Abstract
Background: Transfusion of blood products is often necessary for patients undergoing stem cell transplantation (SCT). The need for red cell and platelet transfusion may vary significantly depending on the type of transplantation and underlying disease.
Methods: In an attempt to evaluate the need and volume of transfusions in patients undergoing SCT at University of Kansas Medical Center, the authors retrospectively evaluated the transfusion data of all patients who received SCT between 2000 and 2005.
Results: A total of 138 (90%) out of 154 patients undergoing autologous SCT and 24 (43%) out of 56 patients with allogeneic SCT exhibited total hematopoietic engraftment and freedom from transfusion (FFT). Time to achieve FFT (median; range) for RBC units for autologous SCT (12; 0–183) was significantly shorter compared with allogeneic SCT (16.5; 0–373). Number of RBC units (median; range) transfused were significantly less in patients undergoing autologous SCT (4; 0–26) compared to patients undergoing allogeneic SCT (6.5; 0–54). The median cost of transfusion was significantly higher in patients undergoing allogeneic SCT (red cell: $2,015; platelet: $4,480) compared to patients undergoing autologous SCT (red cell: $1,240; platelet: $2,520). The authors recognize that this was a retrospective single-center study and practice guidelines may vary from center to center.
Conclusion: Authors conclude that transfusion of blood products is an expensive but integral part of SCT, more so for allogeneic SCT than for patients undergoing autologous SCT. Total FFT is a desirable long-term goal of successful marrow transplantation.
Introduction
Autologous and allogeneic stem cell transplantation (SCT) is a common salvage procedure for advanced hematological and solid organ cancers. For an SCT to be successful, the majority of patients will require multiple red cell and platelet transfusion supportCitation1–5. The need for red cell and platelet transfusion may vary significantly depending upon underlying disease, marrow reserve, and type of transplantationCitation6,7. Transfusional iron overload, allo-immunization, transfusion-associated graft-versus-host disease, transmission of infectious agents, transfusion reactions, and lung injury are adverse consequences of patients receiving blood transfusionCitation8–10. Freedom from transfusion (FFT) is one of the desired endpoints for a successful transplantation and may vary from patient to patient.
In an attempt to evaluate the need, volume, and cost associated with red cell and platelet transfusions among patients undergoing SCT, the role of transfusions in SCT was retrospectively evaluated and analyzed at University of Kansas Medical Center. An effort was also made to establish engraftment kinetics and evaluate time to achieve freedom from transfusion (FFT) after SCT. The impact of transfusion products on survival for both allogeneic and autologous SCT was also evaluated.
Methods
Retrospective data were collected on all patients undergoing autologous and allogeneic transplantation for a period of 5 years, between 2000 and 2005, regarding indications for transplantation, type of SCT, CD34+ stem cell dose, total number of red cell (RBC) and platelet (PLT) units transfused, cost, and time to achieve freedom from transfusion (FFT). For purpose of analysis, the day of stem cell infusion was counted as day 0. Engraftment for white blood cell was defined as absolute neutrophil count more than 500/mm3 for 3 consecutive days and engraftment for PLT was defined as PLT count more than 20,000/μL for 7 consecutive days without transfusion. The trigger for red cell transfusion was hemoglobin lower than 7.0 g/dl. A PLT count less than 12,000/μL was the trigger for PLT transfusion. FFT for RBC was achieved when a patient would maintain hemoglobin level above 7 g/dl without transfusion for at least 2 weeks. FFT for PLT was identical to PLT engraftment by definition.
Granulocyte engraftment, platelet engraftment and freedom from red cell transfusion were the specific endpoints for this study. Overall and disease free survival were analyzed as secondary outcomes.
Statistical analysis
Statistical analysis was performed by SPSS. These analyses included comparisons of medians made using the Mann–Whitney U-test. Overall survival (OS) and disease-free survival (DFS) were calculated from day 0 of stem cell transplantation to the respective event. In the case of DFS, data were measured to the day of disease relapse, or death from any cause. Death was the endpoint for OS from any cause. These event curves were estimated using the Kaplan and Meier methods and significance was corroborated by a Cox regression model.
Cost analysis was performed from the data obtained directly from the hospital and the department of transfusion medicine at the end of the study period. Median and range were used while reporting the cost incurred from the blood product transfusion. Nursing time and cost of ancillary supplies like tubing and flushes were not included in the cost analysis.
Due to inclusion of all patients with different demographics, and underlying diseases, confounding variables were not considered during the regression analysis and this was a recognized limitation of the study. All patients underwent rigorous pre-transplant evaluation to rule out any pre-existing serious co-morbidities. This was done systematically according to standard operating procedure.
Results
In all, 210 patients were transplanted during the study period of which 154 patients (73%) received autologous transplantation and 56 patients (27%) received an allogeneic transplantation.
The median age was 43 years (range: 4–65 years). Indications (n, %) for autologous transplant were breast cancer 64 (42%), non-Hodgkin's lymphoma 40 (26%), multiple myeloma 14 (9%), Hodgkin disease 13 (8%), acute leukemia 12 (8%), solid tumor nine (6%), and chronic leukemia two (1%). Indications for allogeneic transplant were acute leukemia 25 (45%), chronic leukemia 19 (34%), myelodysplastic syndrome (MDS) four (7%), multiple myeloma four (7%), non-Hodgkin's lymphoma 3 (5%), and amyloidosis one (2%) ().
Table 1. Patient characteristics.
Peripheral blood stem cells (PBSC) were used in 153 (99.5%) out of 154 patients undergoing autologous transplantation. Among the patients undergoing allogeneic transplantation, 16 patients (29%) received bone marrow and 40 (71%) received peripheral blood stem cells. The median CD34+ cell count/kg of recipient body weight was 4.2 × 106.
A total of 138 (90%) out of 154 patients who underwent autologous SCT and 24 (43%) out of 56 patients with allogeneic SCT exhibited total hematopoietic engraftment and FFT (both RBC and PLT). The median time for engraftment in days for granulocytes and platelets in autologous SCT was 11 days (range: 2–92) and 14.5 days (range: 0–174), respectively. The median time to engraft for granulocytes and platelets in patients undergoing allogeneic transplantation was 16 days (range: 10–90) and 24.5 days (range: 7–372), respectively (). Time to achieve FFT (median; range) in days for RBC units for autologous SCT (12; 0–183) was significantly shorter compared with allogeneic SCT (16.5; 0–373). Median time to achieve FFT for platelet transfusion for patients undergoing autologous SCT was 14.5 days (range: 0–174) and for allogeneic SCT was 24.5 days (range: 7–372) were the same as PLT engraftment time by definition ().
Table 2. Engraftment data.
Table 3. Transfusion requirement.
The number of RBC units (median; range) transfused were significantly less in patients undergoing autologous SCT (4; 0–26) compared to patients undergoing allogeneic SCT (6.5; 0–54). The number of platelet unit transfusions (median; range) was significantly less for patients undergoing autologous SCT (4.5; 1–37) compared to patients undergoing allogeneic SCT (8; 1–52) ().
Median duration of follow-up was 1.3 years for all patients. Median overall survival after autologous SCT was 2.9 years compared to 3.5 months after allogeneic SCT (p < 0.001). At 5 years, overall survival and disease-free survival for autologous SCT were 35.3%, 32.5%, and 25.3%, and 25.3% for patients with allogeneic SCT, respectively.
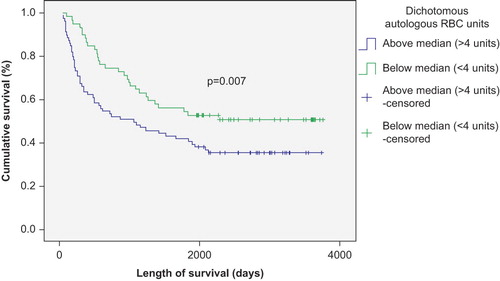
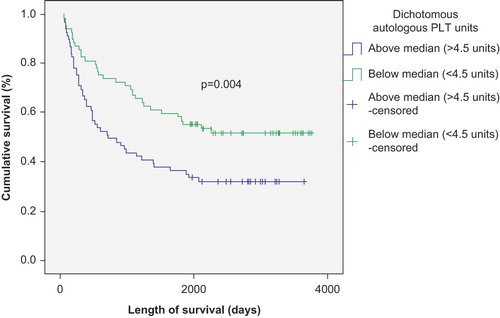
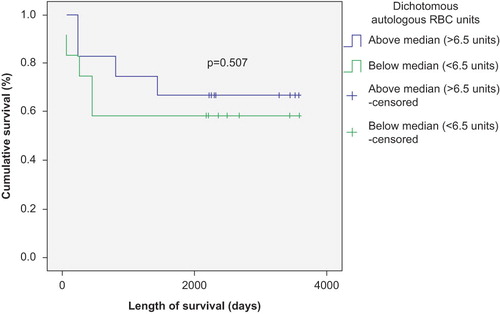
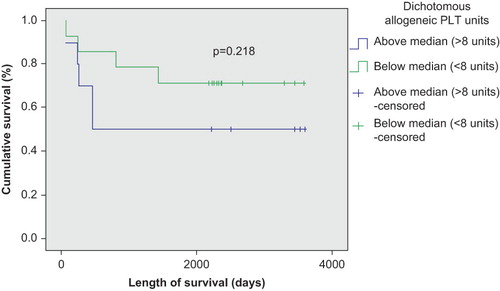
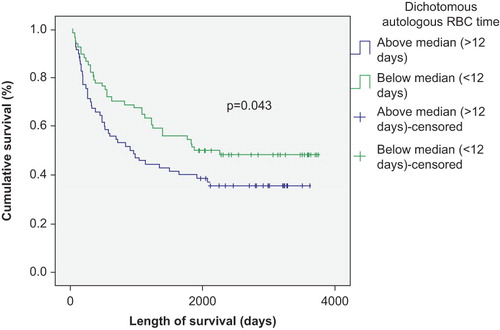
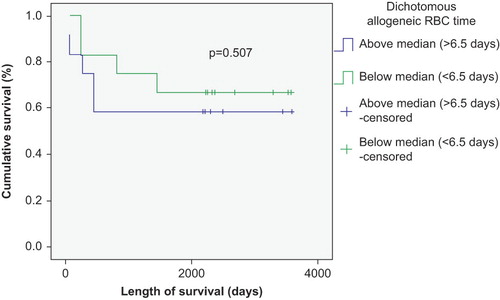
The number of transfused RBC and PLT units negatively correlated with survival in patients with autologous SCT (p < 0.01) ( and ), but was not significantly correlated with survival in patients with allogeneic SCT (p > 0.05) ( and ). This correlation remained strong in both Kaplan–Meier and Cox regression models. There was also a survival advantage noted among autologous SCT patients whose time to achieve FFT from RBC was lower than the median (12 days) compared to those who needed RBC transfusion for longer duration (p < 0.05) (). Such a survival advantage based upon FFT for RBC was not seen among patients undergoing allogeneic SCT ().
The total cost in US$ (median; range) for RBC transfusion for patients undergoing autologous transplantation ($1,240; $0–8,060) was significantly less than patients undergoing allogeneic SCT ($2,015; $0–16,740) (p < 0.01). The cost for PLT transfusion for patients undergoing autologous SCT ($2,520; $560–20,720) was also less compared to patients undergoing allogeneic SCT ($4,480; $560–29,120) (p < 0.05) ().
Table 4. Transfusion unit cost analysis.
Discussion
Conditioning chemo-radiotherapy regimens utilized prior to marrow transplantation leads to a prolonged period of severe pancytopenia. The need for transfusion support during this critical time is essential. Barring rare exceptions, patients undergoing allogeneic transplantation universally require platelet transfusion and the majority will also need red cell transfusionCitation11. Autologous transplantation may require less transfusion but no systematic attempt has been made to explore the role of transfusion in these two different population groupsCitation12.
FFT is a desired goal after any bone marrow transplantation. A total of 138 (90%) out of 154 patients who underwent autologous SCT and 24 (43%) out of 56 patients with allogeneic SCT exhibited total hematopoietic engraftment and FFT (both RBC and PLT). However, several of the patients (10% after autologous transplantation and 57% after allogeneic transplantation) continued to receive RBC transfusion even after they were deemed fully engrafted from a white blood cell and platelet standpoint. The authors consider FFT as a desirable indicator of a successful marrow transplant in the long run.
Time to achieve FFT after marrow transplantation depends on several variables. In the authors' experience, time to achieve FFT for RBC units after autologous SCT was significantly shorter compared with allogeneic SCT by a median of 4.5 days. The median number of platelet transfusions was 3.5 units less in the autologous group compared to allogeneic transplant recipients. Autologous SCT recipients who exhibited total hematopoietic engraftment and FFT achieved granulocyte engraftment a median of 5 days earlier than allogeneic SCT recipients. Autologous SCT recipients achieved platelet engraftment and FFT a median of 10 days earlier than allogeneic SCT recipients.
Among the other variables that have been examined in the past presence of allo-immunization and ABO incompatibility significantly influences transfusion requirementCitation13. The impact of the above variables was not measured in the calculation of time to achieve FFT.
The trigger for prophylactic transfusion of RBC and PLT to this day still provokes debate. A platelet transfusion trigger of 10,000/μl in patients with hematologic malignancy is acceptableCitation14. There is no set guideline for RBC transfusion, but most of the centers accept a hemoglobin level lower than 7 or 8 g/dl as a trigger for red cell transfusion trigger. The transfusion trigger is undergoing debate and reports of successful marrow transplantation in Jehovah's Witnesses underscore the importance of revisiting this issue in a systematic wayCitation15. The other variable for the need of transfusion is pre-transplantation diagnosis, disease status and marrow reserve.
Transfusion prevents hemorrhagic and anemic complications resulting from conditioning regimens, but no study has fully addressed the role of transfusion on overall survival of patients undergoing SCT. Several transfusion-related complications have particular relevance to the transplant settingCitation8–10. This retrospective study suggests that red cell transfusion is associated with decreased survival in patients undergoing autologous SCT. While allograft SCT recipients required a larger number of RBC units spread out over a longer time, this did not seem to affect overall survival. This survival difference was independent of engraftment kinetics of individual patients. The survival difference is likely due to the differences in the underlying diagnosis and disease status, where sicker patients with poorer pre-transplant marrow reserve, secondary to increased numbers of therapy regimens, required more transfusion. Whether exposure to increased number of RBC units in patients undergoing autologous SCT provided an immunomodulatory effect, dampening down the T-effector cells, while the graft-versus-tumor effect is the primary means by which the immune system influences outcomes as far as disease relapse in allo-recipients are open to speculation and may invite future studies involving translational researchCitation16,17. Most animal studies indicate that the mechanisms of immune-modulation are stimulated by leukocytes in the bloodCitation18. The use of universally leukofiltered irradiated blood products in modern day transfusion should to a large extent mitigate any possible antigenic immune modulation.
This may be the first analysis available comparing the cost of transfusion support during SCT until FFT for both autologous and allogeneic SCT. Though the cost of transfusion during transplantation seems high, it was comparable to ongoing transfusion requirement in patients with myelodysplastic syndrome and is definitely less than the cost of transfusion in patients with acute myeloid leukemiaCitation19. As expected, the median cost of transfusion support for both RBC and PLT was less in patients with autologous SCT compared to allogeneic SCT recipients. The cost was obtained directly from the database of the hospital and the department of transfusion medicine. Nursing time, pharmacy time, costs of ancillary supplies were not included during the cost analysis. To avoid practice variation, standard operating procedures clearly mentioning transfusion triggers should be developed for each transplant program. An algorithmic approach will be helpful in cost containment, but keeping the patient safety in the forefront.
Single-center, retrospective design was another limitation of this study in addition to the other limitations addressed above. The authors recognize that practice guidelines vary from center to center. Patient population was not homogeneous as seen in most of the transplant centers. However, a significant attempt was made to streamline practice guidelines and transfusion triggers.
In conclusion, transfusions are commonly performed in support of autologous and allogeneic SCT. FFT for RBC and PLT in addition to WBC engraftment are strong markers for a successful transplant in the long run. Whether decreased survival with more transfusion requirement is related to patient variables or is an independent factor could be sorted out by future prospective trials involving translational research.
Transparency
Declaration of funding
This study was not funded.
Declaration of financial/ other relationships
SG, JPB, JSP, and LT have disclosed that they have no relevant financial relationships.
The JME peer reviewers 1 and 2 have not received an honorarium for their review work on this manuscript. Both have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors wish to acknowledge the help provided by the staff members of the Department of Transfusion Medicine, University of Kansas Medical Center and Professor Barry Skikne for his mentoring.
References
- Mccullough J. The role of the blood bank in transplantation. Arch Pathol Lab Med 1991;115:1195–1200
- Warkentin PI. Transfusion of patients undergoing bone marrow transplantation. Hum Pathol 1983;14:261–266
- Mccullough J, Lasky LC, Warkentin PI. Role of the blood bank in bone marrow transplantation. Prog Clin Biol Res 1984;149:379–412
- Brand A, Claas FH, Falkenburg JH, Blood component therapy in bone marrow transplantation. Semin Hematol 1984;21:141–155
- Clift RA. Cellular support of marrow transplant recipient. Prog Clin Biol Res 1990;337:87–92
- Wulff JC, Santner TJ, Storb R, Transfusion requirements after HLA-identical marrow transplantation in 82 patients with aplastic anemia. Vox Sang 1983;44:366–374
- Weissinger F, Sandmaier BM, Maloney DG, Decreased transfusion requirement for patients receiving nonmyeloablative compared with conventional peripheral blood stem transplants from HLA-identical siblings Blood 2001;98(13):3584–3588
- Hollan SR. Transfusion-associated iron overload. Curr Opin Hematol 1997;4:436–441
- Blumberg N. Allogeneic transfusion and infection: economic and clinical implications. Semin Hematol 1997;34:34–40
- Klein HG. Transfusion in transplant patients: the good, the bad, and the ugly. J Heart Lung Transplant 1993;12:S7–12
- Wandt H, Schaefer-Eckart K, Wilheim M. Two allogeneic hematopoietic stem cell transplantations without the use of blood-product support. Haematologica 2005;90:1292–1294
- Ballen KK, Becker PS, Yeap BY, Autologous stem-cell transplantation can be performed safely without the use of blood product support. J Clin Oncol 2004;22:4087–4094
- Schetelig J, Breitschaft A, Kroger N, ; Co-operative Transplantation Study Group. After major ABO-mismatched allogeneic hematopoietic progenitor cell transplantation, erythroid engraftment occurs later in patients with donor blood group A than blood group B. Transfusion 2005;45(5);779–787
- Diedrich B, Remberger M, Shanwell A, A prospective randomized trial of a prophylactic platelet transfusion of 10 x 10(9) per L versus 30 x 10(9) per L in allogeneic hematopoietic progenitor cell transplant recipients. Transfusion 2005;45(7): 1064-1072
- Mazzas P, Prudenzano A, Amurri B, Myeloablative therapy and bone marrow transplantation in Jehovah's witnesses with malignancies: single center experience. Bone Marrow Transplant 2003;32:433–436
- Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean? Hematology 2005;10(Suppl 1):208–214
- Blajchman MA. Immunomodulatory effects of allogeneic blood transfusions: clinical manifestations and mechanisms. Vox Sang 1998;74(Suppl 2):315–319
- Vamvakas EC. WBC-containing allogeneic blood transfusion and mortality: a meta-analysis of randomized controlled trials. Transfusion 2003;43:963–973
- Gupta P, Leroy SC, Luikart SD, Long-term blood product transfusion support for patients with myelodysplastic syndrome (MDS): cost analysis and complications. Leukemia Res 1999;23:953–959