Abstract
Objective: Opioid users often experience constipation. In this study the impact of constipation on QoL was assessed in patients using opioids either for non-advanced illness or advanced illness.
Methods: Patients using opioids, recruited via public pharmacies, were asked to complete questionnaires on opioid use, constipation and the EuroQol five-dimension questionnaire (EQ-5D). Patients with a severe non-curable disease and relatively short life-expectancy were classified as having an advanced illness; a disabling yet not directly life-threatening condition was defined as non-advanced illness. Constipation was assessed based on questions on opioid side-effects and laxative use. EQ-5D index scores were compared between patients with and without constipation using Wilcoxon two-samples test.
Results: Questionnaires were returned by 588 patients with non-advanced illness, of whom 326 (55%) were classified as having constipation and by 113 patients with advanced illness, of whom 76 (67%) were classified as having constipation. The median EQ-5D index, a weighted health state index score with 1 = full health, was lower in patients with constipation than in patients without constipation (0.31 vs. 0.65, p< 0.01 for non-advanced illness and 0.41 vs. 0.61, p=0.12 for advanced illness).
Conclusion: The results of this study suggest that, in patients using opioids either for non-advanced illness or advanced illness, constipation negatively influences QoL. By separately analysing patients with advanced illness and patients with non-advanced illness, possible selective non-response and confounding was accounted for, but not completely solved.
Key words::
Introduction
Given the negative fundamental effect pain can have on quality of life (QoL), the philosophy underpinning the World Health Organization's three-step analgesic ladder is to free patients from painCitation1. As many cancer patients suffer from moderate-to-severe pain, opioids are the mainstay of analgesic therapy for treating this populationCitation2. Furthermore, opioids are used for the treatment of chronic non-cancer-related pain, mostly musculoskeletal pain. It has been estimated that on a global level a total of 365 million prescriptions were written for opioids in 2005 (235 million prescriptions in the USA, 66 million in the EU and 64 million in the rest of the world)Citation3.
While opioids are the gold standard for treating pain when analgesics such as paracetamol and aspirin do not achieve adequate controlCitation1, adverse effects compromise their therapeutic potential. The gastrointestinal (GI) tract is a significant site of opioid-related adverse effects due to the presence of opioid receptorsCitation4. Activation of these receptors by exogenous opioids disrupts GI motility and secretion, thereby inhibiting normal bowel function. This action commonly causes bothersome GI side-effects, the most common of which is constipation. In Dutch pharmacotherapeutic guidelines, preventive use of laxatives is recommended when opioids are usedCitation5. However, despite the preventive use of laxatives constipation occursCitation6.
Rates of constipation in patients using opioids reported in the literature vary depending on study design, study patients, type of opioid and definition of constipation. In a meta-analysis of randomised placebo-controlled trials of non-cancer patients receiving opioids for moderate-to-severe pain, about 40% of patients experienced constipationCitation7. Rates from the individual trials ranged from 20 to 60%. However, in another meta-analysis also including weak opioids, only 15% of non-cancer patients reported constipationCitation8. A series of studies among cancer patients found that 40–63% of patients had opioid-induced constipationCitation9. The higher rate was observed from prospective patient interviews and the lower rate was obtained from chart audits. Results from a population-based survey of adults using opioids to manage pain unrelated to cancer, show that 57% of participants reported developing constipation in association with opioid useCitation10.
Several studies in different populations and using different QoL measures have shown that in patients with constipation QoL is lower than in non-constipated individualsCitation11,12. Results of a US and European survey in patients taking daily oral opioids for chronic pain (PROBE 1) show that most patients reported that their constipation had at least a moderate negative impact on their overall QoL and activities of daily livingCitation13. The aim of this study was to assess the impact of constipation on QoL measured by the EuroQol five dimension questionnaire (EQ-5D) in patients using opioids. Patients with non-advanced illness and patients with advanced illness were analysed separately as severity of disease may influence the impact of constipation on QoL.
Patients and methods
Study patients
In January 2008, all 1937 public pharmacies registered in the Netherlands were approached to recruit patients using opioids. The study protocol was approved by the privacy committee of the PHARMO Institute, an independent scientific research organisation dedicated to study drug use and outcomes in daily practice. Participating public pharmacies received 40 sets of questionnaires. Eligible patients, i.e. patients visiting the pharmacy for dispensing of a prescribed opioid, were asked by a pharmacy employee to complete a set of questionnaires on opioid use, constipation and QoL (see description below). In case the opioid dispensing was picked up by the patient's caregiver, which is not unlikely for severely ill patients, this caregiver was approached and was given a set of questionnaires and requested to ask the patient to complete these questionnaires. Questionnaires could either be filled in while in the pharmacy or at home, and be returned by mail via a stamped self-addressed envelope. This would take about 30 minutes. When necessary, patients were allowed to be helped with writing down answers. No remuneration was provided for participating in the study. Patients returning questionnaires were included in the study if they reported use of the following opioids that are frequently prescribed in the Netherlands: morphine (including retard formulations), hydromorphone, nicomorphine, oxycodone, pethidine (injectables), fentanyl, alfentanil, sufentanil, piritramide, dextropropoxyphene, pentazocine, buprenorphine, methadone, and tramadol.
Questionnaires
The following questionnaires were used in this study: (1) a generic Dutch questionnaire developed by the PHARMO Institute and Wyeth Pharmaceuticals BV including 21, mainly closed, questions about type, dose, indication and side-effects of opioid use and type, timing and effect of laxative use; (2) the PAC-SYM: validated Dutch translation of a questionnaire about constipation-related symptomsCitation14.
PAC-SYM is composed of 12 items divided into three domains: abdominal symptoms (4 items), rectal symptoms (3 items) and stool symptoms (5 items). For each item the following is asked and rated on a 5-point scale: ‘How severe have each of these symptoms been in the last 2 weeks?’; (3) the PAC-QOL: validated Dutch translation of a questionnaire about the burden of constipation on patients' everyday functioning and well-beingCitation15. PAC-QOL is composed of 28 items, including constipation-related worries and concerns (11 items), physical discomfort (4 items), psychosocial discomfort (8 items) and satisfaction (5 items). For each item, responses related to the last 2 weeks are rated on a 5-point scale; (4) the EQ-5D: validated Dutch translation of a questionnaire on health-related QoLCitation16–18. EQ-5D consists of five dimensions (mobility, self-care, usual activities, pain/discomfort, anxiety/depression) each of which can take one of three responses relating to three levels of severity (no problems/some or moderate problems/extreme problems). Furthermore, it includes a standard vertical 20 cm visual analogue scale (similar to a thermometer) for recording an individual's rating for their current health-related QoL.
Classification
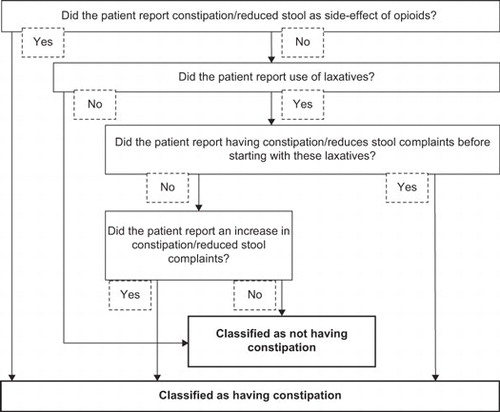
The generic questionnaire about opioid use, constipation and use of laxatives included a question on the indication of opioid use. Based on the indication mentioned, a single person classified patients as having an advanced illness if the indication for opioid use was a severe non-curable disease with a relatively short life-expectancy. Patients were classified as having a non-advanced illness when they were using opioids for (chronic) conditions such as low back pain, which are disabling yet not directly life-threatening. Constipation was assessed based on four questions in this questionnaire on opioid side-effects and use of laxatives. shows how these four questions were used to classify constipation. Patients who reported constipation as a bothersome side-effect of opioid use were directly classified as having constipation. To also include patients who were experiencing constipation, but did not mention it as one of the most bothersome side-effects, three additional questions regarding use of laxatives were used.
Statistical analyses
All questionnaires were processed using SPSS (SPSS Inc., Chicago, IL, USA). Subsequently data was transferred to SAS. Descriptive statistics and a non-parametric test for between-group differences in summary scores for the PAC-SYM, the PAC-QOL and the EQ-5D (Wilcoxon two-samples test, two-sided p-value) were calculated using SAS version 9.1. EQ-5D index (a weighted health state index score) was calculated according to the EQ-5D instruction manual using weights for each dimension and response category (with heaviest weights for middle and high response categories in dimensions Pain and Anxiety). Sum scores for PAC-SYM and PAC-QOL were calculated according to the instruction manuals. Briefly, for total instrument scores, the scores of non-missing items within the instrument were summed and divided by the total number of non-missing items (total score range, 0–4). For PAC-QOL, average scale scores were calculated first and overall score was the average scale score.
Results
Out of 1,937 pharmacies who were invited to the study, 170 pharmacies participated. Questionnaires from 724 patients were received from these 170 pharmacies. Since each pharmacy received 40 sets of questionnaires, which amounts to 6,800 sets of questionnaires distributed to these 170 pharmacies, the response rate at the patient level was 11%. It is likely that not all of these 6,800 questionnaires actually have been handed out, therefore this should be considered a minimum response estimate.
Among the 724 participating patients, 701 patients reported use of any of the aforementioned opioids specific to the Netherlands and were included in the study. Among these 701 users of opioids, 588 (84%) reported back pain and other chronic conditions such as osteoarthritis as the indication for opioid use; these patients were classified as having non-advanced illness. Out of these, 326 (55%) were classified as having constipation, 252 (43%) were classified as having no constipation and for ten patients (2%) constipation was unknown due to failed questions used to determine constipation. Of the 326 patients with constipation, 295 reported constipation as a side-effect of opioids and 31 reported use of laxatives and had constipation complaints at the onset of laxative use. Furthermore, 113 (16%) out of the 701 patients were classified as having advanced illness; this only concerned cancer. Out of these, 76 (67%) were classified as having constipation, 35 (31%) were classified as having no constipation and for two patients (2%) constipation could not be defined. Of the 76 patients with constipation, 64 reported constipation as a side-effect of opioids, 11 reported use of laxatives and had constipation complaints at the onset of laxative use and one patient reported use of laxatives with no constipation complaints at the onset of laxative use but with complaints after starting laxative use.
General characteristics of study patients with non-advanced and advanced illness are shown in , stratified by constipation. Oxycodone and tramadol were the most reported opioids in non-advanced ill patients. In advanced ill patients these were oxycodone and fentanyl. Laxatives were used by 73% of the non-advanced ill patients with constipation and 95% of the advanced ill patients with constipation. Among both patient groups, about 40% of patients with constipation using laxatives developed this constipation after the onset of laxative use. Among patients without constipation, laxatives were used by 12% of the non-advanced ill and 29% of the advanced ill patients. This concerns (successful) preventive use.
Table 1. General characteristics of patients with non-advanced illness and patients with advanced illness, stratified by constipation.
In accordance with the classification of constipation, patients with constipation had a statistically significant higher PAC-SYM sum score (indicates worse symptoms) than patients without constipation (1.25 vs. 0.21, p< 0.01 for non-advanced illness and 0.88 vs. 0.42, p< 0.01 for advanced illness). Similarly, patients with constipation had a statically significant higher PAC-QOL sum score (indicates worse constipation-related QoL) than patients without constipation (1.42 vs. 0.66, p< 0.01 for non-advanced illness and 2.06 vs. 1.56, p< 0.01 for advanced illness).
shows the results of the EQ-5D questionnaire for patients with non-advanced and advanced illness, stratified by constipation. For patients with non-advanced illness, the largest difference in an individual dimension of the EQ-5D between patients with constipation and patients without constipation was observed for self-care, usual activities, pain/discomfort and anxiety/depression. The median EQ-5D index, a weighted health state index score with 1 = full health, was lower in patients with constipation than in patients without constipation (0.31 vs. 0.65, p< 0.01). This index has a bi-modal distribution as shown in . For patients with advanced illness a similar distribution of the EQ-5D index was observed (data not shown). The median EQ-5D index was lower, although not statistically significant, in patients with constipation than in patients without constipation (0.41 vs. 0.61, p=0.12). The largest difference in individual dimension of the EQ-5D between advanced ill patients with constipation and advanced ill patients without constipation was observed for usual activities and anxiety/depression.
Figure 2. Distribution of the EQ-5D index in patients with non-advanced illness, stratified by constipation.
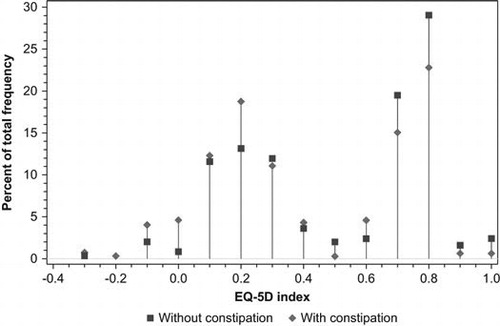
Table 2. Results of the EQ-5D questionnaire for patients with non-advanced illness and patients with advanced illness, stratified by constipation.
Discussion
In this population-based study among users of opioids, QoL was lower in patients with constipation than in patients without constipation, although not statistically significant among advanced ill patients. The median EQ-5D was about similar in constipated patients with non-advanced illness and constipated patients with advanced illness (0.31 and 0.41, respectively). Similarly, non-constipated patients with and without advanced illness had the same median EQ-5Ds (0.61 and 0.65, respectively). These data suggest that the impact of constipation on QoL was similar for patients with advanced illness and patients with non-advanced illness.
The proportion of patients with constipation was 55% among non-advanced ill patients and 67% among advanced ill patients. These percentages are closely comparable to other population-based studies in the literature. In a survey by Cook et alCitation10, 57% of adults using opioids to manage pain unrelated to cancer reported developing constipation. Prospective patient interviews among cancer patients revealed that 63% of patients had opioid-induced constipationCitation9. About 40% of our study patients developed constipation after the onset of laxative use. This may be related to several factors among which the type and dose of laxative prescribed and the compliance of laxative use by the patient. Bell et alCitation13 also found that in individuals taking daily oral opioids for chronic pain, constipation frequently occurred despite the use of laxatives.
The EQ-5D index was lower in patients with constipation than in patients without constipation. In advanced ill patients the difference in EQ-5D was not statistically significant, but this is likely to be the result of the smaller patient numbers in this group. Apparently, even in patients with a life-threatening disease constipation is a major detriment to QoL. There are no studies in the literature reporting EQ-5D index in relation to opioid-induced constipation. However, several studies in different populations and using different QoL measures have shown that in patients with constipation QoL is lower than in non-constipated individuals and treatments for constipation improve QoLCitation11,12.
Some methodological remarks are to be made to the current study. First, the minimum response rate to the survey was 11%. As it is likely that participating in a survey and filling out questionnaires poses a heavier burden on patients with more severe disease, the non-response was probably selective toward patients with more advanced illness and worse quality of life. This issue is partly solved as patients with advanced illness and patients with non-advanced illness were analysed separately. The observed proportions of advanced ill and non-advanced ill patients with constipation, however, may still be an underestimate as (severely) constipated patients may have been less likely to participate in the study. Also, the impact of constipation on QoL in the two patient groups may therefore be underestimated. Alternatively, patients who did not have any problems with their constipation may have had the feeling that this questionnaire was not applicable to their situation and have been less likely to participate. Second, the type of study design does not provide evidence as to a causal relationship between QoL and constipation.
Furthermore, although patients with advanced illness and patients with non-advanced illness were analysed separately, no further analyses were conducted to isolate constipation from other factors potentially influencing QoL because of lacking information. Including questions on these numerous factors would have resulted in a too large questionnaire. It is however not likely that the observed differences in median EQ-5D index (0.31 vs. 0.65 with non-advanced illness and 0.41 vs. 0.61 with advanced illness) will be completely attributable to other factors than constipation. Third, patients who did not report constipation as bothersome side-effect and did not report using laxatives were classified as not having constipation, but may suffer from (very) mild constipation. The likelihood of such misclassification, however, is very small in view of the impact of constipation on QoL observed in this study. Besides, it will only have resulted in an underestimation of the impact of constipation on QoL. Lastly, it should be noted that this study was about constipation in general among users of opioids, and not about opioid-induced constipation specifically.
This study, as well as other available information in the literatureCitation4,11,12,19, shows why prevention of (opioid-induced) constipation is so important. First, as observed in our study as well as by Wald et alCitation12 constipation has a negative influence on QoL. Second, although very uncommon, constipation may result in GI obstruction, dilation, perforation and in some cases even deathCitation4,19. Another important consequence of (opioid-induced) constipation, especially in patients with an advanced illness, may be insufficient absorption of oral medication. Third, constipation can impact the use of opioidsCitation4. Patients may discontinue opioid therapy because of constipation, which can pose challenges in achieving pain therapy goals. In the PROBE studyCitation13 a third of patients reported to have missed, decreased or stopped using opioids in order to make it easier to have a bowel movement. Fourth, constipation poses an economic burden on the individual and societyCitation11. Resource utilisation associated with the diagnosis and management of constipation is a significant cost driver, whereas constipation prevention programmes have demonstrated cost savings. In addition, it is likely that constipation also has an impact on caregivers.
New therapeutical options such as selective peripheral opioid antagonists, that target the cause of the opioid-induced constipation could contribute to the treatment of constipation and thereby improve overall quality of lifeCitation20,21.
In conclusion the results of this study suggest that, in patients using opioids either for non-advanced illness or advanced illness, constipation negatively influences QoL. A targeted therapy addressing (opioid-induced) constipation might be of importance for patients using opioids.
Transparency
Declaration of funding: This study was financially supported by an unrestricted grant from Wyeth Pharmaceuticals BV, Hoofddorp, The Netherlands. No limitations were set with regard to the conduct of the study and the writing of the manuscript. Wyeth is now a part of Pfizer, Inc., the merger of local entities may be pending and is subject to full local regulatory approval.
Declaration of financial/other relationships: F.J.A. Penning-van Beest. P. van den Haak and R.M.C. Herings have disclosed that they are employees of the PHARMO Institute, which receives grants from several pharmaceutical companies, including Wyeth. R.M. Klok and Y.F.D.M. Prevoo have disclosed that they are employees of Wyeth Pharmaceuticals BV.
The JME peer reviewers 1 and 2 have not received an honorarium for their review work on this manuscript. Both have disclosed that they have no relevant financial relationships.
Acknowledgements: The authors would like to thank M.W. van der Linden for his work in the design and conduct of the study.
In addition, the results have been presented on a poster at the 11th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR, 8–11 November 2008, Athens, Greece).
References
- World Health Organization. WHO's pain ladder. Available at: www.who.int/cancer/palliative/painladder/en/index.html.
- Quigley C. The role of opioids in cancer pain. BMJ 2005;331: 825-829.
- Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract 2007;61:1181-1187.
- Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs 2007;16:181-194.
- Nederlands Huisartsen Genootschap. Farmacotherapeutische Richtlijn Pijnbestrijding. Oktober 2007. Available at: nhg.artsennet.nl/upload/104/standaarden/FTR/Pijnbestrijding.html.
- van Schieveen S, Kramer P. Omgaan met obstipatie door sterke pijnmedicatie. Onderzoek onder patiënten en mantelzorgers. Available at: http://www.dubbelop.info/Portals/2/multimedia/TNSNIPOOmgaanmetoic.pdf.
- Kalso E, Edwards JE, Moore RA, McQuay HJ. Opioids in chronic non-cancer pain: systematic review of efficacy and safety. Pain 2004;112:372-380.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther 2005;7:R1046-1051.
- McMillan SC. Assessing and managing opiate-induced constipation in adults with cancer. Cancer Control 2004;11:3-9.
- Cook SF, Lanza L, Zhou X, Gastrointestinal side effects in chronic opioid users: results from a population-based survey. Aliment Pharmacol Ther 2008;27:1224-1232.
- Dennison C, Prasad M, Lloyd A, The health-related quality of life and economic burden of constipation. Pharmacoeconomics 2005;23:461-476.
- Wald A, Scarpignato C, Kamm MA, The burden of constipation on quality of life: results of a multinational survey. Aliment Pharmacol Ther 2007;26:227-236.
- Bell TJ, Panchal SJ, Miaskowski C, The prevalence, severity, and impact of opioid-induced bowel dysfunction: results of a US and European patient survey (PROBE 1). Pain Med 2009;10:35-42.
- Slappendel R, Simpson K, Dubois D, Keininger DL. Validation of the PAC-SYM questionnaire for opioid-induced constipation in patients with chronic low back pain. Eur J Pain 2006;10: 209-217.
- Marquis P, De La Loge C, Dubois D, Development and validation of the Patient Assessment of Constipation Quality of Life questionnaire. Scand J Gastroenterol 2005;40:540-551.
- The EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16: 199-208.
- Brazier J, Jones N, Kind P. Testing the validity of the EuroQol and comparing it with the SF-36 health survey questionnaire. Qual Life Res 1993;2:169-180.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72.
- World Health Organization. Symptom relief in terminal illness. Geneva: World Health Organization, 1998;24-33.
- Fakata KL, Cole BE. Peripheral opioid antagonists: a therapeutic advance for optimizing opioid gastrointestinal tolerability. J Fam Pract 2007;56:S3-12.
- Moss J, Rosow CE. Development of peripheral opioid antagonists' new insights into opioid effects. Mayo Clin Proc 2008;83: 1116-1130.