Abstract
Objective: This analysis assesses the cost-effectiveness of memantine for the treatment of moderate-to-severe Alzheimer's disease (AD) in the UK.
Methods: This cost-utility analysis was based on a Markov model. The model simulated 5-year progress of patients with AD until they need full-time care (FTC), defined as a patient becoming either dependent or institutionalised. Transition probabilities were based on a predictive equation, derived from the London and South-East Region epidemiological study. Resource use, utilities and mortality were obtained from the same study. Memantine efficacy was based on a meta-analysis of six large trials. The model compared memantine to its alternative in the UK, i.e. no pharmacological treatment or background therapy with acetylcholinesterase inhibitors.
Results: Memantine was found to delay the need to FTC by 6 weeks compared with current practice in the UK. It was associated with increased quality-adjusted life-years and cost savings to the healthcare system (probability of this outcome was 96%). The projections were made assuming that benefits from the 6-month treatment were sustained over time, which is regarded as the main limitation. The model underwent extensive sensitivity analyses, which confirmed the base-case findings.
Conclusions: The model suggests that memantine delays the need for FTC and decreases cost. It can be regarded as a cost-effective choice in the management of moderate and severe AD.
Introduction
It has been estimated that 24.3 million people worldwide were living with dementia in 2001, with 4.6 million new cases of dementia occurring every yearCitation[1]. This estimate was based on a Delphi panel approach where 12 international experts estimated the prevalence of dementia guided by a systematic review of the evidence. As the prevalence increases with age, the burden of this condition is expected to rise with the ageing population. The incidence rate of dementia for people aged of 65–69 years in England and Wales in 2005, has been estimated at 7.4 [95% CI 3.6–16.1] per 1000 person-years, increasing by 5-year age bands to 84.9 [95% CI 63.0–107.8] per 1000 person-years for those 85 years or olderCitation[2]. This equates to approximately 180,000 new cases per year. Alzheimer's disease (AD) is the most common form of dementia and is thought to account for between 50 and 60% of all cases of dementiaCitation[3].
The burden of dementia and specifically AD is high, both clinically for the patient and their carers, and also from an economic perspective. The economic burden rises with increasing disease severityCitation[4]. One review estimates the median annual costs of care of dementia in Europe as €28,000 per patientCitation[4]. The direct costs of AD in the UK in 1999 were estimated to be £23 billion annuallyCitation[5]. Given the high economic burden of AD, there is a need for the effective treatments with proven cost effectiveness to enable efficient allocation of healthcare resources.
Memantine is a moderate-affinity uncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist approved for the treatment of moderate-to-severe AD in the UK. The cost-effectiveness of memantine in the UK setting from the NHS and Personal Social Services perspective has previously been estimatedCitation[6]. This analysis utilised a Markov model to simulate patient progression through a series of health states related to disease severity, dependency and residential setting. Data on resource use and baseline patient characteristics were taken from the London and South-East Region (LASER–AD) longitudinal epidemiological studyCitation[7]. Clinical data from a single randomised controlled trial of memantine compared to placebo was usedCitation[8]. In that analysis memantine was reported to be the dominant option compared to no treatment, being associated with lower costs and greater clinical effectiveness.
An alternative approach to the economic evaluation of AD treatments involves modelling the time to full-time care (FTC)Citation[9],Citation[10], a composite outcome defined in terms of the amount of supervision and care required by a patient on a daily basis, regardless of the locus of care and who the caregiver is. Delay of FTC is clinically and economically relevant for decision makers, carers and patients. Particularly in patients with very advanced AD, evaluating the impact of treatment based on the time to reach a more severe state is no longer appropriate; time to FTC represents the most, and perhaps only, relevant outcome for patients with advanced AD and their carers.
The framework of time to FTC has been widely used for the economic evaluations of the acetylcholinesterase inhibitors (AChEIs) in the treatment of mild and moderate AD11. Although similar in method to these assessments, the model used here is based on a new predictive equation developed to address some of the limitations of the historical models. The new predictive equation considers all clinical manifestations of AD on the core domains – cognition, functioning and behaviour – and is derived from a representative cohort of people with AD12. The aim of this cost-utility analysis was to undertake a robust evaluation of the cost effectiveness of memantine in the UK healthcare setting. The model is based on pivotal clinical trials data and UK specific epidemiological, health outcomes and resource use data and uses the new predictive equation of time to FTC.
Methods
This economic evaluation was conducted to assess the long-term health and economic impact of memantine for the treatment of patients with moderate and severe AD. This was a cost-utility analysis employing a three-state Markov model, which simulated 5-year evolution of pre-FTC patients in terms of time until requiring FTC, quality-adjusted life-years (QALYs), and cost. The central event of the model was a need for FTC, which was defined based on locus of care (community or institutions) and on patient's dependency status as per an assessment of patient's physical and functional disability. Memantine was compared to the established clinical practice in the UK for management of moderate and severe AD, i.e. either no pharmacological treatment or background therapy with AChEIs. The model combines two major data source: six large multicentre randomised controlled clinical trials (RCT) for memantine, and a UK epidemiological study, named the London and South-East Region (LASER–AD) study. The analyses were conducted from the National Health Service and Personal Social Services (NHS and PSS) perspective, covering routine patient management, hospitalisation, social community services, institutionalisation and AD medications. The indirect or societal costs, e.g. carer's loss of productivity and leisure time, were not part of this analysis. The costs were reported in 2009 GBP and euros to facilitate cross-country comparison of the results.
Model
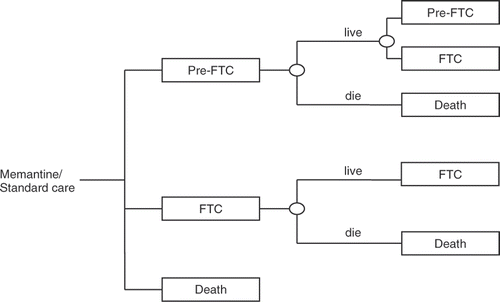
The Markov model consisted of three states, pre-FTC, FTC and death (). Full-time care state was defined as a patient becoming either dependent or institutionalised and represents an assessment of a patient's physical and functional disability and their location of care. Within the model, each Markov cycle was 1 month and the total time frame for the model was 5 years (i.e., 60 cycles). A 5-year time horizon was chosen for the model because AD is a chronically progressive disease with a reduced life expectancy. This is in line with previous pharmaco-economic evaluation in AD that used the same FTC conceptual frameworkCitation[11].
The transition process from the pre-FTC state to FTC state was driven by a new predictive equation that estimates the probability of a pre-FTC patient with AD to require full-time care based on cognitive, functional and behavioural assessments. The predictive equation was derived from a UK longitudinal epidemiological study, LASER–ADCitation[7]. Data was available for 117 pre-FTC patients over 54-month follow-up period, generating 320 eligible observations for the analysis. This analysis is described in detail in Rive et al. 2010Citation[12].
A key feature of the predictive equation used in the model was that it included assessments on all core AD domains: cognition; functioning and behavioural. The predictive equation models the time to FTC based on the patient's cognition, functioning and behavioural status at baseline. These data inputs are the AD Assessment Scale cognitive sub score (ADAS–cog) at baseline, the AD Cooperative Study Activities of Daily Living scale (ADCS–ADL) at baseline and the Neuropsychiatric Inventory total score (NPI) at baseline. The equation also considers the rate of change over time of cognition and functioning and therefore models differences patient disease progression over time. The predictive equation is presented below (Equation 1).
Equation 1: Equation to predict length of time to FTC in AD patients
In the LASER–AD cohort, the assessments were made at baseline, 6 months and every 12 months thereafter, generating five intervals. As the model had a 5-year time horizon, an extrapolation beyond 54 months was made. The monthly probabilities of reaching the FTC state were then calculated from each interval probability, assuming constant risk of FTC within each time interval:
Equation 2: Estimating monthly probabilities of reaching FTC statewhere
is the monthly probability derived from the interval probability Pj and length (j) is the length of the corresponding interval.
The probabilities of dying were also estimated from the LASER–AD cohort. The monthly probability of survival of pre-FTC patients (i.e., the population of interest at entry in the model) was computed as a function of time with a Weibull parameterisation. The probability of dying was assumed to be the same in both treatment groups to avoid indirect treatment effect on mortality.
Equation 3: Monthly death probabilitieswhere time is time in days from the model start.
Then, the probability of remaining alive between two cycles denoted i and i + 1 was calculated as S(i + 1)/S(i), and thereby, the probability of death between i and i + 1 is 1 − S(i + 1)/S(i).
Numerical examples of calculating the transition probabilities between the states are presented in Appendix A. The model is also represented as a probability transition matrix in Appendix A, .
Comparator
The model compared treatment with memantine to established clinical practice in the UK setting for the management of moderate and severe AD, i.e. either no pharmacological treatment or background therapy with AChEIs, which we will refer to as ‘standard care’. Standard care for patients not on an AChEI was considered to be receipt of social support and assistance with day-to-day activities.
Model inputs
Predictive equation parameters at baseline and memantine effect
The transition probabilities for the standard care group were obtained by entering baseline patient characteristics from the LASER–AD cohort (, Appendix A).
Table 1. Predictive equation parameters at baseline.
In the memantine group, the baseline characteristics of patients were adjusted based on a treatment effect observed in the six large multicentre 6-month RCTs for memantine. The model made the same assumption regarding treatment effect as that in previously published AChEIs modelsCitation[11]: ‘eligible patients start treatment immediately and benefits from treatment are assumed to have an immediate effect, modifying patients' time-related risk of progression from pre-full-time care to the full-time care health state’. This was implemented by deducting treatment effect on ADAS–cog, ADCS–ADL and NPI total from the patients’ baseline data at the start of the economic model. As the clinical trials were not designed to assess the disease-modifying effect of therapy, memantine was assumed to have no impact on the rate of change in cognition and functioning over time, and the input values in the predictive equation for these parameters are the same in both the memantine and the standard care group ().
Clinical data for memantine was obtained from the meta-analysis of six large multicentre RCTs with a similar designCitation[13]. The trials compared memantine to placebo given as a monotherapy or as in adjunct to AChEI over a period of 24–28 weeksCitation[8],Citation[14–18]. As weighted mean differences (WMD) were required to be able to input the data into predictive equation, the transformation of clinical data were undertaken. The ADCS–ADL19 (range 0–54) scores were rescaled into ADCS–ADL23 (range 0–78) scores and cognition outcomes assessed using the Severe Impairment Battery (SIB) scale were converted to ADAS-cog scores using a linear regression model computed on data from the LASER–AD study:
Equation 4: Relationship between SIB and ADAS-cog scores
The RCitation[2] of the model was 75.6%, thereby indicating good predictive properties.
The meta-analysis was performed using classical inverse variance weighting methodsCitation[19]. The fixed effect approach was used by default. In case of evidence of heterogeneity (p-value from test for heterogeneity below 0.10, in line with published Winblad (2007) meta-analyses)Citation[13], or in the case of moderate heterogeneity (identified as ICitation[2] > 50%, as per current recommendationsCitation[20]), a random-effect approach was applied instead. The clinical data as WMD is summarised in .
Table 2. Treatment effect with memantine, results of the meta-analysis of six pivotal RCTs.
Numerical examples of calculating the transition probabilities between the states are presented in Appendix A.
Health-related quality of life weights
In accordance with the recommendations of the recent guidelineCitation[21], the effects of memantine were quantified in terms of health related quality of life (HRQoL) by estimating EQ-5D health utility weights for the pre-FTC and the FTC state. The five dimensions of the EQ–5D instrument items were mapped from the Twelve-Item Health Status Questionnaire (HSQ-12)Citation[22], the Ferm's D–testCitation[23] and the quality of life scale in Alzheimer's disease (QoL–AD)Citation[24] using the following algorithmCitation[25]:
‘Mobility’ was mapped using item 4 from HSQ–12
‘Self-care’ was mapped using items 4 and 6 from D–test
‘Usual activities’ was mapped using item 5 from HSQ–12
‘Pain/discomfort’ was mapped using item 8 from HSQ–12
‘Anxiety/depression’ was mapped using item 3 from QoL–AD
To capture accurately the HRQoL of patients in the pre-FTC state and the change in utility as the patient approaches FTC, utilities in the pre-FTC state were modelled according to the patient's socio-demographic profile, comorbidities and clinical characteristics (severity and MMSE score, ADAS-cog, ADCS–ADL and NPI total scores, NPI domain scores and presence/absence of symptoms). Firstly, these variables were tested in a univariate generalised linear model; variables that were significant predictors of utility were then combined in a stepwise procedure. The final model included only the ADCS–ADL total score as this was by far the strongest predictor for utility in the pre-FTC health state. The utility for pre-FTC was calculated using the baseline ADCS–ADL score, as well as the ADCS–ADL monthly slope to model its evolution through time.
Equation 5: Equation to predict utility in the pre-FTC state
The health utilities for the patients in pre-FTC in each model cycle were obtained by entering their baseline characteristics and the length of time elapsed since beginning of the evaluation. The first evaluation was at the model start, i.e. month (0). The utility in the FTC state was 0.336 (SE = 0.043), based on sub-group analysis of 98 FTC moderate-to-severe patients in LASER–AD. The utility for death was assumed to be 0.
Resource utilisation and cost
In line with the guideline on technology appraisalsCitation[21], the valuation of cost was performed from NHS and PSS perspective, i.e. only direct medical and non-medical costs such as routine patient management, hospitalisation, social community services, institutionalisation costs and AD medications were included. Although informal care represents a large portion of AD care, the indirect or societal costs such as carer's loss of productivity and leisure time were not part of this analysis.
The data on resource utilisation was collected within the LASER–AD study. The Client Service Receipt Inventory (CSRI)Citation[27], adapted for use with the elderly, was used to record the utilisation of formal and informal services, such as social work assistance, home care, community nurses, general practitioner assessments, inpatient and outpatient appointments and informal care. As the data was collected on the volume of service consumed over the preceding three months, the resource volumes were divided by 3 to fit monthly cycles of the model, assuming homogeneous resource utilisation across the recall period. For patients residing in the community, all resource use was accounted for in estimating the total costs. In the case of patients within an institutional setting, only the cost of hospitalisation (for the duration of time hospitalised) and the cost of the residential care or nursing home (for the duration of time not hospitalised) were recorded. This assumes that all other costs, such as physician costs and cost of meals are included within the overall institutionalisation cost. The monthly resource utilisation volumes are reported in . Costs per model state were transformed into 2009 GBP using the unit costs from the ‘Unit Costs of Health and Social Care’Citation[28]. The mean (SE) cost of care per month was £724 (£217) in the pre-FTC state compared to £3267 (£284) in the FTC state. A cost of 0 was applied to death.
Table 3. Monthly resource use and costs by pre-FTC and FTC state.
The monthly treatment cost with memantine was estimated as £64.80 based on a cost of £2.16 per defined daily dose (British National Formulary, September 2009, BNF 58). In addition, the additional cost associated with monitoring patients in the memantine group was included. This consisted of an initial visit to a psychiatrist or a GP for the treatment initiation then by follow-up visits to the GP every 6 months. The monitoring costs were therefore £124.28 for the first visit (psychiatrist visit for initiation) and £36 for the subsequent visits (GP visit for renewal). The cost associated with memantine treatment was assumed to occur until patients reach the FTC state. This assumption was relaxed in a scenario analysis, where these costs were applied to a typical treatment duration with memantine in the UK (16 months)Citation[29].
Drug acquisition costs of AChEIs were not included in the model, as it was assumed that the proportion of patients receiving AChEIs was the same in the two treatment arms and would therefore not affect cost differences between the treatments.
Discount rates
An annual discount rate of 3.5% was applied to both costs and health benefits occurring beyond the first year, reflecting the present recommendationCitation[21]. Half-cycle correction was applied on all outcomes of the economic model (costs and health benefits) to account for transition between health states, which may occur anytime within each cycle.
Base-case analysis
The base-case analysis presents the results of the deterministic and probabilistic models. The treatment alternatives are compared in terms of time to FTC, quality-adjusted life-years (QALYs) and costs. To facilitate cross-country comparison of the results, the costs expressed in 2009 GBP were converted to EUR by applying the average exchange rate of £1 : €1.2042Citation[30]. All results are presented per patient.
Sensitivity analyses
The model underwent extensive stochastic and one-way scenario analyses across clinical inputs, discount rates, costs and utilities.
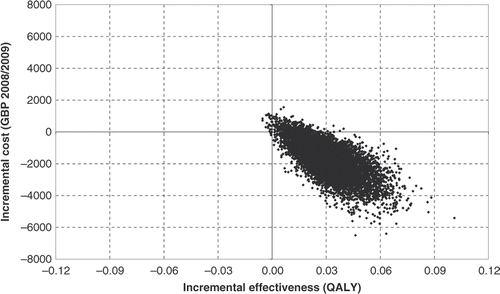
Monte Carlo simulations were undertaken by simultaneously varying all model parameters according to the pre-assigned distributions. Observed distributions around the parameter in the equation (baseline scores and slopes), memantine efficacy and health utilities were compatible with Gaussian distributions in terms of symmetry and shape. Normal distributions were therefore chosen as a priori for each of these parameters. Gamma distributions were assigned to cost inputs in order to reflect the positive skewness observed. A Monte Carlo simulation was used to evaluate outcomes based on 10,000 repeated random computations, which attributed a value to each of the a priori distributions. Cost and health outcomes were then estimated and their distribution calculated on the basis of the 10,000 repetitions. The results were used to calculate the probabilities for memantine to be more effective, less costly and cost effective at both the £20,000 and £30,000 per QALY thresholds. A joint distribution of incremental costs and effects was presented as a scatter plot on the cost-effectiveness plane.
The scenario analyses were also performed to further test the robustness of the results and conclusions. The parameters were independently varied within their plausible ranges to identify those having the greatest impact on the results. These were:
Computation of memantine efficacy on clinical outcomes using last observation carried forward analysis (LOCF) instead of observed cases (OC)
Computation of cost associated with memantine treatment (drug acquisition cost and patient monitoring) over the typical treatment duration observed in the UK, 16 months
Variation of cost per state by ±20% and ±30%
Alternative utility values per model state from published literature: 0.60 for pre-FTC and 0.34 for FTCCitation[11]
Alternative discount rates (0% and 6% per annum as per the NICE guidance)Citation[21]
Results
This model showed that patients not treated with memantine spent an average of 19.8 months (1.65 years) in the pre-FTC state. Overall costs in this group were estimated at around £94,800 (€114,100) over the 5-year evaluation period, or £19,000 (€22,800) annually. QALYs in this group were estimated at 1.5, which was 30% of full health ().
Table 4. Base-case model results.
Treatment with memantine prolonged time to FTC on average by 6 weeks per patient compared to standard care over the 5–year evaluation period, representing a 6.6% increase in the time to FTC compared to standard treatment. Memantine was also associated with a gain of 0.031 QALYs compared to routine care. The health benefits with memantine translated into cost savings that completely offset the additional cost of memantine above that of standard care. The cost saving per patient was −£1700 (−€2100). Lower costs in the memantine group were due to the prolonged pre-FTC period. When cost of memantine treatment was calculated as per the typical treatment duration in the UK, the cost saving increased to −£2200 (−€2670).
Probabilistic sensitivity analysis demonstrated that memantine was more effective than standard care in 99.8% of simulations. Memantine was the dominant treatment strategy in 96.3% of the 10,000 simulations. When considering willingness to pay thresholds of £20,000 (€24,100) and £30,000 (€36,000) memantine was a cost effective treatment in 98.1% and 98.5% of cases, respectively compared with standard care. The results of the probabilistic analysis, i.e. outcomes of the 10,000 Monte Carlo simulations are presented on the cost-effectiveness plane ().
The robustness of the model is demonstrated by the extensive sensitivity analysis. Memantine was the dominant strategy in all sensitivity analyses on memantine efficacy, cost, utility and discount rates ().
Table 5. Summary of scenario analyses.
Discussion
The high burden of dementia in the UK has been most recently highlighted by the results of a study commissioned by the Alzheimer's Research Trust and published in January 2010Citation[5]. This analysis reports that there are an estimated 821,884 people living with dementia in the UK. The total annual costs of dementia, including health care, social care, informal care and productivity losses is estimated at £23 billion in the UK compared to £12 billion for cancer and £8 billion for heart disease. The majority of the total costs for dementia are associated with the need for FTC; 37% of dementia patients living in long-term care institutions contribute over £9 billion in social care costs to the total, and informal care from unpaid carers accounts for a further £12.4 billion. Given the very high economic burden of dementia alongside the high clinical burden on patients and their carers it is unsurprising that the improved management of dementia patients is a key health priority in the UK; the National Dementia Strategy for England in development since 2007, was finalised by 2010 with a 5-year deadline for full implementationCitation[31]. The need for clinically effective treatments in dementia and AD that are also cost-effective is of increasing importance as the prevalence of these conditions increases.
Memantine, as the only treatment for severe AD in the UK, addresses an unmet clinical need, and the clinical efficacy across standard outcome measures has been well-documented in the clinical trial setting. The analysis presented here considers the cost-effectiveness of memantine using a novel predictive equation that models the time to FTC. The time to FTC assesses the maintenance of independent living and is an important and relevant measure in AD, particularly for patients with advanced, severe disease.
One of the strengths of the predictive equation used here is the ability to model the need for FTC regardless of the location of care. The new equation represents an advance in the assessment of the economic impact of treatments in AD as it contains a functional component in addition to cognition and behavioural disturbances. This is important, since the needs of AD patients are greatly affected by their functional ability and by the presence of psychotic and behavioural symptoms, with functional status determining dependency and therefore the need for FTC. The predictive equation used here is ideally placed for economic evaluation from the UK perspective as the equation is built on UK data, using the LASER–AD study. The same study was used to generate the costs and utilities in this model. Noteworthy, the LASER–AD study was designed to be representative to general AD population. Patients were selected on the basis of gender, residential setting, and severity of disease using independent quotas methods.
The cost-utility analysis shows that memantine is a more effective and less costly treatment for moderate-to-severe AD in the UK setting compared to standard care. Validity and robustness of the results were demonstrated by extensive sensitivity analysis. Although this model employs a different framework, results of this analysis are in line with previously published evaluations for memantine in the UKCitation[6].
Previous economic evaluations employing the concept of time to FTC were performed for AChEI for the treatment of mild-to-moderate ADCitation[32],Citation[33]. The average time to FTC was estimated as 38.4 months, which is twice as long as that in this analysis, which was conducted in moderate and severe patients. This confirms a clinical fact that patients at more advanced stages of the disease have a higher need for FTC. Despite the fact that these evaluations cannot be directly compared due to differences in the severity of the included patients, it is interesting to note that prolonged time in a pre-FTC state was of a similar magnitude. In mild-to-moderate patients treated with AChEIs, time to FTC was prolonged by 3–12% as compared to no treatment. This analysis showed that for patients with more advance AD, treatment with memantine prolonged time to FTC by 7% when compared to standard care in the UK for this patient population, i.e. no pharmacological treatment or background medication with AChEIs. Furthermore the studies reported that not all AChEIs were the dominant treatment choice compared to no treatment. The difference in costs ranged from a marginal cost saving to an additional cost over 10 years of model time horizon.
The model is associated with some limitations. The clinical inputs for the model are based on RCTs of 6 months duration and, as no longer-term data is available, the treatment benefit of memantine at baseline is assumed to be sustained over time. This is an approach used in other models where efficacy inputs are based on short-term clinical trialsCitation[34]. Although the benefits of memantine are assumed to be maintained over time, the model also assumes that there is no further improvement, which is a conservative assumption. Furthermore some of the data from the memantine clinical trials were collected using scales different to those in the equation, requiring transformation of data that could reduce the accuracy of the prediction. As is frequently the case in evaluations of AD treatments, selection of utility inputs in the model is not straightforward. Within the LASER–AD study, utilities were not directly measured using the EQ–5D and were estimated through a mapping process. The limitation of the cost data included in the model was that it was based on a 3-month recall period which could have introduced recall bias. It is worth noting that memantine treatment was the cost-effective option across all of the sensitivity analyses conducted on clinical, utility and cost outcomes.
The results of this model, employing the new predictive equation, support the use of memantine in the treatment of moderate to severe AD in the UK. As the costs of AD increase with disease severity the need for cost-effective treatments is especially great in the severe population.
Conclusions
This model-based economic evaluation demonstrates that memantine delays the need to FTC in moderate-to-severe AD patients in the UK when compared to standard care. The treatment is associated with increased benefits at no additional costs relative to its alternative, and can be regarded as a cost-effective treatment choice for management of the moderate and severe AD.
Transparency
Declaration of funding: Lundbeck SAS provided financial support for data gathering assistance for the LASER–AD study.
Declaration of financial/other relationships: B.R., M.G., C. G.–G. and C.F. have disclosed that they are full time employees of Lundbeck SAS; C.K. has disclosed that he has received grant money from Lundbeck SAS for his role in the LASER–AD study and has also been in receipt of speaker honoraria and consultancy fees from Lundbeck SAS; G.L. has disclosed the she received from Lundbeck SAS the grant money and a gift to UCL for data gathering for the LASER–AD study; M.T. has disclosed that he was a full time employee of Lundbeck SAS at the time of the LASER–AD study.
Supplementary Material
Download PDF (84.6 KB)References
- Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366: 2112–2117
- Matthews F, Brayne C. The incidence of dementia in England and Wales: findings from the five identical sites of the MRC CFA Study. PLoS Med 2005; 2: e193
- Blennow K, de Leon MJ, Zetterberg H. Alzheimer's disease. Lancet 2006; 368: 387–403
- Jönsson L, Wimo A. The cost of dementia in Europe: a review of the evidence, and methodological considerations. Pharmacoeconomics 2009; 27: 391–403
- Luengo- Fernandez R, Leal J, Gray A. Dementia 2010 – The economic burden of dementia and associated research funding in the United Kingdom. Health Economics Research Centre, University of Oxford for the Alzheimer's Research Trust, 2010, www.dementia2010.org
- Jones RW, McCrone P, Guilhaume C. Cost effectiveness of memantine in Alzheimer's disease: an analysis based on a probabilistic Markov model from a UK perspective. Drugs Aging 2004; 21: 607–620
- Livingston G, Katona C, Roch B, et al. A dependency model for patients with Alzheimer's disease: its validation and relationship to the costs of care – the LASER-AD study. Curr Med Res Opin 2004; S7: 1007–1016
- Reisberg B, Doody R, Stöffler A, et al. Memantine in moderate-to-severe Alzheimer's disease. N Engl J Med 2003; 348: 1333–1341
- Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA 1997; 277: 806–812
- Caro JJ, Getsios D, Migliaccio-Walle K, et al. Assessment of health economics in Alzheimer's disease (AHEAD) based on need for full-time care. Neurology 2001; 57: 964–971
- Green C, Picot J, Loveman E, et al. Modelling the cost effectiveness of cholinesterase inhibitors in the management of mild to moderately severe Alzheimer's disease. Pharmacoeconomics 2005; 23: 1271–1282
- Rive B, Le Reun C, Grishchenko M, et al. Predicting time to full-time care in AD: a new model. J Med Econ 2010; 13: 362–370
- Winblad B, Jones RW, Wirth Y, et al. Memantine in moderate to severe Alzheimer's disease: a meta-analysis of randomised clinical trials. Dement Geriatr Cogn Disord 2007; 24: 20–27
- Peskind ER, Potkin SG, Pomara N, et al. Memantine treatment in mild to moderate Alzheimer disease: a 24-week randomized, controlled trial. Am J Geriatr Psychiatry 2006; 14: 704–715
- Porsteinsson AP, Grossberg GT, Mintzer J, et al. Memantine treatment in patients with mild to moderate Alzheimer's disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res 2008; 5: 83–89
- Bakchine S, Loft H. Memantine treatment in patients with mild to moderate Alzheimer's disease: results of a randomised, double-blind, placebo-controlled 6-month study. J Alzheimers Dis 2007; 11: 471–449
- van Dyck CH, Tariot PN, Meyers B, et al. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis Assoc Disord 2007; 21: 136–143
- Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA 2004; 291: 317–324
- Hasselblad V, McCrory DC. Meta-analytic tools for medical decision making: a practical guide. Med Decis Making 1995; 15: 81–96
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560
- NICE - Guide to the methods of technology appraisal. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf
- Radosevitch DM, Pruitt M. Twelve-item health status questionnaire (HSQ–12) cooperative validation project: comparability study. Poster presented at the AHSR FHSR Annual Meeting, 1996.
- Ferm L. Behavioural activities in demented geriatric patients. Study based on evaluations made by nursing staff members and on patients’ scores on a simple psychometric test. Gerontol Clin (Basel) 1974; 16: 185–194
- Logsdon RG, Gibbons LE, McCurry SM, et al. Quality of life in Alzheimer's disease: patient and caregiver reports. J Ment Health Ageing 1999; 5: 21–32
- Sapin C, Livingston G, Katona C, et al. Main factors influencing preference-based utility values in Alzheimer's disease: results from the LASER–AD Study. Poster presented at the 7th International Conference on Alzheimer's and Parkinson's Disease (AD/PD), Sorrento, Italy, March 9-13, 2005.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997; 35: 1095–1108
- Beecham J, Knapp MRJ. Costing psychiatric intervention. Measuring Mental Health Needs, GJ Thornicroft, CR Brewin, JK Wing. Gaskell, London 1992
- Curtis L. Unit Costs of Health and Social Care 2009, Personal Social Services Research Unit. University of Kent, Canterbury 2009
- Lundbeck Data on File.
- Foreign exchange rates: European Union http://www.hmrc.gov.uk/exrate/european-union.htm
- Department of Health – National Dementia Strategy http://www.dh.gov.uk/en/socialcare/deliveringadultsocialcare/olderpeople/nationaldementiastrategy/index.htm
- Caro J, Getsios D, Migliaccio-Walle K, et al. AHEAD Study Group. Rational choice of cholinesterase inhibitor for the treatment of Alzheimer's disease in Canada: a comparative economic analysis. BMC Geriatr 2003; 3: 6
- Ward A, Caro JJ, Getsios D, et al. AHEAD Study Group. Assessment of health economics in Alzheimer's disease (AHEAD): treatment with galantamine in the UK. Int J Geriatr Psychiatry. 2003; 18: 740–747
- Caro JJ, Salas M, Ward A, et al. Assessment of Health Economics in Alzheimer's Disease. Economic analysis of galantamine, a cholinesterase inhibitor, in the treatment of patients with mild to moderate Alzheimer's disease in the Netherlands. Dement Geriatr Cogn Disord 2002; 14: 84–89