Abstract
Objective: Reduction in health-related quality of life is common in children born small for gestational age (SGA) or children with growth hormone deficiency (GHD). Growth hormone treatment with somatropin in these children leads to normalisation of height. The aim of this study was to determine whether somatropin is a cost-effective treatment option for short children born SGA and GHD children in Sweden.
Methods: A Markov decision-tree model was used to calculate the relative costs and health benefits associated with somatropin treatment over the lifetime of SGA and GHD children, compared with no treatment. The analysis was undertaken from a Swedish Health Service perspective. As quality-adjusted life-year (QALY) data were not obtained directly in the clinical studies, a degree of uncertainty is related to these results. Sensitivity analyses assessed the degree of uncertainty surrounding central parameters.
Results: For short children born SGA, somatropin treatment was associated with an additional 3.29 QALYs at an incremental cost of 792,489 SEK (Swedish Krona), compared with no treatment. For GHD, somatropin treatment resulted in 3.25 additional QALYs at an incremental cost of 391,291 SEK. This equates to an incremental cost per QALY of 240,831 SEK and 120,494 SEK for SGA and GHD, respectively, below a cost-effectiveness threshold of 500,000–600,000 SEK/QALY.
Conclusions: Somatropin is a cost-effective treatment strategy in Sweden for children with GHD and SGA. To overcome present study limitations future clinical research should incorporate appropriate quality of life questionnaires.
Introduction
Small for gestational age (SGA) is defined as a birth weight and/or birth length that is less than −2 standard deviation scores (SDS) from the meanCitation1. SGA is associated with an increased risk of not reaching normal adult heightCitation2. The condition also carries an elevated risk of developing cardiovascular complications, obstructive pulmonary disease, type 2 diabetes mellitus, renal insufficiency and impaired reproductive functionsCitation3–8. Approximately 5% of all newborns are born SGACitation9. Of those born SGA, most achieve appropriate catch-up growth by 2 years of age, but about 15% do not catch up to a height above −2 SDS from the meanCitation3,10.
Growth hormone deficiency (GHD) is generally due to the pituitary gland being unable to produce sufficient growth hormone (GH) to generate the growth process. The diagnosis of GHD in childhood is a complex process involving comprehensive clinical and auxological assessment (primarily looking at height standard deviation scores (HSDS)). The GHD diagnosis is usually confirmed by appropriate GH provocation tests, which evaluate the GH reserve of the children. GHD is associated with different problems at various ages. In newborn infants the primary manifestations may be hypoglycaemia or micropenis. In later infancy and childhood, growth failure may be the major effect. Adults with growth hormone deficiency may have diminished lean body mass and poor bone density and a number of physical and psychological symptoms, including poor memory, social withdrawal, and even depressionCitation11.
Growth failure for GHD children or the failure to achieve appropriate catch-up growth for SGA children results in persistent short stature and may be associated with a significant psychosocial impairment. A recent study of the general UK population has demonstrated the negative impact that short stature can have on a person's quality of lifeCitation12. The study linked HSDS and health-related quality of life (HRQoL), which combines a person's physical, psychological and social well being into a single health-related outcome measure or utility scoreCitation13. Health technology assessment agencies, such as the Pharmaceutical Benefits Board (TLV) in Sweden and the National Institute for Health and Clinical Excellence (NICE) in the UK, utilise HRQoL data in order to evaluate the cost-effectiveness of medical interventions and therefore provide national guidance on the promotion of good health. These analyses capture outcomes in the form of a cost per quality adjusted life year (QALY). This outcome combines the cost of an intervention with the quantity and quality of life that the treatment provides and indicates the additional costs required to generate an additional year of perfect health (one QALY)Citation14. In this way cost/QALY ratios can be used to assess and compare the benefits afforded by various healthcare interventions across different disease states. In Sweden, a suggested threshold for cost-effectiveness is 500,000–600,000 SEK per QALYCitation15.
Growth hormone is essential for normal growth in children and acts by increasing growth via direct action on the growth plates and by production of insulin-like growth factors. GH is currently used to treat various conditions associated with growth failure or retardation, including GHD, Turner syndrome, chronic renal insufficiency, idiopathic short stature and SGA. Patients with proven GHD and SGA should be treated with recombinant GH as soon as possible after the diagnosis is made (SGA children should be ≥4 years according to EMEA approved indication). The primary objectives of the GH therapy of GHD and SGA children are normalisation of height during childhood and attainment of normal adult heightCitation1,11.
The present study set out to determine the cost- effectiveness of GH therapy (somatropin) compared with ‘no treatment’ in children with GHD and short children born SGA in line with the treatment options available in clinical practice. The study is founded upon a systematic review of GHD literature and a long-term RCT of somatropin (Norditropin) in SGA childrenCitation16,17. The clinical data (start and final HSDS) are linked to HRQOL dataCitation12 to calculate the cost/QALY, and as far as the authors are aware, this is the first study of its kind in Sweden.
Methods
A Markov decision-tree model was constructed in Microsoft Excel to compare the cost-effectiveness of a somatropin treatment regimen with no intervention, for the treatment of GHD and SGA children, from a Swedish health service perspective. The model included health states for ‘alive and not treated’, ‘alive and treated’ and ‘dead’. The model assumes that a daily subcutaneous injection of somatropin is administered for the duration of treatment, unless a patient completes treatment or dies. Swedish population mortality rates were applied in each cycle, the mortality rate was assumed equal for treated and untreated patients. The model had 74 yearly cycles from the initiation of treatment, based on the average age of the treated GHD and SGA children patients and Swedish life tablesCitation18. The transition probabilities (the duration of treatment) were estimated from clinical trials or observational studies. The daily somatropin dose was calculated according to the child's weight. Cost of monitoring the children was applied to both the treatment and no treatment cohorts. Patients in the treatment cohort received a benefit of an additional HSDS gain relative to patients in the no treatment cohort. In each yearly cycle, HRQoL is estimated based upon the HSDS. Individuals are assumed to maintain the same HRQoL after treatment has stopped for the rest of their lifetime. However, the additional utility value was only applied after 2 years of treatment to mimic the normal catch-up growth durationCitation19. In each cycle the total costs and QALYs are calculated by multiplying the individual costs and HRQoL by the number of people in the cohort still alive for the treatment and no treatment cohorts. The model calculates the total discounted QALY gain, and overall cost of treatment for the treatment and no treatment cohorts. Although some clinical evidence is available, the model did not include reduced risk of morbidities, such as osteoporosis fracture, birth by caesarean section or diabetes in patients treated with somatropin. Both costs and utility outcomes, occurring beyond the first year of treatment, were discounted at a rate of 3.0% as stipulated in the guidelines for economic evaluations from the Swedish Pharmaceutical Benefits BoardCitation20. The effect of varying the discount rate was assessed in the sensitivity analysis.
The GHD analysis is based on clinical data obtained in a systematic literature review. The SGA analysis is based on clinical data from a long-term, multicentre, double-blind, randomised, two-arm clinical trial comparing the effects of somatropin on long-term growth and final (adult) height in children born SGACitation16,17.
Data sources
SGA population
The SGA patient population modelled reflects the patient population from the somatropin trialCitation17, previously reported by van Pareren et alCitation19 and Bannink et alCitation16. In accordance with the SGA indication approved by the European Medicines Agency (EMEA), the evaluated somatropin dose was 0.033 mg/kg/day for SGA. A higher dose of somatropin (0.067 mg/kg/day) was also evaluated in the SGA RCTCitation17. However, this dose regimen is not included in the present study because the higher dose is not recommended by EMEA for the treatment of SGA childrenCitation21. Moreover, the present cost-effectiveness analysis only evaluates the clinical data for SGA children whose height at the time of inclusion in the clinical study was < −2.5 height SDS in accordance with the EMEA approved indication. The trial enrolled 79 children who were randomised to receive a regimen of low- (0.033 mg/kg/day) or high-dose (0.067 mg/kg/day) somatropinCitation19. In all, 26 of the 79 children had both GHD and SGA and were consequently excluded from the analysis set. A total of 38 children completed the study and finalised treatment. Six children were classified as still growing (height velocity > 2 cm per year). Nine withdrew from treatment for other reasons (e.g. moving from area). No patients withdrew due to adverse events. The 38 children were classified as appropriate for the intent-to-treat (ITT) adult height analysis at the end of the study. A total of 19 children were excluded from the present analysis as they were treated with high-dose somatropin (0.067 mg/kg/day) which is not licensed in Sweden. Demographics of the 19 patients included in the SGA study population are summarised in Citation17.
Table 1. Patient demographics.
GHD population
To obtain clinical data for the GHD children a systematic review was performed. The search strategy was comparable to the one used by NICE in their 2002 appraisal of somatropin, expect it was limited to GHDCitation22. Search terms for ‘somatropin’ included: somatropin, somatotropin, somatotrophin, growth, hormone, growth hormone, genotropin, humatrope, norditropin, saizen, zomacton, nutropin. Search terms for ‘GHD’ included: Growth hormone deficiency, growth hormone deficien*, GH-deficien*, GHD. Search terms for ‘height outcomes’ included: Adult height, Final height, HSDS, Height SDS. The searches were conducted on March 2, 2009 and no date restrictions were applied (inclusive search dates were 1966 – March 2, 2009). The initial search identified 27 relevant studies. To ensure validity and quality of the selected studies only studies with n> 300 and data on long-term (> 2 years) treatment duration, start height and final height were included. For studies which reported updates of the same database/registry the latest available publications were selected. For the purpose of this study, only studies reporting ‘final height’ were considered. Data on ‘age at treatment start’, ‘gender’, ‘pre treatment HSDS’, ‘treatment duration’, ‘dosing’ and ‘final HSDS’ were extracted and analysed. The four publications finally selected covered data in the KIGS registryCitation23, the NCGS registryCitation24, the French GHD registryCitation25 and the Dutch GHD registryCitation26. Demographics of the GHD study population are summarised in .
Efficacy
In the SGA analysis, HSDS data for SGA patients treated with somatropin were obtained from the somatropin clinical trial dataCitation17. A placebo controlled (‘no treatment’) group was not included in the clinical trial as this was considered unethical, given the well known beneficial treatment effects of growth hormone. Therefore, to enable a comparison of somatropin regimens with ‘no treatment’ in this analysis, it was assumed that SGA patients in the ‘untreated’ arm started on the same growth curve as the treated group and gained +0.3 height SDS score as determined from a cohort of matched untreated short children born SGA described by van Pareren et al (2003)Citation19.
For the GHD analysis starting and final HSDS were obtained from the systematic literature review and meta-analysis. To enable a comparison of somatropin regimens with ‘no treatment’ in this analysis, it was assumed that GHD patients in the ‘no treatment’ arm stayed on the same growth curve. Although children with GHD may have a HSDS within the normal range initially, as the deficiency continues they fall further and further behind in growth. Thus the assumption that untreated children with GHD would have a final HSDS equal to their pre-treatment HSDS is conservative as untreated children often demonstrate ‘fall away’ in height and decrease in height if they remain untreated. This assumption is supported by Harris et alCitation27.
For both the GHD and SGA analyses it was assumed that no patients discontinued treatment due to adverse events. This is based on the fact that no child stopped treatment due to adverse events in the long-term SGA clinical trialCitation16. Moreover the EMEA SPC states ‘Adverse reactions in children are rare……Headache has been reported with an incidence of 0.04 per patient per year’Citation28.
Non-responders to somatropin therapy in SGA and GHD are very rare. However, a few children may stop treatment although adult height has not been reached (but a satisfying height is reached). The suboptimal height of these children are reflected in the mean HSDS results as well as in the average treatment duration used.
Drug cost and resource use
The analysis was conducted from the perspective of the Swedish healthcare system. Costs were obtained from published sources and based on 2009 pricesCitation29,30. The per-patient cost of somatropin is calculated based on a cost of 234.14 SEK per mgCitation30. The dosage for SGA was 0.033 mg/kg/day as is in the SGA somatropin RCTCitation19. For GHD the literature review revealed a mean dose of 0.023 mg/kg/day. The mean weight of the children was obtained from the SGA somatropin RCTCitation17 and was assumed equal for both GHD and SGA children. As expected with a cohort of children, the mean population weight increased annually throughout the treatment period ()Citation17.
The proportion of children requiring somatropin treatment every year following initiation of treatment determined the total cost of treatment. For the GHD children the literature review revealed an average treatment duration of 5.1 years (SD: 1.8).
Table 2. The increase in patient's weight and age over the course of somatropin treatmentCitation17.
No data on treatment compliance was available from the somatropin trial. However, poor compliance has been identified as a reason for suboptimal results in the treatment of GH-deficient childrenCitation31. An evidence based analysis of GH efficacy has estimated treatment compliance to be 90.1%, corresponding to approximately three missed injections per month and this assumption was applied to the cost-effectiveness analysisCitation31.
All children were assumed to visit an endocrinologist four times per year, for the duration of somatropin therapy, irrespective of their treatment. The number of visits required per year was based on the treatment plan defined in the SGA clinical trialCitation17. Each endocrinologist visit was assumed to cost 2,945 SEK based on a level 1 endocrinology visit in SwedenCitation29.
Health-related quality of life (HRQoL)
Utility data was derived from a recent UK based study which assessed the relationship between short stature and HRQoL in 14,416 adults (aged ≥18 years)Citation12. During this study, HRQoL was measured using the EQ-5D (EuroQoL) questionnaire to assess five dimensions of health (mobility, self-care, usual activities, pain/discomfort, anxiety/depression)Citation32. Each dimension comprised three levels (no problems, some/moderate problems, extreme problems). Using an EQ-5D scoring algorithmCitation33, the five domains were summarised into a single utility score for different HSDS ranges ().
Table 3. Utility scores associated with height SDS categoriesCitation12.
In this analysis utility scores were used to calculate the improvement in HRQoL for the different treatment regimens, over the lifetime of the patient, according to the study by Christensen et alCitation12. The study of Christensen et alCitation12 used banded HSDS. This however, is limited due to banding occurring at 0.5 HSDS increments, and the large band increment means that utility benefits relating to increases in height would not be captured if a patient failed to move into a higher HSDS band. Consequently, in order to more accurately capture the benefit in utilities associated with somatropin treatment, a linear interpolation was performed using the midpoint of the published HSDS bandsCitation12 to determine a utility value for each 0.01 increment in HSDS. The utility value remained at 0.687 if HSDS fell below −3, in line with the utility value for −3 HSDS as described in the study by Christensen et alCitation12. This approach is conservative since unless HSDS exceeds −3, the utility benefit associated with increases in height would not be captured.
Base-case analysis
A utility value was assigned to the patient population on initiation of GH treatment according to the average HSDS at the start of the somatropin treatment (). During treatment patients will increase in height and approach that of the normal height growth curve. In the base-case analysis it is assumed that maximum catch-up growth will occur at 2 years and after this time point, patients will remain at their final HSDS for the remainder of their life (). This is based on evidence that short children born SGA undergoing somatropin treatment catch up with the normal height growth curve (> −2 HSDS from the mean) after approximately 2 years of treatmentCitation19. Therefore, it was assumed that these patients continued to grow according to the normal height growth curve, until a final height was achieved and treatment ended. Since an attainment of catch-up growth will not be instantaneous a linear increase in height over the 2-year time period has been assumed. At each annual time point a utility value was assigned to the patient population according to the average HSDS attained. The impact upon cost-effectiveness which resulted from variations in the period of time over which catch-up growth to within a normal HSDS range occurs was assessed in sensitivity analysis.
Alternative scenario analyses
Timing of HRQoL benefit
In a more optimistic approach (scenario one), treatment was assumed to result in an immediate increase in height that would bring them in line with their optimum height. As such, changes in HRQoL associated with final outcomes were attributed to patients following the first year of treatment. In a more conservative approach (scenario two), changes in HRQoL, associated with final outcomes, were assumed to take place in the years following completion of somatropin treatment.
Impact of adverse events
Adverse events were not included in the base-case analysis as adverse events in children treated with somatropin are rare and mild to moderate in intensity. However, in an alternative scenario it was assumed that all patients treated with somatropin would require an additional endocrinologist visit per year due to adverse events.
Univariate sensitivity analysis
Univariate or one-way sensitivity analyses were performed, in which all model parameters including efficacy and costs were systematically and independently varied using a realistic minimum and maximum value (). This type of analysis was used to demonstrate whether the cost-effectiveness results were particularly sensitive to any of the model parameters.
Table 4. Variables used in the univariate and probabilistic sensitivity analyses.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis (PSA) quantified the degree of uncertainty surrounding the results, based on the precision with which input parameters were estimated. In this analysis, all model parameters were simultaneously varied over the distribution of values that each parameter could potentially take and the incremental costs and benefits for each treatment were calculated ().
Results
Base-case results
Somatropin treatment of short children born SGA was associated with an incremental drug acquisition cost of 764,357 SEK per patient, compared with no treatment (; discounted costs). Clinician visits amounted to an average of 79,882 SEK per treated patient (; discounted costs). These additional costs were offset by the clinical benefit achieved over the life-time of the SGA patient, manifested as an increase in height and represented by an incremental QALY gain for somatropin of 3.29, compared with no treatment (; discounted QALYs). Somatropin therapy in SGA was associated with a discounted cost/QALY of 240,831 SEK (116,242 SEK, undiscounted), compared with no treatment.
Table 5. Base-case results: per-patient costs and benefits associated with somatropin therapy.
Somatropin treatment of GHD children was associated with an incremental drug acquisition cost of 372,949 SEK per patient, compared with no treatment (; discounted costs). Clinician visits amounted to an average of 51,837 SEK per treated patient (; discounted costs). These additional costs were offset by the clinical benefit achieved over the life-time of the GHD patient, manifested as an increase in height and represented by an incremental QALY gain for somatropin of 3.25, compared with no treatment (; discounted QALYs). Somatropin therapy in GHD was associated with a discounted cost/QALY of 120,494 SEK (53,733 SEK, undiscounted), compared with no treatment.
Alternative-scenario analyses results
Timing of HRQoL benefit
Variation in the time at which patients achieved quality of life benefits as a result of treatment was tested in two alternate scenario analyses. In both cases incremental costs associated with drug acquisition and clinician visits remained unchanged from the base-case scenario (; discounted costs).
In scenario one, patients were assumed to achieve improvements in quality of life following the first year of somatropin treatment. This resulted in an increased QALY gain compared with base case for somatropin therapy for SGA and GHD (3.32 QALYs for SGA and 3.38 QALYs for GHD) and an associated reduction in cost/QALY (discounted) to 238,698 SEK and 119,688 SEK for SGA and GHD, respectively.
In scenario two, patients were only deemed to have achieved improvement in HRQoL following completion of their somatropin therapy, resulting in a smaller QALY gain compared to base case (2.90 QALYs for SGA and 3.12 QALYs for GHD). As a result the cost/QALY (discounted) for somatropin compared with ‘no treatment’ increased to 273,126 SEK and 125,437 SEK for SGA and GHD, respectively.
Impact of adverse events
Assuming that all patients treated with somatropin have one additional endocrinologist visit per year to treat adverse events increases the overall treatment cost by 25,875 SEK for SGA patients and 16,432 SEK for GHD patients. Consequently, this increases the cost/QALY (discounted) to 248,694 SEK and 125,546 SEK for SGA and GHD, respectively.
Univariate sensitivity analyses
Univariate sensitivity analyses showed that the cost- effectiveness of somatropin was affected by changes in the utility scores associated with the HSDS bands; the HSDS band < −3.0 from the mean was particularly sensitive to changes. These results are unsurprising, since most patients occupy this height SDS band prior to receiving treatment. An increase in the HRQoL associated with this height SDS band in isolation would reduce the QALY gain observed upon somatropin treatment. However, all utility scores were obtained from the same study suggesting that if uncertainty surrounded one utility score then all utility scores employed in the analysis would be associated with the same level of uncertainty. As such, the impact of changing one utility score in isolation should be viewed with caution.
Analyses were very sensitive to the discount rates employed, in particular for outcomes; increasing discount rates to 5% for outcomes resulted in an increase in the ICER for all treatment comparisons. For SGA, the base-case ICER was increased to 354,806 SEK. Simultaneously increasing the discount rate for both outcomes and costs to 5% increased the base-case ICER to 321,894 SEK. Using a lower discount rate for both outcomes and costs results in a reduction in the cost/QALY obtained. This has been demonstrated in the base-case analysis where results are presented for undiscounted outcomes and costs (0%; ).
In the base-case analysis it was assumed that patients had a compliance rate of 90.1% for somatropin treatment. Although increased treatment compliance would increase drug costs; even when 100% treatment compliance was assumed, somatropin was still cost-effective compared with no treatment. The cost/QALY (discounted) was 266,640 SEK and 133,235 SEK for SGA and GHD, respectively.
The cost/QALY estimates for both GHD and SGA remained below 600,000 SEK when all other model parameters including efficacy rates were varied.
Probabilistic sensitivity analysis
Simultaneous variation of all modelling parameters within realistic ranges revealed a high level of certainty (73.4%, ), that somatropin was cost-effective for the treatment of children born SGA, when the willingness-to-pay threshold of 500,000 SEK was consideredCitation15. Assuming a willingness-to-pay threshold of 600,000 SEK per QALY, there was a 77.6% chance that somatropin was cost-effective. The willingness-to-pay threshold would need to drop below 260,000 SEK for the certainty of cost-effectiveness to drop below 50%.
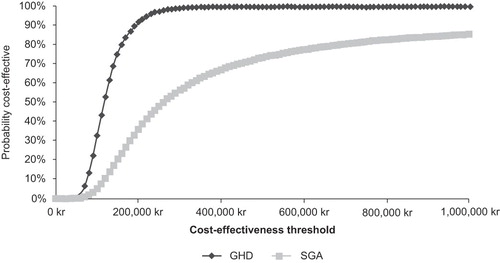
Simultaneous variation of all modelling parameters within realistic ranges revealed a high level of certainty (99.9%, ) that somatropin was cost-effective for the treatment of children with GHD when the willingness-to-pay threshold of 500,000 SEK was consideredCitation15. The willingness-to-pay threshold would need to drop below 117,000 SEK for the certainty of cost-effectiveness to drop below 50%.
Discussion
The advent of recombinant human growth hormone (GH) has transformed the clinical management of children and adolescents who are short as a result of endocrinological conditions. GH therapy has been shown to have a good safety profile and to be highly effective at promoting growth in GHD children and children born SGA who fail to demonstrate spontaneous catch-up growthCitation17,19. However, as far as the authors are aware this is the first cost-effectiveness study carried out to assess the effects of GH therapy (somatropin), in the treatment of GHD and SGA children from a Swedish perspective.
Until recently limited data existed to determine the effect of short stature on health-related quality of life (HRQoL) due to underpowered studies and the fact children find self reported questionnaires difficult to completeCitation34. HRQoL data is increasingly recognised as an important measure of the impact of a disease or therapeutic outcome. Although NICE approved GH for the treatment of children with growth failure in 2002, it had reservations about the calculations of the likely utility gain and so recommended further studies to clarify the clinical impact of GH treatment on quality of lifeCitation35.
HRQoL data collected during the pivotal somatropin clinical trial demonstrated that children born SGA, when treated with long-term GH therapy, showed significant improvements in many aspects of quality of life, compared to children receiving no treatment, including physical abilities (p=0.002) and contact with adults (p=0.002)Citation36. A recent UK based study has confirmed that quality of life is positively correlated with increasing height and for the purposes of determining cost-effectiveness has provided a single measure of the quality of life (utility scores) associated with different height rangesCitation12. By mapping utility scores to the proven height gain achieved by GHD and SGA children treated with somatropin, valuable improvements in HRQoL have been demonstrated. Overall, this economic evaluation has shown that somatropin is a cost-effective treatment for GHD and SGA in Sweden, costing 240,831 SEK/QALY for SGA and 120,494 SEK/QALY for GHD (discounted, base case), relative to no treatment.
Cost/QALY estimates remained below 600,000 SEK when all base-case assumptions were independently changed within realistic ranges. Probabilistic sensitivity analysis, in which all parameters within the model were varied at the same time, showed the economic analysis to be extremely robust with a high probability that somatropin was cost-effective (using a Swedish willingness-to-pay threshold of 500,000 – 600,000 SEK).
The difference in the cost-effectiveness results for GHD and SGA is hardly surprising given the fact that the SGA children are treated with a higher dose of somatropin for a longer duration of time, resulting in a higher cost per QALY. However, somatropin therapy for both indications are considered ‘good value for money’ when compared with the willingness-to-pay threshold of 500,000–600,000 SEK/QALY in Sweden.
The current findings are supported by a recent multinational study of the cost-effectiveness of height screening programmes in children aged 4–11 yearsCitation37, which demonstrates that quality of life benefits are associated with early screening and treatment of various height-related conditionsCitation37.
When conducting an economic analysis of this kind, the uncertainty surrounding the robustness of the data used should always be tested through sensitivity analyses. Analysis was carried out to assess whether ‘time to attainment of quality of life benefits’ as a result of somatropin treatment had an effect on cost-effectiveness. Assuming that patients did not achieve an improvement in HRQoL until completion of their somatropin therapy, a smaller QALY gain compared to ‘no treatment’ was observed and the cost/QALY for somatropin compared with ‘no treatment’ increased slightly. However, this scenario appears to be unrealistic; patients treated with somatropin have been shown to achieve improvements in height within 2 years of treatment initiationCitation19 and this would be expected to be mirrored by quality of life benefits.
Although several publications have demonstrated improved treatment satisfaction and improved HRQOL in children as a result of somatropin treatmentCitation38, very few studies have evaluated utility results. The QALY results in the present study are consequently based on the pivotal assumption that utility can be mapped onto HSDS scores. Although criticism may be levelled at the study design since utility scores were estimated and not directly obtained from study participants, the indirect assessment of utility applied in the study provides a reasonable method of obtaining utility data in this special population. First of all, it should be considered that when the clinical studies of GHD and SGA were initiated (April 1991 for the SGA study) HRQoL was in its infancy and was not commonly investigated in clinical trials and the questionnaires included in the SGA trial can unfortunately not be used to obtain utility scores. For example, in the SGA study a HRQoL questionnaire (the TACQOL-S) was included which demonstrated positive results for the treated childrenCitation36, however, this questionnaire does not capture all dimensions relevant for a utility assessment. New utility data obtained from a new prospective long-term clinical study would take another 10–15 years to obtain. Secondly, utility data from young children are inherently unreliable as the questionnaires are difficult to complete and the validity of, especially baseline data, is often questionableCitation34. Thus, mapping utility data onto clinical data may provide more reliable results in this special population. Finally, it should also be considered that the most compelling utility data would be provided by a new randomised controlled clinical trial comparing GH treatment with no treatment, an approach that is currently ethically unjustifiable given the known clinical benefits of GH in the SGA and GHD population.
Moreover, it can be discussed whether utilities measured in a UK population can be used for a Swedish population. The European Disability Weight Group has previously demonstrated that utility values are fairly similar in the UK, France, the Netherlands, Spain and Sweden. Although minor differences in utilities were observed between Sweden and the UK, it important to notice that the ranking of disease states was very similar – this becomes important as the present analysis deals with the marginal utilityCitation39.
The data used in the health economic model is sub-set of the published dataCitation16,17 which is appropriate for the health economic analysis (corresponding with the EMEA approved dose and indication). The sub-group of children (n=19) may seem limited and could indicate a limited generalisability. However, the ∼15-year-long clinical study is unprecedented in this therapeutic area and no other clinical trial in SGA children report final ‘adult height’ – most SGA studies only run for 1–2 years and do not capture final adult height. Moreover similar (slightly better) results were demonstrated in the children treated with the higher dose (not EMEA approved and therefore not included in the present analysis)Citation16,17.
Children were assumed to require an endocrinologist visit four times per year for the duration of their somatropin treatment, based on the treatment plan defined in the clinical trialCitation17. This may not be reflective of clinical practice where, according to guidelines on the treatment of children with GHCitation40, patients should be assessed 4 times a year for the first year and then every 4–6 months thereafter. The cost of endocrinologist visits may therefore have been overestimated within the model and consequently the overall cost of somatropin therapy may be lower in clinical practice. However, these same guidelines recommend that patients receiving somatropin therapy be monitored annually for thyroid function, bone age and pituitary status, the costs of which were not included in the analysisCitation40.
The costs of IGF-1 tests (which are approximately 200 SEK per test) were not considered in the analysis. According to expert opinion, the management of patients receiving somatropin therapy should include IGF-1 testing. However, as the costs of these tests are relatively low, their exclusion from the model would not have a significant impact upon the cost-effectiveness of somatropin therapy. Furthermore, the analysis did not include monitoring of children who were in the ‘no treatment’ arms, although clinical practice would incur such costs, suggesting that the cost-effectiveness of somatropin therapy may be greater than reported due to the conservative approach taken.
The economic analysis represents a simple approach to determine the cost-effectiveness of somatropin. Although the model only mapped increasing height to improvements in quality of life, other potential health benefits could be considered. For example, short children born SGA are thought to have an increased risk of diabetes and cardiovascular eventsCitation3 and there is evidence to show that women of short stature have a twofold risk of caesarean sections when giving birth, compared with taller patients (p< 0.001)Citation41.
Moreover, there is a considerable impact of short stature on social factors such as academic achievement, social class and risk of suicide. Evidence on the association between short stature and intelligence among children was systematically reviewedCitation42. Studies that directly compared short children with average height controls found that short children had significantly lower academic achievement test scores or a greater likelihood of low scores than controlsCitation42. Similar results were found in a number of studies that evaluated intelligenceCitation42. The early school progress of boys is also reported to be influenced by height. A study of 2,848 children aged 5–12 years found that of boys who were experiencing difficulty at school, those of short stature were more likely to have to repeat a gradeCitation43. It was suggested that this was an example of a societal perception of childhood ability that was biased against smaller childrenCitation43. A population based study of 168,068 Swedish males found that males born SGA had an increased risk of subnormal ability in four dimensions of intellectual performance; logical, spatial, theoretical and verbal capacity. SGA males with short adult stature or a small head circumference at birth were particularly associated with the risk of subnormal logical performanceCitation44.
Slow growth in infancy has also been linked to a lower income in later life. A study of 4,630 boys concluded that irrespective of the social class into which they were born, those who grew slowly between birth and 1 year of age had poor educational achievements, a lower occupational status and a lower income than those who grew more rapidlyCitation45.
A strong inverse relationship has been shown between height and risk of suicide. In a population of 1,299,177 Swedish men between the ages of 18 and 49, there was a twofold increased risk of suicide in short men than tall men. A 5 cm increase in height was found to be associated with a 9% decrease in suicide riskCitation46. A further study linked short birth length to the increased risk of violent suicide attemptsCitation47.
Conclusion
In conclusion, based on a simple decision-tree modelling approach the authors have demonstrated that somatropin is a cost-effective treatment strategy for GHD and SGA children which leads to a normalisation of height in childhood and improvement in HRQoL. Somatropin therapy was associated with costs per QALY which from a Swedish perspective represent ‘good value for money’ (assuming a willingness-to-pay threshold of 500,000–600,000 SEK/QALY). It is clear from sensitivity analyses that base-case parameters for somatropin therapy compared with no treatment are robust. Additional analyses that consider the other potential health benefits of somatropin therapy may provide further evidence of the cost-effectiveness of this treatment.
Transparency
Declaration of fundingThis study was funded by Novo Nordisk, Denmark.
Declaration of financial/other relationshipsT.C. and C.D. have disclosed that they are employees of Novo Nordisk. C.F. and A.B. have disclosed that they are employees of Abacus International, a company that received funding from Novo Nordisk to conduct this study.
The JME peer reviewers 1 and 2 have not received an honorarium for their review work on this manuscript. Both have disclosed that they have no relevant financial relationships.
References
- Clayton PE, Cianfarani S, Czernichow P, Management of the child born small for gestational age through to adulthood: a consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J Clin Endocrinol Metab 2007;92:804-10.
- Barker DJ, Hales CN, Fall CH, Type 2 (non-insulin- dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993;36:62-7.
- Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res 1995;38:733-9.
- Hinchliffe SA, Lynch MR, Sargent PH, The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol 1992;99:296-301.
- Hales CN, Barker DJ, Clark PM, Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019-22
- Osmond C, Barker DJ, Winter PD, Early growth and death from cardiovascular disease in women. BMJ 1993;307:1519-24
- Ibanez L, Potau N, Ferrer A, Reduced ovulation rate in adolescent girls born small for gestational age. J Clin Endocrinol Metab 2002;87:3391-3.
- Barker DJ, Godfrey KM, Fall C, Relation of birth weight and childhood respiratory infection to adult lung function and death from chronic obstructive airways disease. BMJ 1991;303:671-5
- Novo Nordisk Limited. Available at: http://www.novonordisk.co.uk/documents/article_page/document/ght_facts_children_sga.asps. s(Last accessed June 2007)
- Hokken-Koelega AC, De ridder MA, Lemmen RJ, Children born small for gestational age: do they catch up? Pediatr Res 1995;38:267-71.
- Growth Hormone Research Society. Consensus guidelines for the diagnosis and treatment of growth hormone (GH) deficiency in childhood and adolescence: summary statement of the GH Research Society. J Clin Endocrinol Metab 2000;85:3990-3.
- Christensen TL, Djurhuus CB, Clayton P, An evaluation of the relationship between adult height and health-related quality of life in the general UK population. Clin Endocrinol 2007;67:407-12.
- Verrips G, Vogels A, Koopman H, Measuring health related quality of life in a child population. Eur J Publ 1999;9:188-93.
- Phillips C, Thompson G. What is a QALY? Available at http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/QALY.pdf. (Last accessed November 2009)
- Persson U, Hjelmgren J. Health services need knowledge of how the public values health. Lakartidningen 2003;100:3436-7.
- Bannink EM, van Doorn J, Mulder PG, Free/dissociable insulin-like growth factor (IGF)-I, not total IGF-I, correlates with growth response during growth hormone treatment in children born small for gestational age. J Clin Endocrinol Metab 2007;92:2992-3000.
- Novo Nordisk Limited. GHRETARD/BPD/14-20-21/NL clinical trial. Unpublished data 2007
- Statistics Sweden (Statistiska centralbyran). Available at: http://www.scb.se/. (Last accessed January 2010).
- van Pareren Y, Mulder P, Houdijk M, Adult height after long-term, continuous growth hormone (GH) treatment in short children born small for gestational age: results of a randomized, double-blind, dose-response GH trial. J Clin Endocrinol Metab 2003;88:3584-90.
- The Pharmaceutical Benefits Board. General guidelines for economic evaluations from the Pharmaceutical Benefits Board (LFNAR 2003:2). Available at: http://www.ispor.org/peguidelines/source/Guidelines_in_Sweden.pdf. (Last accessed November 2009)
- Novo Nordisk Limited. Norditropin summary of product characteristics. Available at: http://emc.medicines.org.uk/medicine/2760/SPC/Norditropin SimpleXx 5 mg/1.5 ml, Norditropin SimpleXx 10 mg/1.5 ml, Norditropin SimpleXx 15 mg/1.5 ml. (Last accessed January 2010)
- Bryant J, Cave C, Mihaylova B, Clinical effectiveness and cost-effectiveness of growth hormone in children: a systematic review and economic evaluation. Health Technol Assess 2002;6:1-168.
- Cutfield WS, Karagiannis G, Reiter EO. GH treatment to final height in idiopathic GH deficiency: The KIGS experience. In: Ranke MB, Price DA, Reiter EO, eds. Growth Hormone Therapy in Pediatrics – 20 years of KIGS. Basel: Karger 2007;145-62.
- August GP, Julius JR, Blethen SL. Adult height in children with growth hormone deficiency who are treated with biosynthetic growth hormone: the National Cooperative Growth Study experience. Pediatrics 1998;102:512-16.
- Carel JC, Ecosse E, Nicolino M, Adult height after long term treatment with recombinant growth hormone for idiopathic isolated growth hormone deficiency: observational follow up study of the French population based registry. BMJ 2002;325:70
- de Ridder MA, Stijnen T, Hokken-Koelega AC. Prediction of adult height in growth-hormone-treated children with growth hormone deficiency. J Clin Endocrinol Metab 2007;92:925-31.
- Harris M, Hofman PL, Cutfield WS. Growth hormone treatment in children: review of safety and efficacy. Paediatr Drugs 2004:6:93-106
- The European Agency for the Evaluation of Medicinal Products. Committee for Proprietary Medicinal Products (CPMP) opinion following an article (75) referal - Norditropin. Available at: http://www.ema.europa.eu/pdfs/human/referral/norditropin/347803en.pdf. (Last accessed January 2010)
- Endocrinology list prices in Sweden. Available at: http://www.externt9.lul.se/svn/akademiska09.xls. (Last accessed November 2009)
- FASS. Available at: http://www.fass.se/LIF/home/soktraffar_all.jsp?searchtext1=norditropin&searchtext2=&allCategories=false&searchtype=. (Last accessed November 2009)
- Lustig RH. Optimizing growth hormone efficacy: an evidence-based analysis. Horm Res 2004;62(Suppl 3):93-7.
- The EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy 1990;16: 199-208.
- Dolan P, Gudex C, Kind P, A social tariff for EuroQol: results from a UK general population survey. Working Papers 138, September 1995. Centre for Health Economics, University of York. Available at: http://www.york.ac.uk/inst/che/pdf/DP138.pdf. (Last accessed November 2009)
- Pal DK. Quality of life assessment in children: a review of conceptual and methodological issues in multidimensional health status measures. J Epidemiol Community Health 1996;50:391-6.
- The National Institute for Health and Clinical Excellence (NICE). Guidance on the use of human growth hormone (somatropin) in children with growth failure. Technology Appraisal No. 42, May 2002. Available at: http://www.nice.org.uk/nicemedia/pdf/HGHinChild-42-ALS.pdf. (Last accessed November 2009).
- Bannink EM, van Pareren YK, Theunissen NC, Quality of life in adolescents born small for gestational age: does growth hormone make a difference? Horm Res 2005;64:166-74.
- Fayter DA, Nixon J, Hartley S, Effectiveness and cost-effectiveness of height screening programmes during the primary school years: a systematic review. Arch Dis Child 2007;93:278-84.
- Sandberg D, Colsman, M. Assessment of psychosocial aspects of short stature. Growth Genet Horm 2005;21:17-25.
- Schwarzinger M, Stouthard ME, Burstrom K, Cross-national agreement on disability weights: the European Disability Weights Project. Popul Health Metr 2003;1:9
- Kirk J, Butler G, (BSPED). Treatment of Children with Recombinant Human Growth Hormone (r-hGH). Shared Care Guidelines April 2006. Available at: http://www.bsped.org.uk/professional/guidelines/docs/SharedcareGH-BSPED.pdf. (Last accessed November 2009).
- Sheiner E, Levy A, Katz M, Short stature – an independent risk factor for Cesarean delivery. Eur J Obstet Gynecol Reprod Biol 2005;120:175-8.
- Wheeler PG, Bresnahan K, Shephard BA, Short stature and functional impairment: a systematic review. Arch Pediatr Adolesc Med 2004;158:236-43.
- Wake M, Coghlan D, Hesketh K. Does height influence progression through primary school grades? Arch Dis Child 2000;82: 297-301.
- Lundgren EM, Cnattingius S, Jonsson B, Birth characteristics and different dimensions of intellectual performance in young males: a nationwide population-based study. Acta Paediatr 2003;92:1138-43.
- Barker DJ, Eriksson JG, Forsen T, Infant growth and income 50 years later. Arch Dis Child 2005;90:272-3.
- Magnusson PK, Gunnell D, Tynelius P, Strong inverse association between height and suicide in a large cohort of Swedish men: evidence of early life origins of suicidal behavior? Am J Psychiatry 2005;162:1373-5.
- Mittendorfer-Rutz E, Wasserman D, Rasmussen FF. Fetal and childhood growth and the risk of violent and non-violent suicide attempts: a cohort study of 318,953 men. J Epidemiol Commun Health 2008;62:168-73.