Abstract
Objective: To assess the resource implications and budget impact of managing cow milk allergy (CMA) in the Netherlands from the perspective of the healthcare insurers.
Methods: A model was constructed depicting the management of CMA in the Netherlands using information obtained from interviews with youth healthcare doctors (n = 14), general practitioners (n = 6) and paediatricians (n = 11) with relevant clinical experience of managing CMA. The model was used to estimate the expected level of healthcare resource use and corresponding cost (at 2007/08 prices) attributable to managing 4,382 new CMA sufferers.
Results: The expected cost of healthcare resource use attributable to managing 4,382 new CMA sufferers up to 1 year of age following initial consultation with a community-based physician at a mean 3 months of age was estimated to be €11.28 (95% CI: €7.82; €14.33) million. Clinical nutrition preparations emerged as the primary cost driver accounting for 91% of the total cost and clinician visits collectively accounted for a further 5%. The time taken for CMA sufferers to be put on an appropriate diet and achieve symptom resolution was estimated to be 30 (95% CI: 27; 32) days. Sensitivity analysis showed that the costs would increase by ∼16% if all new CMA sufferers were to undergo a double-blind placebo-controlled cow milk challenge in a hospital setting, as is currently being proposed. It is not clear how this proposal would affect time to symptom resolution since this would depend on the efficiency of hospitals being able to deal with the increased workload.
Limitations: The intolerance rates were derived from a 1-year follow-up study among 1,000 infants with CMA in the UK, healthcare resource use was not collected prospectively and the study period was censured at 1 year of age and does not consider the impact of CMA in subsequent years. However, most children outgrow this form of allergy during their second year.
Conclusion: Within the model's limitations, CMA imposes a substantial burden on the Dutch healthcare system. Moreover, initiating a double-blind placebo-controlled cow milk challenge for all CMA sufferers will potentially increase clinicians’ workload and use of limited resources within paediatric hospital departments in the Netherlands.
Introduction
The Dutch healthcare system is characterised by private health insurance with social conditions. Under the Health Insurance Act (Zorgverzekeringswet), all residents of the Netherlands are obliged to have health insurance and all insurers are obliged to offer a policy to any individual who applies, regardless of age or healthCitation[1]. Individuals who cannot afford the premium for a health plan receive a subsidy financed by taxation. The Government compensates insurers for providing coverage to the elderly and other high-risk individuals by granting ‘risk-equalization’ paymentsCitation[1],Citation[2]. The basic health plan provides essential curative care which has been benchmarked against the criteria of demonstrable efficacy, cost effectiveness and collective financingCitation[3]. Individuals are at liberty to purchase additional optional packages from insurers to cover treatments that are not covered in the basic plan. Premiums for children up to 18 years of age are financed by the Government. The insurers reimburse a range of costs including clinicians’ fees, examinations, treatment charges, hospitalisations, medications and clinical nutrition preparationsCitation[3].
In the Netherlands, primary healthcare is provided by general practitioners (GPs) who are gatekeepers of the system. Hence, access to a specialist physician or the hospital is via a GP referral. In addition, children between 0 and 4 years of age are seen regularly by youth healthcare doctors (YHDs), based at a ‘Consultatie Bureau’ (CB). CBs provide first-line medical and preventative care. The CBs also provide other services involving vaccination of infants, managing developmental problems and dealing with other infant-related problems including food allergies. Secondary and tertiary care is mainly provided by medical specialists in hospitals, through inpatient and outpatient services. More than 90% of hospitals are private, non-profit facilities and the others are public university hospitalsCitation[3].
Cow milk allergy (CMA) is an adverse reaction to protein in cow's milk, involving the immune system. Its incidence in infancy in western industrialised countries has been estimated at 2–3%Citation[4]. Symptoms of CMA generally appear within the first few months of life. An infant can experience symptoms either very quickly after feeding (rapid onset) or not until 7–10 days after consuming cow milk protein (slower onset)Citation[5–7]. The slower-onset reaction is more common and symptoms may include loose stools (possibly containing blood), vomiting, refusing food, irritability, colic, skin rashes and failure to thrive. This type of reaction is more difficult to diagnose because the same symptoms may occur with other health conditions. Most children will outgrow this form of allergy by 2 years of age. Rapid-onset reactions appear suddenly with symptoms that can include irritability, vomiting, wheezing, swelling, hives and bloody diarrhoea. In rare cases anaphylaxis can occur, however this is more common in nut and shellfish allergiesCitation[8]. Several tests can be used to inform a diagnosis of CMA, including a stool test, radioallergosorbent test (RAST), skin prick test, and patch testingCitation[7],Citation[9]. However, these tests may yield both false positve and false negative results.
Nutritional formulas for infants with CMA include soy-based milk, extensively hydrolysed formulas (eHF) and amino acid formulas (AAF)Citation[10],Citation[11]. Extensively hydrolysed formulas contain milk proteins which have been pre-digested, making them less allergenic than the whole proteins contained in regular formulasCitation[11]. Amino acid formulas (such as NeocateFootnote*) contain only amino acids in the absence of any protein and thus are not allergenicCitation[12–14]. Partially hydrolysed formulas are not recommended for use in infants with CMA since they are not considered truly hypoallergenic and can still provoke significant allergic reactionsCitation[15].
Mothers of affected infants who are being breast-fed are advised to eliminate dairy products from their diet under the close supervision of a dietician, because a strict diet must be followed to ensure adequate intake of nutrients while eliminating cow milk protein. In many European countries, and in Australia and New Zealand, mothers of bottle-fed infants are often advised to feed either a soy formula or an eHF to those >6 months of age, but to initially feed only an eHF to those <6 months of age. Those infants who cannot tolerate soy would generally be switched to eHF and those who cannot tolerate eHF would generally be switched to an AAF. However, due to the possibility of co-allergies and oestrogenic effects only eHF and AAF are used in the Netherlands.
In the Netherlands, diagnosis of CMA is confirmed by open challenge either by GPs or YHDs and affected infants are initially treated with an eHF. Infants who remain symptomatic are generally referred to a university hospital where diagnosis would be confirmed using a double-blind placebo-controlled cow milk challenge (DBPCCMC). However, it is being proposed that a DBPCCMC should be used by general hospitals to establish a diagnosis in all cases of suspect CMA and if a diagnosis cannot be established at a general hospital, then affected infants should be referred to a university hospital where the process would be repeated under more strict conditions. Performing a DBPCCMC in all suspect CMA sufferers could potentially create additional workload, in terms of clinicians’ time and healthcare resource use, and lead to delays in diagnosis and treatment as well as reducing the efficiency of healthcare delivery in paediatric departments in public hospitals. Against this background, the study assessed the resource implications and budget impact of managing CMA in the Netherlands.
Methods
Study objective
The study objective was to assess the resource implications and budget impact of managing CMA in the Netherlands from the perspective of the healthcare insurers.
Modelling the management of CMA
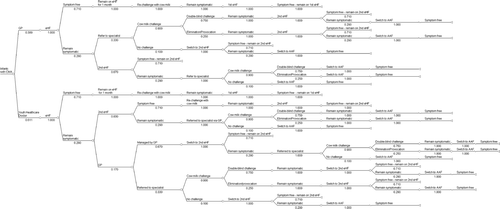
A computer-based model () was constructed using TreeAge Pro 2007 depicting the treatment patterns and associated resource use attributable to managing newly-diagnosed infants at the time of presentation with the symptoms of CMA. The model was used to estimate the cost of managing a cohort of new CMA sufferers up to 1 year of age following initial presentation to a GP or YHD in the Netherlands.
Figure 1. Model depicting the management of cow milk allergy sufferers following initial presentation to a general practitioner or youth healthcare doctor. Numbers denote the probability of an infant following a particular path according to current practice. Note: eHF, extensively hydrolysed formula; AAF, amino acid formula; GP, general practitioner.
Model inputs – clinical outcomes
Based on the findings of a 1-year follow-up study the authors have undertaken among 1,000 infants <1 year of age with CMA in the UK, it was estimated that 29% of infants would be intolerant to an eHFCitation[16].
Model inputs – resource use
A systematic literature search was performed using available databases, however no publications were identified that quantified healthcare resource use associated with managing CMA in the Netherlands. Therefore, mean values of resource use incorporated in the model were estimated using information obtained from interviews with a randomly selected sample of 14 YHDs and 6 GPs. In addition, 11 paediatricians (8 general paediatricians and 3 specialist paediatricians) with relevant clinical experience were asked for an interview and all contacted paediatricians agreed. The interviews used a structured questionnaire and interviewees were asked objective questions about diagnosis, patient management, resource utilisation, use of prescribed clinical nutrition preparations, use of prescribed drugs and time to symptom resolution based on their own practice together with general questions about the infrastructure of the healthcare system in the Netherlands. The information elicited from the interviewees was inputted into an Excel spreadsheet and analysed using descriptive statistics to obtain mean parameter values, as outlined below.
The aim of the interviews was to establish the treatment paths and associated resource use attributable to managing CMA sufferers in the Netherlands. From the interviews it was estimated that GPs and YHDs are the first point of contact for 38% and 62% of CMA sufferers respectively. Patient management and choice of clinical nutrition preparation depends on the initial clinician seen by a CMA sufferer.
Management of CMA sufferers by YHDs
The 6 interviewed YHDs collectively saw ∼100 new cases of CMA per year. An infant would see a YHD at the first clinic visit, a nurse at the second clinic visit and then alternate between a YHD and nurse for subsequent visits. A diagnosis of CMA is generally based on history and presentation of clinical signs and symptoms. In addition, the infants would undergo an open challenge. All suspected bottle-fed CMA patients would be initially treated with an eHF, followed by cow milk provocation either 2 weeks later (17%) or 4 weeks later (83%). The infants would be re-challenged either at the clinic (67%) or at home (33%). It was also found that YHDs did not perform any other tests or double-blind challenges. Following diagnosis, CMA sufferers who tolerate an eHF would remain on it for ∼12 months. Those infants who remain symptomatic following initial treatment would either be switched to a second eHF (83%) or referred to a GP (6%) or referred to a hospital specialist via a GP (11%). None of the interviewed YHDs prescribed soy or an AAF.
It was estimated from the interviewees that ∼30% of mothers breast feed for ∼3-4 months only. All breast-feeding mothers would be asked to eliminate cow milk protein from their diet for ∼4 weeks. If the symptoms remit following elimination and re-appear following an open challenge, a diagnosis of CMA would be made. These mothers would be advised to eliminate cow milk protein-containing foods from their diet until the baby starts a formula. Mothers would never be asked to stop breast-feeding. However, if symptoms persist despite maternal elimination of cow milk, the infant would be referred to a GP or hospital specialist via a GP for further diagnostic tests.
It was also estimated that 83% of breast-feeding mothers and 50% of bottle-feeding mothers would be referred to a dietician. Mothers would be provided with dietary information and may also be prescribed dietary supplements. Referred mothers would have a mean one visit with a dietician. In addition, all CMA sufferers would have a mean one visit with a dietician when solid foods are being introduced into their diet. According to the YHDs, ∼67% CBs have dietician facilities.
Management of CMA sufferers by GPs
The 6 interviewed GPs collectively saw ∼104 new cases of CMA per year. Infants do not have routine visits with their GP and are only seen by them if they experience health problems or if referred by a YHD. A GP would generally make a diagnosis of CMA based on history and presentation of clinical signs and symptoms. In addition, all patients would undergo an open challenge. In ∼67% of cases this would be conducted by a GP, but the other ∼33% of cases would be referred to a YHD for an open challenge. All suspected bottle-fed CMA infants would be initially treated with an eHF followed by an open challenge 2 weeks later (33%), 4 weeks later (33%), 6 weeks later (17%) or 8 weeks later (17%). Approximately 50% of infants would be re-challenged at their GP's clinic and the other 50% at home. None of the interviewed GPs performed other diagnostic tests or double-blind challenges.
If a CMA diagnosis is established, the infants would remain on their initial eHF for ∼12 months. Infants remaining symptomatic (29%) following initial treatment would either receive a second eHF (67%) or be referred to a hospital specialist for further diagnostic tests (33%). None of the interviewed GPs prescribe soy or an AAF. Breast-fed infants would be diagnosed and managed in the same way by GPs as by YHDs.
GPs would refer 67% of breast-feeding mothers and none of the bottle-feeding mothers to a dietician. Mothers would be provided with dietary information and may also be prescribed dietary supplements. Referred mothers would have a mean one visit with a dietician. In addition, all patients with CMA would have a mean one visit with a dietician when solid foods are being introduced into their diet. Only ∼17% of interviewed GPs had dietician facilities available in their clinics.
Management of CMA sufferers by paediatricians
The 11 paediatricians collectively saw ∼500 new CMA patients per year. All patients were referred to general paediatricians by GPs. Specialist paediatricians get ∼60% of their referrals from other paediatricians and ∼40% from GPs. In all cases a diagnosis of CMA would be based on history and presentation of clinical signs and symptoms. Additionally, approximately 22% of sufferers would undergo an open challenge, ∼68% would undergo a DBPCCMC and ∼10% would be initially put on an AAF. In addition, ∼90% of suspected CMA sufferers would have a RAST test, ∼60% a skin prick test and ∼50% a skin patch test. Following diagnosis, ∼85% of CMA sufferers would be subsequently managed by a paediatrician, whereas 15% would be discharged back to their GP.
The treatment adopted by paediatricians depends on the treatment initiated by the referring physician. If a referred CMA sufferer is already on a second eHF a paediatrician would initiate treatment with an AAF. Otherwise paediatricians generally try a second eHF before prescribing an AAF. It was also estimated that ∼47% of patients remain on a GP-initiated or YHD-initiated diet following referral to a paediatrician. Patients would be re-challenged with cow milk either every 6 months (90%) or every 12 months (10%). Breast-fed infants would be diagnosed and managed by paediatricians in the same way that they are by YHDs and GPs.
Approximately 38% of CMA sufferers would be seen by a dietician, 31% by a paediatric allergist, 15% by a paediatric dermatologist, 8% by a paediatric gastroenterologist and 8% by a paediatric pulmonologist. In the Netherlands, paediatricians work in teams, therefore at the same visit a patient may be seen by more than one specialist. None of the interviewed paediatricians performed procedures such as endoscopies, gastroscopies or X-rays on suspected CMA sufferers. Also, there would be negligible prescribing of medications for symptomatic relief of symptoms to CMA sufferers. However, if patients present with gastro-oesophageal reflux they could be prescribed a proton pump inhibitor and/or an H2-antagonist, although it was not routine practice.
Model outputs
The time taken for CMA sufferers to be put on an appropriate diet and achieve symptom resolution was estimated depending on whether they were initially seen by a GP or YHD and whether they remain symptom-free or symptomatic following the initial diet.
The number of infants born in the Netherlands was estimated to be 175,000 per annumCitation[4]. The estimated incidence of CMA among infants is 2.5%Citation[5]. Hence, the annual number of new CMA sufferers in the Netherlands was estimated to be 4,382 infants. The model estimated the expected levels of healthcare resource use attributable to managing 4,382 new CMA sufferers up to 1 year of age following initial consultation with a community-based physician. Unit resource costs in Euros at 2007/2008 () obtained from private healthcare insurers in the Netherlands were applied to the resource utilisation estimates within the model. This enabled an estimation of the expected cost of healthcare resource use associated with managing 4,382 new CMA sufferers following initial consultation with a GP or YHD, from the perspective of the insurers.
Table 1. Unit resource costs at 2007/08 prices.
The analysis assumed that a private healthcare insurer would only reimburse a clinical nutrition preparation after a confirmed diagnosis of CMA has been made by a clinician using either elimination/provocation or DBPCCMC. Consequently, all diagnosed CMA sufferers would have their clinical nutrition preparations reimbursed. Moreover, according to the interviewees a patient's mean age at the time of initial presentation to a GP or YHD is ∼3.0 months.
Sensitivity analyses
In order to assess uncertainty within the model, probabilistic sensitivity analysis was undertaken using Monte Carlo iterations (5,000 iterations of the model) by simultaneously varying the probabilities, resource use values and unit costs. A beta distribution was used to represent uncertainty in the probability values by assuming a 20% standard deviation around the mean values. The resource use and unit cost estimates were varied randomly according to a lognormal distribution by assuming a 20% standard deviation around the mean. The 20% variability in each parameter was considered reasonable since it more than covered the variance in clinicians’ responses to individual questions. Additionally, deterministic sensitivity analyses were performed to identify how the results would change by varying different assumptions in the model (as described in ).
Sensitivity analysis also assessed the budget impact of implementing the following strategy: all suspected CMA sufferers presenting with repeated bloody diarrhoea, persistent growth retardation, gastrointestinal symptoms, eczema, asthma, angioedema and anaphylaxis would undergo a DBPCCMC at a general hospital. Moreover, sufferers with inconclusive or delayed reactions would undergo a DBPCCMC at a university hospital. All diagnosed CMA sufferers would be treated according to current practice (i.e., all infants would be initially treated with an eHF and those intolerant to an eHF would be treated with an AAF).
Results
Resource implications and expected healthcare costs
The expected cost per CMA infant managed according to current practice up to 1 year of age following an initial visit to a GP or YHD was estimated to be €2,567 (95% CI: €1,794; €3,365) per patient. Of this, 72% of the cost (€1,846) was due to eHF preparations and a further 19% (€497) was due to AAF preparations. Clinician visits accounted for ∼5% of the total cost. However, the cost varied according to whether a patient responded to their diet. Those who responded to their initial diet were estimated to cost ∼€2,154 (95% CI: €1,436; €2,964) per patient, whereas those who did not respond to their initial diet were estimated to cost ∼€3,572 (95% CI: €2,746; €4,449) per patient. Moreover, the expected cost per CMA infant managed according to current practice up to 1 year of age following an initial visit to a GP was estimated to be €2,561 (95% CI: €1,890; €3,318) per patient, whereas it was estimated to be €2,568 (95% CI: €1,896; €3,349) per patient following an initial visit to a YHD.
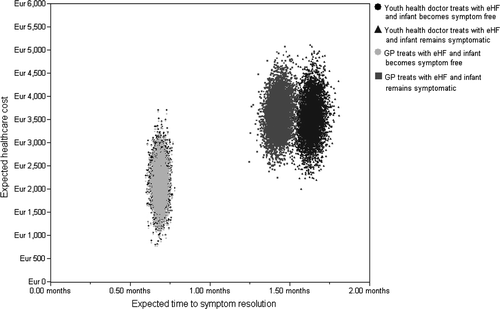
The expected length of time for a CMA sufferer to be put on an appropriate diet and achieve symptom resolution was estimated to be 30 (95% CI: 27; 32) days. Probabilistic sensitivity analyses highlighted the variance in time to symptom resolution and cost according to the speciality of the initial clinician and whether a patient initially responded to diet (). The expected time to symptom resolution was estimated to be:
14 (95% CI: 13; 16) days per symptom-free patient and cost an estimated €2,130 (95% CI: €1,354; €2,920) following an initial visit to a GP.
37 (95% CI: 34; 39) days per symptomatic patient and cost €3,614 (95% CI: €2,834; €4,405) following an initial visit to a GP.
14 (95% CI: 13; 16) days per symptom-free patient and cost €2,169 (95% CI: €1,336; €2,946) following an initial visit to a YHD.
43 (95% CI: 40; 45) days per symptomatic patient and cost €3,546 (95% CI: €2,661; €4,444) following an initial visit to a YHD.
Budget impact of managing CMA
The expected levels of healthcare resource use attributable to managing 4,382 CMA sufferers up to 1 year of age following an initial consultation with a GP or YHD are shown in , from which it can be seen that there are 50% more visits with YHDs than GPs for the management of CMA. This could be due to the fact that most of the GPs refer their patients back to YHDs for open challenges and for any subsequent management following a confirmed diagnosis of CMA.
Table 2. Expected levels of healthcare resource use attributable to managing 4,382 new cow milk allergy sufferers up to 1 year of age following initial consultation with a general practitioner or youth healthcare doctor.
The expected cost of healthcare resource use attributable to managing 4,382 new CMA sufferers according to current practice up to 1 year of age following an initial consultation with a community-based physician was estimated to be €11.28 (95% CI: €7.82; €14.33) million (). eHF preparations emerged as the primary cost driver accounting for 72% of the total cost and AAF accounted for a further 19%. Clinician visits collectively accounted for <5% of the total cost.
Table 3. Expected cost (euros at 2007/2008 prices) of healthcare resource use (95% confidence intervals) attributable to managing 4,382 new cow milk allergy sufferers up to 1 year of age following initial consultation with a general practitioner or youth healthcare doctor.
The expected cost varied according to whether patients were initially seen by a GP or YHD. If all patients were initially seen by a GP, the expected cost of healthcare resource use would be an estimated €11.22 (95% CI: €8.28; €14.50) million and if all patients were initially seen by a YHD it would be an estimated €11.25 (95% CI: €8.30; €14.67) million.
Sensitivity analyses
Deterministic sensitivity analyses () demonstrated that the model is relatively robust and the expected costs of managing 4,382 newly diagnosed CMA sufferers is most sensitive to the acquisition costs of eHF and AAF. As the cost of eHF is varied the expected cost up to 1 year of age following an initial visit to a GP or YHD was found to range from ∼€8.68 to ∼€14.21 million. Similarly, as the acquisition cost of AAF is varied the expected cost ranges from ∼€9.50 to ∼€19.84 million. Sensitivity analyses also showed the expected healthcare cost to be sensitive to the probability of being intolerant to eHF. This is because those who are intolerant to eHF are switched to AAF which is more expensive than eHF. However, the healthcare costs were found to be insensitive to changes in the number of visits that CMA sufferers have with a GP or YHD and to other changes to the model's inputs.
Table 4. Sensitivity analyses.
The length of time for a CMA sufferer to be put on appropriate diet and achieve symptom resolution was not affected by changes to any of the model's inputs with the exception of whether a sufferer is initially seen by a GP or YHD ().
Resource implications and budget impact of all patients having a double-blind challenge
Sensitivity analyses modelled the impact of managing 4,382 newly-diagnosed CMA sufferers up to 1 year of age following initial consultation with a community-based physician according to the strategy described in the Methods section, instead of current practice.
It was estimated that 16% of CMA sufferers are currently referred to paediatricians. Of these 50% (i.e., 8% of all sufferers) undergo a DBPCCMC at university hospitals. According to the interviewees, the challenge period is approximately 1 week for both the placebo and test formulae and that a DBPCCMC involves two day-case admissions (i.e., 1 day for each formula). The model assumed that a DBPCCMC would be performed using the same protocol in general hospitals as in university hospitals. It was further assumed that CMA sufferers would be discharged to their referring community-based physician after a CMA diagnosis had been made and would have the same frequency of follow-up visits as diagnosed CMA sufferers have presently.
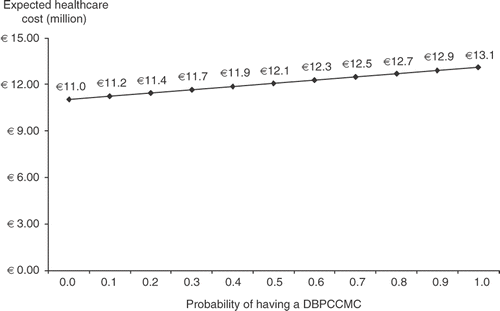
According to the model, the expected cost to manage 4,382 newly-diagnosed CMA sufferers up to 1 year of age following initial consultation with a community-based physician would rise from ∼€11.28 (95% CI: €7.82; €14.33) million to ∼€13.14 (95% CI: €9.15; €16.76) million (). Accordingly, the incremental cost of managing all 4,382 CMA sufferers undergoing a DBPCCMC was estimated to be €1.86 (95% CI: €1.33; €2.43) million. This represents a ∼16% increase in the cost of managing the annual cohort of new CMA sufferers. The increase is primarily due to a 1,150% increase in the number of day-case admissions at a cost of ∼€1.03 million, and an increase in the number of consultations with paediatricians at a cost of ∼€0.3 million ().
illustrates how the expected cost to manage 4,382 newly-diagnosed CMA sufferers up to 1 year of age following initial consultation with a community-based physician at a mean age of 3 months changes in accordance with changes to the probability of having a DBPCCMC.
Discussion
There are no published studies assessing healthcare resource use attributable to managing CMA in the Netherlands. Hence, the current study examined the health economic impact of CMA in the Netherlands using a decision-modelling approach that incorporated clinical outcomes and resource utilisation estimates derived from interviews with healthcare professionals involved in the management of CMA as well as previous studies conducted by the authors on CMACitation[16–18]. Decision analysis is a systematic approach to decision making under uncertainty that uses mathematical relationships to define a series of possible consequences that flow from a set of options being evaluatedCitation[19]. Hence, a key advantage of using this approach is that it allows for variability and uncertainty associated with model inputs, as depicted by the Monte Carlo simulationsCitation[19].
According to the model, the cost to Dutch healthcare insurers of managing an annual cohort of 4,382 new CMA sufferers up to 1 year of age following initial consultation with a community-based physician was estimated to be €11.28 million. By adapting the model to consider an alternative scenario whereby all CMA sufferers undergo a DBPCCMA in a hospital setting, it was estimated that the expected cost to the Dutch healthcare insurers would increase by ∼€1.86 million. This represents an ∼16% increase in the cost of managing the annual cohort of new CMA sufferers and is primarily driven by an increase in the number of outpatient visits with hospital physicians and admissions onto day-wards. Nevertheless, this increase is likely to be an underestimate of the real impact of all CMA sufferers undergoing a DBPCCMA as the model has not considered the impact of (1) the increased workload associated with all suspected CMA infants having a DBPCCMC or (2) increased waiting times to see a hospital physician and (3) increased administrative costs. More importantly, the model has also excluded the cost and consequences of managing potential sufferers who do not have a diagnosis of CMA, but nevertheless have undergone a DBPCCMC. Consequently, the cost of healthcare resource use associated with all suspect CMA sufferers undergoing a DBPCCMC will have been underestimated. Neither did the model consider that use of a DBPCCMC may have a higher likelihood of getting a correct diagnosis of CMA and patients getting a more appropriate treatment. Notwithstanding this, some guidelines have changed and GPs and YHDs have recently been allowed to start prescribing an AAF. However, our sample of GPs and YHDs advised that they would not prescribe an AAF, but continue to refer patients who do not respond to an eHF to a paediatrician for further investigation. Furthermore, the period of the study considers expected costs up to 1 year of age following presentation to a community-based physician and does not consider the impact of CMA in subsequent years. However, most children outgrow this form of allergy during their second yearCitation[5–7].
It is not clear how time to symptom resolution would be affected by the introduction of a DBPCCMC as a diagnostic tool for CMA as this would depend on the efficiency of clinicians dealing with their increased workload and waiting times. However, it was found that time to symptom resolution is quicker for CMA sufferers who respond to their initial diet and it is also quicker if the initial consultation is with a GP rather than a YHD. The latter could be explained by the fact that at present patients not responding to their initial treatment who need to be referred to a paediatrician can only be referred by a GP, thus prolonging the time it takes to put a CMA sufferer on appropriate diet and achieve symptom resolution.
The authors have previously reported that Finland's Social Insurance Institution adopted new guidelines as the basis for reimbursing clinical nutrition preparations for the treatment of CMA without prior validation in clinical practice, and were found to increase the use (and corresponding cost) of paediatric healthcare resources in Finland's public hospitals, which could have been used elsewhere within the systemCitation[17]. Clinical guidelines are systematically developed statements designed to help clinicians and patients make informed decisions about appropriate healthcare for specific circumstancesCitation[20]. The benefits of usable clinical guidelines enabling patients, practitioners, scientists, and purchasers to make more informed decisions and share information more effectively has been well-documentedCitation[21]. However, before healthcare organisations adopt guidelines, they have an onus of responsibility to validate them and ensure they are clinically effective and cost effective. Adopting recommendations from guidelines without appropriate validation can lead to a waste of resourcesCitation[22]. Similarly, guidelines for the use of clinical nutrition preparations in managing CMA in Australia have been developed by a panel of clinicians specialising in the management of infants with this allergyCitation[23]. However, these guidelines may require adaptation before they can afford health economic benefits to the paediatric healthcare system in AustraliaCitation[18].
Our results could also be confounded by other limitations within the model. The intolerance rate to eHF was based on a 1-year follow-up study among 1,000 infants with CMA who were managed in the community in the UKCitation[16] and not on the Dutch infants. Resource use was not collected prospectively, but estimated from interviews with 31 clinicians across the Netherlands with experience of managing CMA. Consequently, the model used resource estimates for the ‘average patient’ and does not take into account such factors as age, gender, co-morbidities and other disease-related factors. However, the inherent variability and uncertainty of using data from sources, other than that collected prospectively, is depicted by the Monte Carlo simulations. Furthermore, changes in the quality of life and improvements in general well-being of sufferers and their parents as well as parents’ preferences have been excluded in the model. Also excluded are changes in patients’ behaviour. Hence, our study may have underestimated some of the impact of the treatment strategies that were evaluated.
Conclusion
Within the study's limitations, the model indicates that CMA imposes a substantial burden on the Dutch healthcare system. If a GP was the initial point of contact rather than a YHD, the cost of treatment would be slightly less and time to symptom resolution would be quicker for patients who are intolerant to an eHF. Moreover, if every suspected infant with CMA-related symptoms underwent a DBPCCMC, healthcare resource use and corresponding cost of managing CMA sufferers up to 1 year of age is expected to increase.
Transparency
Declaration of funding: This study was funded by SHS International Ltd, Liverpool, UK, and Nutricia, Zoetermeer, the Netherlands. However, neither SHS International Ltd nor Nutricia had any input into the study design or data analysis. Neither did they review the results or have any input into writing this manuscript.
Declaration of financial/other relationships: E.S. and J.F.G. have disclosed that they have no relevant financial relationships.
Acknowledgements: The authors would like to thank the following YHDs for their contributions: Dr A.M. Ablas-Oskam, Oegstgeest; Dr D.M. Coo, Veldhoven; Dr C. Holsheimer-Fritz, Alkmaar; Dr A. Molnar, Zaanstraak; Dr D. Oers, Einthoven; Dr C.A. Veerman, Amsterdam. The authors would also like to thank the following GPs for their contributions Dr A.A. Armburst van den Berg, Eemnes; Dr A.S. Cooman, Bilthoven; Dr A.M. Leeuwen, Hazerswoude Rydnyk; Dr A.A. Matser, Beuningen; Dr A. Mohazzab, Soesterberg; Dr A.E. Weselling, Groningen. Last but not least, the authors would also like to thank the following consultants for their contributions Dr P.L.P. Brand, Princess Amalia Children’s Clinic, Zwolle; Prof A.E.J. Dubois, University Hospital, Groningen; Dr M. Fick, Saint Jansdal Hospital, Hardewyrk; Dr T. Hendriks, Catharina Hospital, Eindhoven; Dr M. Hoekstra, Wilhelmina Childrens Hospital, Utrecht; Dr C.M.F. Kneepkens, V.U. University Hospital, Amsterdam; Dr J.P. Leusink, Bernhoven Hospital, Oss; Dr T.W. Slok, Kennermer Gasthuis Hospital, Harlem; Dr J. Verhage, Rijnstate Hospital, Arnhem; Dr J.T.C.M. Verhallen-Dantuma, St Franciscus Gasthuis Hospital, Rotterdam; Dr J.M.B. Wennink, St Lucas Andreas Hospital, Amsterdam.
Notes
*Neocate, SHS International Ltd, Liverpool, UK.
References
- Maarse H, Health reform – one year after implementation. Health Policy Monitor, 2007. Available from http://www.hpm.org/survey/nl/a9/1
- Maarse H, Okma K. Dutch got private in health insurance reform. Euro Observer 2004; 6: 7–8
- Health insurance system. Hague: Ministry of Health, Welfare and Sport; 2008. Available from http://www.minvws.nl/en/themes/health-insurance-system/default.asp
- World Health Organization. World Health Statistics 2007. World Health Organization, Geneva 2007
- Høst A, Halken S. A prospective study of cow milk allergy in Danish infants during the first 3 years of life. Clinical course in relation to clinical and immunological type of hypersensitivity reaction. Allergy 1990; 45: 587–96
- Bishop J, Hill DJ, Hoskings CS, et al. Natural history of milk allergy: clinical outcome. J Paediatr 1990; 116: 862–7
- Hill DJ, Duke AM, Hoskings CS, et al. Clinical manifestations of cow's milk allergy. The diagnostic value of skin tests and RAST. Clin Allergy 1998; 18: 481–90
- Action Against Allergy. Available from http://www.actagainstallergy.co.uk
- Walker-Smith JA. Diagnostic criteria for gastrointestinal food allergy in childhood. Clin Exp Allergy 1995; 25: 20–2
- Osborn DA, Sinn J. Soy formula for prevention of allergy and food intolerance in infants. Cochrane Database Syst Rev 2004; 3: CD003741
- Seppo L, Korpela R, Lonnerdal B, et al. A follow-up study of nutrient intake, nutritional status, and growth in infants with cow milk allergy fed either a soy formula or an extensively hydrolysed whey formula. Am J Clin Nutr 2005; 82: 140–5
- de Boissieu D, Matarazzo P, Dupont C. Allergy to extensively hydrolyzed cow milk proteins in infants: identification and treatment with an amino acid-based formula. J Pediatr 1997; 131: 744–7
- Hill DJ, Heine RG, Cameron DJS, et al. The natural history of intolerance to soy and extensively hydrolyzed formula in infants with multiple food protein intolerance (MFPI). J Pediatr 1999; 135: 118–21
- Kanny G, Moneret-Vautrin DA, Flabbee J, et al. Use of an amino-acid based formula in the treatment of cow's milk protein allergy and multiple food allergy syndrome. Aller Immunol (Paris) 2002; 34: 82–4
- Moneret-Vautrin DA, Hatahet R, Kanny G. Protein hydrolysates: hypoallergenic milks and extensively hydrolysed formulas. Immuno-allergenic basis for their use in prevention and treatment of milk allergy. Arch Pediatr 2001; 8: 1348–57
- Sladkevicius E, Nagy E, Lack G, Guest JF. Resource implications and budget impact of managing cow milk allergy in the UK. J Med Econ 2010; 13: 119–28
- Guest JF, Valovirta E. Modelling the resource implications and budget impact of new reimbursement guidelines for the management of cow milk allergy in Finland. Curr Med Res Opin 2008; 24: 1167–77
- Guest JF, Nagy E. Modelling the resource implications and budget impact of managing cow milk allergy in Australia. Curr Med Res Opin 2009; 25: 339–49
- Morris S, Devlin N, Parkin D. Economic Analysis in Health Care. John Wiley, London 2007
- Field MJ, Lohr KN, (eds), Guidelines for Clinical Practice. From Development to Use. Washington, DC: National Academy Press, 1992
- Feder G, Eccles M, Grol R, et al. Using clinical guidelines. BMJ 1999; 318: 728–30
- Royal College of General Practitioners. The development and implementation of clinical guidelines: report of the Clinical Guidelines Working Group. Exeter: Royal College of General Practitioners, 1996 (Report from practice 26.).
- Kemp AS, Hill DJ, Allen KJ, et al. Guidelines for the use of infant formulas to treat cows milk protein allergy: an Australian consensus panel opinion. Med J Aust 2008; 188: 109–12