant medication during the pre- and post-index date periods.
Healthcare resource utilization and costs
Mean healthcare resource utilization and costs per patient-year are summarized in . A significant reduction in overall inpatient visits was observed post-index date (–1.86 visits/patient/year, p< 0.05), and this was reflected in a $6,336 reduction in costs related to inpatient visits. The number of diabetes-related inpatient visits also decreased after initiation of insulin aspart from 2.19 visits/patient/year to 1.70 visits/patient/year (–0.50 visits/patient/year, p< 0.05). The observed decrease in diabetes-related inpatient visits led to a numerically large, although not statistically significant, decrease in costs of $3,019. Moreover, a significant decrease in diabetes-related office visits was observed after starting insulin aspart (–0.77 visits/patient/year, p< 0.0001), and this corresponded to a significant reduction in costs associated with diabetes-related office visits ($86, p=0.0001).
Table 4. Mean healthcare resource utilization and costs during the pre- and post-index date periods.
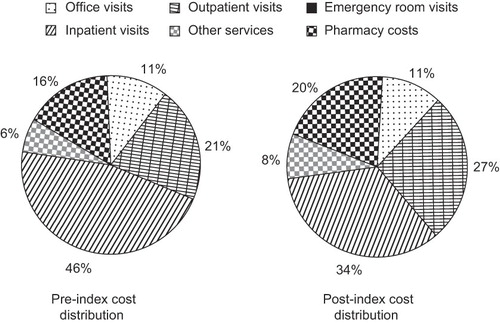
Cost distribution
The majority of healthcare costs are accounted for by inpatient visits (). Indeed, inpatient visits contributed to 46% of healthcare costs. However, this percentage decreased to 34% after initiation of insulin aspart. Outpatient visits are another major contributor to healthcare costs, with 21% and 27% of costs associated with outpatient visits pre- and post-index date, respectively. A small increase was observed in the proportion of costs accounted for by pharmacy costs after insulin aspart initiation (16 vs. 20%).
Discussion
This was a retrospective analysis of adult patients with type 2 diabetes, who were enrolled in a health plan and intensified their previous basal insulin treatment regimen with insulin aspart between April 2007 and September 2008. The patient cohort was self-compared in terms of glycemic control and healthcare costs between the pre- and post-index periods. A small increase in the mean daily dose of basal insulin (NPH insulin, insulin glargine, or insulin detemir) was observed after initiation of insulin aspart treatment. Insulin aspart was prescribed at a mean dose of 32 U/day. After initiation of insulin aspart therapy, an improvement in glycemic control and a non-significant reduction in the incidence of claims-generated hypoglycemia were observed. Indeed, mean HbA1c was reduced by 0.5% in the post-index period compared to the pre-index period (p< 0.01); while the incidence of hypoglycemic events was reduced from 0.87 to 0.53 events/patient-year. These results are in line with other studies that have shown that insulin aspart is effective and well-tolerated. For example, in a randomized study of 231 patients with type 2 diabetes comparing efficacy and safety of insulin aspart to human insulin (HI) and human premix (MIX), a 0.91% decrease in HbA1c was observed for insulin aspart compared with a 0.73% and 0.65% decrease for HI and MIX, respectivelyCitation9. Hypoglycemic events per month were 0.4, 0.56, and 0.19 for insulin aspart, HI, and MIX, respectivelyCitation9.
Treatment intensification, such as add-on of insulin aspart to basal insulin therapy might be considered to be expensive to the healthcare payer. However, in this study significant reductions in overall (p< 0.0001) and diabetes-related (p< 0.001) healthcare costs were observed when diabetes treatment was intensified by adding insulin aspart. These reductions can mainly be attributed to the large reduction in overall and diabetes-related medical costs, even though a small increase was seen for overall and diabetes-related pharmacy costs. Interestingly, non-insulin diabetes-related pharmacy costs decreased after initiation of insulin aspart treatment. Overall, these results may imply that intensification of diabetes treatment with insulin aspart is cost effective or even cost saving. Recently, the cost effectiveness of insulin aspart was compared with that of HI in four European countries (Sweden, Spain, Italy, Poland), using a pharmacoeconomic model based on data from patients in the PREDICTIVE studyCitation13. Insulin aspart as part of a basal-bolus regimen was shown to improve quality-adjusted life expectancy and to reduce the incidence of diabetes-related complications compared with human insulin in all four countries. Furthermore, over a 35-year modeling period, insulin aspart was both more effective and cost saving compared with HI in Sweden and Spain, and would be considered cost effective in Italy, but was not cost effective in Poland, possibly due to a high baseline prevalence of diabetes-related cardiovascular complicationsCitation13.
Major healthcare costs, including diabetes-related costs, are accounted for by inpatient visits. The American Diabetes Association (ADA) has estimated that ∼50% of total diabetes-related costs can be attributed to hospital inpatient careCitation2. In this study cohort 46% of total costs were associated with inpatient visits prior to insulin aspart initiation. The reductions in overall and diabetes-related inpatient visits observed in the present study were not statistically significant but represented a large amount of money and therefore probably accounted for the majority of the cost savings observed with insulin aspart.
A significant reduction was observed for total OAD use after initiation of insulin aspart therapy (p< 0.0001), and this was reflected in a significant decrease in non-insulin diabetes-related pharmacy costs (p< 0.0001). The discontinuation of expensive OADs, such as thiazolidinediones and third-generation sulfonylureas (glimepiride) probably also contributed to the decrease in healthcare costs observed with insulin aspart.
In addition, intensification of diabetes treatment with insulin aspart improved glycemic control, which may lead to a lower incidence of diabetes-related complications over time, and this in turn may reduce healthcare costs. Pharmacoeconomic modeling studies do indeed suggest that the use of analogs saves complication-related costs in the long termCitation13,14. However, the time scale of the present study is too short to have revealed similar findings.
A reduction in the incidence of hypoglycemic events was observed after initiation of insulin aspart, which may again contribute to a reduction in healthcare costs. This decrease was not significant, probably due to the very small number of events observed (0.87 and 0.53 events/patient-year pre- and post-initiation, respectively). However, it is reassuring that insulin intensification did not result in any increase in hypoglycemia, and the reduction in incidence may have played a role in reducing inpatient visits.
This retrospective analysis may be associated with several limitations. A potential limitation is that the follow-up period was not long enough to investigate the long-term effects of intensifying basal-oral therapy by adding insulin aspart, on glycemic control and healthcare costs. This must be taken into consideration when interpreting these results. Furthermore, patients were not randomized, which may imply selection bias. The statistical power of this study may also be limited due to the rather small size of the study population. The choice of patients was solely driven by the design of the study and its inclusion and exclusion criteria. This study has also used a more conservative approach in that the pre- and post-index observation time varied, and this must be taken into account when interpreting these results. Moreover, the absence of a control group in this pre-post analysis may have resulted in exposure to a regression to the mean, and self-comparisons are often limited by time period bias. Finally, the observational retrospective study design limits causal inferences about the results; however, the results highlight an important relationship between initiation of insulin aspart and healthcare resources that warrants further research.
Conclusion
In this managed-care population of patients with type 2 diabetes who intensified their basal insulin regimen with insulin aspart, a significant improvement in glycemic control and a significant decrease in the use of concomitant OAD therapy were observed after initiation of insulin aspart. In addition, patients incurred significantly lower overall and diabetes-related healthcare costs. These results imply that intensification of existing diabetes treatment with insulin aspart may prove to be cost effective.
Acknowledgments
Declaration of interests: The preparation of this article was supported by Novo Nordisk Inc., Princeton, USA. M.A. and W.L. have disclosed they are employees of Novo Nordisk, Inc, and M.A. is also a stock holder. E.M. has disclosed that she is employed by Watermeadow Medical, which received funding from Novo Nordisk, Inc. for editorial assistance and data analysis.
References
- Centers for Disease Control and Prevention, Department of Health and Human Services. Diabetes data and trends. National Diabetes Fact Sheet 2007. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed July 2009
- American Diabetes Association (ADA). Economic costs of diabetes in the US in 2007. Diabetes Care 2008;31:1–20
- Narayan KMV, Boyle JP, Geiss LS, Impact of recent increase in incidence on future diabetes burden. US, 2005–2050. Diabetes Care 2006;29:2114–2116
- American Diabetes Association (ADA). Economic costs of diabetes in the US in 2002. Diabetes Care 2003;26:917–932
- Nathan DM, Buse JB, Ferrannini E, Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193–203
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853
- Holman RR, Paul SK, Bethel MA, Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2008;359:1565–1576
- Leichter S. Is the use of insulin analogues cost-effective? Adv Ther 2008;25:285–299
- Bretzel RG, Arnolds S, Medding J, A direct efficacy and safety comparison of insulin aspart, human soluble insulin, and human premix insulin (70/30) in patients with type 2 diabetes. Diabetes Care 2004;27:1023–1027
- Poulsen MK, Henriksen JE, Hother-nielsen O, The combined effect of triple therapy with rosiglitazone, metformin, and insulin aspart in type 2 diabetic patients. Diabetes Care 2003;26:3273–3279
- Perriello G, Pampanelli S, Porcellati F, Insulin aspart improves meal time glycaemic control in patients with type 2 diabetes: a randomized, stratified, double–blind and cross-over trial. Diabet Med 2005;22:606–611
- Liebl A, Prager R, Binz K, Comparison of insulin analogue regimens in people with type 2 diabetes mellitus in the PREFER study: a randomized controlled trial. Diabetes Obes Metab 2009;11:45–52
- Palmer JL, Goodall G, Nielsen S, Cost-effectiveness of insulin aspart versus human soluble insulin in type 2 diabetes in four European countries: subgroup analyses from the PREDICTIVE study. Curr Med Res Opin 2008;24:1417–1428
- Ray JA, Valentine WJ, Roze S, Insulin therapy in type 2 diabetes patients failing oral agents: cost-effectiveness of biphasic insulin aspart 70/30 vs insulin glargine in the US. Diabetes Obes Metab 2007;9:103–113