Abstract
Objective:
To study outcomes of multiple sclerosis (MS) patients treated with either glatiramer acetate (Copaxone) or interferon beta-1a for once-weekly, intramuscular administration (Avonex).
Methods:
An ‘intent-to-treat’ (ITT) cohort (n = 1282) was established, consisting of patients diagnosed with MS who began therapy on either glatiramer acetate (GA) or intramuscular interferon beta-1a (IFN beta-1a-IM) and had continuous insurance coverage from 6 months before to 24 months after the date when they began taking the medication. A ‘persistent use’ (PU) cohort (n = 639) was also constructed, consisting of individuals who, in addition to the criteria listed above, had a claim for GA or IFN beta-1a-IM within 28 days of the end of the 2-year post-period. Data were obtained from the i3 InVision Data Mart Database from July 2001 to June 2006. Multivariate regressions were used to examine both the 2-year total direct medical costs and the likelihood of relapse associated with the use of each of these alternative MS medications. A relapse was defined as either being hospitalized with a principal diagnosis of MS or having an outpatient visit with a MS diagnosis followed within 7 days by a claim for a corticosteroid. All regressions controlled a wide range of factors that may potentially affect outcomes.
Results:
In the ITT cohort, patients who started therapy on GA had a significantly lower 2-year risk of relapse (10.01 vs. 5.18%; p = 0.0034) as well as significantly lower 2-year total medical costs ($44,201 vs. $41,121; p = 0.0294). In the PU cohort, patients who used GA also had a significantly lower 2-year risk of relapse (7.25 vs. 2.16%; p = 0.0048) as well as significantly lower total medical costs ($67,744 vs. 63,714; p = 0.0445).
Limitations:
The analyses relies on an administrative claims database of an insured population and hence, may not be generalizeable to other populations. In addition, such a database precludes measurement of lost work time, unemployment, caregiver burden or other costs associated with MS.
Conclusions:
Results from this study indicate that the use of GA is associated with significantly lower probability of relapse as well as significantly lower 2-year total direct medical costs than IFN beta-1a-IM.
Introduction
Multiple sclerosis (MS) is the most common neurologically disabling condition for young adults in industrialized countriesCitation1,Citation2.Of the 2.5 million people around the world who have MSCitation3, an estimated 400,000 live in the United StatesCitation4,Citation5, where about 200 individuals are diagnosed with MS each weekCitation4. The disease is generally categorized into benign, relapsing-remitting, secondary progressive and primary progressive MS, although all types are characterized by progressive damage and destruction to myelin, the protective sheath surrounding nerve fibers of the central nervous systemCitation3. It results in a wide range of physical and cognitive symptoms, including weakness, fatigue, ataxia, physical dysfunction, bladder and bowel problems, sensory effects, visual impairment and short term memory lossCitation6. While symptoms vary widely, all MS patients face an unpredictable course of the diseaseCitation7 and a reduction in their quality of lifeCitation8,Citation9.
With no cure available, the goals for treating MS include slowing the progression of the disease, reducing the frequency of relapses, and treating symptomsCitation10. In 1993, US authorities approved interferon beta-1b as the first disease-modifying therapy (DMT) for the treatment of relapsing-remitting multiple sclerosis, and since that time, DMTs have been used to reduce the frequency of relapses and to gain a beneficial effect on the Expanded Disability Status Scale (EDSS)Citation11,Citation12. The US Food and Drug Administration has approved for first-line therapy (i.e., the first treatment that may be prescribed) for patients with relapsing-remitting multiple sclerosis, glatiramer acetate (GA), known by the brand name CopaxoneFootnote*, and the beta interferons (IFNs), which include IFN beta-1a for intramuscular administration (Avonex), IFN beta-1a for subcutaneous administration (Rebif), and IFN beta-1b (Betaseron in the United States; Betaferon§ in Europe)Citation13.
Placebo-controlled clinical trials of GA and IFN beta-1a-IM and a 10-year open-label extension study of GA have demonstrated that these therapies can reduce the frequency of relapsesCitation14–16. GA does not require safety lab monitoring necessary for the interferon productsCitation17,Citation18 and has been shown to be an effective treatment alternative for MS patients who cannot tolerate treatment with the beta interferons due to IFN-related side-effectsCitation19.
Literature examining the medical costs of individuals using the DMTs is scant. Early cost-effectiveness analyses of the DMTs generally concluded that the drugs had unfavorable cost-effectiveness ratiosCitation20,Citation21. More recent cost-effectiveness research has produced more favorable cost-effectiveness ratios for both the DMTs in general and for GA, in particularCitation22,Citation23. It has been noted that the earlier studies extrapolated long-term costs from short-term data, while the more recent studies benefited from the availability of longer term data or used a primary outcome of relapses averted in modeling the short-term placebo-controlled trialsCitation24.
In order to expand the research comparing the DMTs, particularly in the area of costs-of-use, a retrospective, multivariate analysis was conducted to compare the outcomes of patients treated with either GA or IFN beta-1a-IM. Specifically, the analyses evaluated relapse rates and direct medical costs associated with each of these commonly prescribed treatments for relapsing-remitting MS. The analyses also explored the impact of the persistent use of these drugs over a 24-month period.
Methods
Data for this study were obtained from the i3 InVision Data Mart Database. This retrospective database is fully de-identified and Health Insurance Portability and Accountability Act (HIPAA) compliant. The database contains laboratory test results, medical claims, and pharmacy data for more than 20 million de-identified individuals. The data collected spanned the period from July 1, 2001 to June 30, 2006.
This analysis included two distinct cohorts. In the ‘intent-to-treat’ (ITT) cohort, patients were included if they had a diagnosis of multiple sclerosis (International Classification of Diseases, Ninth Revision [ICD-9] code of 340.xx), a procedure code or outpatient prescription for daily use of GA or once-weekly administered IFN beta-1a-IM (with the first such date identified as the index date), and insurance coverage extending continuously from 6 months before through 24 months after the index date. In addition, the index date was required to be between January 1, 2002 and July 1, 2004, with the dates dictated by the time period of data collection as well as the requirements of a 6-month pre-period and 24-month post-period. A total of 1282 individuals were identified in the ITT cohort – 613 who received GA and 669 who received IFN beta-1a-IM.
In addition to the ITT cohort, a ‘persistent use’ subgroup was also constructed. These individuals met all the inclusion/exclusion criteria of the ITT cohort. Moreover, they were required to have used no other disease-modifying therapies other than the intent-to-treat medication during the 2-year post-period and were also required to have received a procedure or prescription for the ITT medication in the last 28 days in the post-periodCitation25. There were 639 individuals who met all of the above conditions; 308 received GA and 331 received IFN beta-1a-IM.
The major outcomes of interest in this analysis were costs and the probability of relapse. Costs were calculated as direct medical costs, including inpatient, outpatient, and prescription drug services. Cost estimates were based upon paid amounts of adjudicated claims, including insurer and health plan payments, copayments, and deductibles. All medical costs were converted to 2006 values using the medical component of the Consumer Price Index. Consistent with previous research which has examined relapses in a managed-care settingCitation26 or with claims databasesCitation27, relapse was defined as either a hospitalization with a diagnosis of MS or an outpatient visit with a diagnosis of MS accompanied by a prescription of corticosteroids within 7 days after the outpatient visits.
Five classes of independent variables were used to predict the total medical costs and probability of relapse for individuals with MS treated with either GA or IFN beta-1a-IM. Specifically, it was hypothesized that outcomes would be influenced by a patient’s demographic characteristics, general health status, medical comorbidities, general medications used, and specific immunomodulatory medication. Patient demographic characteristics included the patient’s age, gender, region of residence, and insurance coverage. Region was categorized as Northeast, South, Midwest, or West, while insurance coverage was categorized as commercial or Medicaid/Medicare. The analyses also controlled for general health status, hypothesizing that individuals who were more likely to use medical resources in the past may be more likely to have higher expenses or to experience a relapse. To capture general health, variables were constructed that measured the number of previous medical diagnoses (as counted by the number of unique ICD-9 codes at the three digit level, excluding ‘V’ codes), the number of previous prescription drugs prescribed, and whether an individual was hospitalized with a diagnosis of MS in the 6 months prior to the index date.
Furthermore, it was hypothesized that individuals who had more comorbidities may be more costly or be more likely to experience a relapse. The analysis therefore controlled for comorbid diagnoses of anxiety (based upon ICD-9 codes of 300.0x, 300.01, 300.02, or 300.21), depression, (296.2x, 296.3x, or 300.4x) diabetes (250.xx), hyperlipidemia (272.0x), and hypertension (401.xx) which occurred in the 6 months prior to the index date. The analysis also controlled for the medications an individual took in the 6 months before the index date. Indicator variables were used to control for the use of general medications, such as corticosteroids, anticholinergics, anticonvulsants, antivirals, CNS stimulants, genitourinary treatments, and musculoskeletal agents (e.g., anti-spasticity agents). Finally, the analyses examined the impact of the type of immunomodulatory medication on patient outcomes, by comparing the effect of treatment with GA to that of therapy with IFN beta-1a-IM.
A logistic regression was used to examine the impact of immunomodulatory therapy on the probability of relapse in the 2-year post-period, while controlling for the factors discussed above. Ordinary least squares was used to examine the impact of specific MS therapy on costs, using the log of costs as the dependent variable in order to account for the skewed nature of cost data. As with the logistic regressions, the least squares analysis controlled for demographic characteristics, general health status, comorbid medical conditions, and the use of symptomatic medications. All analyses were conducted using SAS Version 9.1. For each cohort, statistical significance was examined using a significance level of 0.05, adjusted for multiple comparisons using a Hochberg adjustmentCitation28.
Results
presents the descriptive statistics for the ITT cohort. Among the 1,282 individuals included in this cohort, the mean age was 43 years, 80% were female, and the vast majority (97%) were commercially insured. In the 6 months prior to beginning DMT medication, the most commonly diagnosed comorbidity was hypertension, and patients most commonly received an outpatient prescription for a corticosteroid, an anticonvulsant, or a musculoskeletal agent. Comparing the individuals who received GA to those who received IFN beta-1a-IM revealed few statistically significant differences between the groups. However, individuals treated with GA were significantly more likely to have been prescribed a musculoskeletal agent, an anticonvulsant, or a genitourinary agent in the pre-period. In addition, the GA users among the ITT cohort were significantly more likely to be diagnosed with comorbid depression.
Table 1. Patient characteristics - intent-to-treat cohort.
The descriptive statistics of the PU cohort, shown in , were similar to those of the ITT cohort. Specifically, the mean age of the PU cohort was 44 years, 79% were female, and 98% were commercially insured. Moreover, an examination of the 6 months prior to medication initiation revealed that for this group, as in the ITT cohort, the most commonly diagnosed comorbidity was hypertension, and patients most commonly received an outpatient prescription for a corticosteroid, an anticonvulsant, or a musculoskeletal agent. As with the ITT cohort, there were few statistically significant differences in patient characteristics when comparing individuals who initiated therapy on GA to those who initiated therapy with IFN beta-1a-IM. However, in the PU cohort, patients who were treated with GA were significantly more likely to be prescribed a CNS stimulant in the post-period compared to individuals treated with IFN beta-1a-IM.
Table 2. Patient characteristics - persistent use cohort.
and present the main results of the study. presents the impact of the use of GA compared to IFN beta-1a-IM on the probability of relapse. For both the ITT and PU cohorts, the probability of relapse in the 2 years post-medication initiation is significantly lower for patients who initiated therapy on GA compared to those who began treatment with IFN beta-1a-IM. Specifically, after controlling for patient demographic characteristics, general health status, comorbidities, and use of symptomatic medications, patients who initiated therapy on GA in the ITT cohort had a 2-year estimated relapse rate of 5.18% compared to a 10.01% estimated relapse rate associated with the use of IFN beta-1a-IM (p = 0.0034). When comparing the results for patients in the PU cohort to results for the ITT cohort, there are three main findings. First, as with the ITT cohort, the use of GA was associated with a significantly lower estimated 2-year risk of relapse, as compared to the use of IFN beta-1a-IM (p = 0.0048). Second, compared to patients in the ITT cohort, patients in the PU cohort had lower risks of relapse. For example, the risk of relapse associated with the use of IFN beta-1a-IM in the ITT cohort was 10.01%, while for the PU cohort the 2-year risk of relapse was 7.25%. Similarly, the use of GA in the ITT cohort was associated with an estimated 2-year risk of relapse rate of 5.18% compared to an estimated 2-year risk of relapse of 2.16% in the PU cohort. Finally, the persistent users of GA experienced lower risk of relapse than did the persistent users of IFN beta-1a-IM. Specifically, persistent users of GA had a 3.02% lower 2-year risk of relapse rate than did ITT users of GA. In contrast, the reduction in the 2-year relapse rate associated with IFN beta-1a-IM was 2.76%.
Figure 1. Impact of medication on probability of relapse. *P < 0.05 indicates a significant difference between the GA and IFN beta-1a-IM cohorts. Interpretation of coefficients – after controlling for other factors in a multivariate model, in an ITT cohort, use of GA was associated with a 2-year risk of relapse of 5.18%, while use of IFN beta-1a-IM was associated with a 2-year risk of relapse of 10.01%.
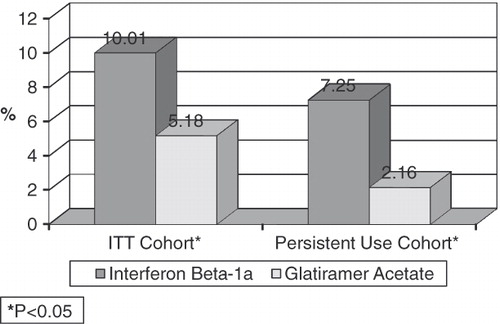
Figure 2. Impact of medication on total medical costs. *P < 0.05 indicates a significant difference between the GA and IFN beta-1a-IM cohorts. Interpretation of coefficients – after controlling for other factors in a multivariate model, in an ITT cohort, use of GA was associated with 2-year total direct medical costs of $41,121, while use of IFN beta-1a-IM was associated with 2-year total direct medical costs of $44,201.
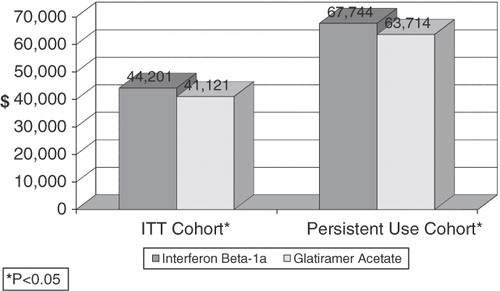
Other factors which had a statistically significant impact on the probability of relapse included patient characteristics, general health status, medical comorbidities, and other medication use. For example, in the ITT cohort, patients with commercial insurance were 62% less likely to relapse compared to those insured via Medicare or Medicaid (p = 0.0365). In addition, patients with a prior hospitalization were significantly more likely to relapse (odds ratio = 2.07; p = 0.0193), as were patients diagnosed with depression (odds ratio = 2.65; p = 0.0028) or those treated with an anticonvulsant in the pre-period (odds ratio = 2.18; p = 0.0198). focuses on the 2-year direct medical costs associated with the use of either GA or IFN beta-1a-IM, after controlling for patient demographic characteristics, general health status, comorbidities, and the use of symptomatic medications. In the ITT cohort, the total direct medical costs associated with the use of GA are statistically significantly lower than the total direct medical costs associated with the use of IFN beta-1a-IM (p = 0.0294). As in the ITT cohort, the use of GA compared to the use of IFN beta-1a-IM in the PU cohort was associated with a significantly lower estimated 2-year direct medical costs (p = 0.0445). This cost differential represents a 6.33% reduction in total 2-year direct medical costs. Compared to patients in the ITT cohort, patients in the PU cohort had higher 2-year direct medical costs. For example, the costs associated with the use of GA in the ITT cohort was $41,121, while for the PU cohort, the 2-year total direct medical costs were $63,714. Similarly, the use of IFN beta-1a-IM in the ITT cohort was associated with estimated 2-year total direct medical costs of $44,201 compared to estimated costs of $67,744 in the PU cohort.
Patient characteristics, general health, comorbidities, and use of other medications also were found to be associated with patient costs. For example, compared to patients who resided in the West, patients in the ITT cohort who resided in the South had 12% higher costs (p = 0.0199), while patients in the PU cohort who resided in the Northeast had 21% higher costs (p = 0.0255). For both the ITT and PU cohorts, an increase in the pre-period number of diagnoses (p < 0.0001; p = 0.0006, respectively) or number of prescription medications prescribed (p < 0.0001; p = 0.0051, respectively) were associated with a 2% increase in total costs. In the ITT cohort, a pre-period diagnosis of diabetes was associated with a 19% increase in total costs (p = 0.0211), while a pre-period diagnosis of depression was associated with a 16% increase in total costs in the PU cohort (p = 0.0449). Furthermore, pre-period receipt of a musculoskeletal agent or anticonvulsant medication was associated with 14% and 10% higher total costs in the ITT cohort, respectively (p = 0.0051; p = 0.0457), while pre-period receipt of a cerebral stimulant in the PU cohort was associated with 14% higher total costs (p = 0.0302).
As a test of the robustness of the results, the analyses were re-examined omitting the patients with non-commercial insurance. The results were not sensitive to this alternative specification and hence, are not reported.
Discussion
This analysis compared the 2-year risk of relapse and direct medical costs of patients who began therapy with either glatiramer acetate (GA) or interferon beta-1a for intramuscular administration (IFN beta-1a-IM). An examination of the descriptive statistics ( and ) revealed that the ‘typical’ patient treated with either GA or IFN beta-1a-IM in this study was a woman in her early- to mid-40s. If diagnosed with a co-morbidity, these patients were most frequently diagnosed with comorbid hypertension, and were frequently the recipient during the pre-period of an outpatient prescription for a corticosteroid, an anticonvulsant, or a musculoskeletal agent. These demographic characteristics are consistent with current literature about MS, which reports that MS is more prominent among women than among menCitation29; that most people are diagnosed with MS when they are between 20 and 50 years oldCitation30; that hypertension is one of the most common comorbidities associated with MS, reportedly affecting 30% of the MS patients surveyed in a recent studyCitation31; and that corticosteroids are the typical treatment for flares of symptoms (relapses) associated with MS. The prescription of an anticonvulsant or a musculoskeletal agent may have been for the management of MS co-morbidities or symptoms, such as epilepsy and/or pain. Previous research has noted that, seizures occur more frequently in patients with MS than in the general populationCitation32. In addition, among MS patients, ‘Painful paroxysmal symptoms such as trigeminal neuralgia (TN) … are treated with antiepileptics as first choice,’ and that patients with MS frequently experience ‘back or muscle and joint pain’Citation33. While there were few statistical differences between GA and IFN beta-1a-IM users, GA users among the ITT cohort were significantly more likely to have been prescribed a musculoskeletal agent, anticonvulsant, or genitourinary agent in the pre-period. More prescriptions among GA users may indicate that GA was prescribed more often to patients with comorbidities, while IFN beta-1a-IM was prescribed to patients who were less ill. GA users among the ITT cohort were also significantly more likely to be diagnosed with comorbid depression. This difference may reflect a preference to prescribe GA rather than IFN beta-1a-IM to patients with a comorbid diagnosis of depression, possibly due to reports of an association between IFN beta-1a and depressionCitation34,Citation35.
While the unadjusted patient statistics raise questions for future research, the multivariate analyses produced the main results of this study. These analyses examine the probability of relapse and total costs associated with the use of GA or IFN beta-1a-IM, while controlling for a wide range of factors that may also impact such outcomes. One of the main findings from the multivariate analyses was that the probability of relapse was significantly lower among the patients who initiated therapy on GA than among those who initiated therapy on IFN beta-1a-IM (see ), both in the intent-to-treat (ITT) cohort and in the persistent-use (PU) cohort. These results support previous research from retrospective studies that found a significantly higher reduction in relapse rate among GA users as compared to IFN beta-1a-IM usersCitation36,Citation37. Further, while persistent users of both GA and IFN beta-1a-IM experienced lower relapse rates relative to their intent-to-treat counterparts, persistent use over 24 months led to a greater degree of improvement among patients treated with GA. Specifically, persistent users of GA had a 3.02% lower 2-year risk of relapse than their ITT counterparts, whereas persistent users of IFN beta-1a-IM had a 2.76% lower 2-year risk of relapse than ITT users of IFN beta-1a-IM. While no previous study has directly compared the effects of persistent use of GA or IFN beta-1a-IM, studies looking at each drug independently have shown that relapse rates decline with persistent use. As an example, at 6 years in the open-label extension study of the pivotal trial for GA it could be determined that, for the persistent users of the medication, the mean annual relapse rate continued to drop steadily over the 6-year periodCitation38. Likewise, at a mean of 10 years of follow-up it was noted that ‘patients who remain on long-term GA therapy continued to do well’ over time, and that ‘relapse rates dropped by approximately one-half in the first year of treatment and by years 9–12, had declined more than 80%’Citation14. Moreover, the study by Ford et al.Citation14 indicated that the decline in relapse rates over time likely did not indicate progression of the disease to secondary progressive MS, since EDSS scores remained stable or improved over time. Similarly, research has indicated a sustained effect on relapse rates over a 4-year period among users IFN beta-1a-IMCitation39.
In addition to experiencing lower risks of relapse, users of GA also had lower 2-year direct medical costs (see ). Moreover, while persistent use (relative to ITT use) increased the cost of treatment with either drug, the costs associated with persistent use were less on GA therapy than on IFN beta-1a-IM therapy. The finding that GA use is associated with lower direct medical costs than IFN beta-1a-IM use is consistent with two recent studies. In one of these studies, the researchers analyzed over 10,000 MS patient records and found the annual MS-related treatment costs of a patient using GA to be lower than for a patient using IFN beta-1a-IM ($16,928 vs. 17,987; p < 0.001)Citation40–42. The other study, which used a pharmacoeconomic model to project costs based on clinical data, determined that GA was the most cost effective out of four immunomodulatory therapies, including IFN beta-1a-IM, when each was compared to symptomatic treatment alone, and that the use of GA resulted in better patient outcomes than the use of symptomatic treatment aloneCitation23.
As with any research, the findings presented here should be interpreted within the context of the limitations of the study’s design. First, this analysis was conducted using an administrative claims database and included only patients with medical and prescription benefit coverage. It may not be possible, therefore, to draw general conclusions about other populations based upon these results. Second, it is less rigorous to rely upon diagnostic codes rather than upon formal diagnostic assessments for identifying patients. Third, the use of medical claims data precludes the use of patient assessments; as a result, the analysis cannot examine quality of life, functioning, or any patient-reported clinical outcomes. Finally, this study focused only on direct medical costs, although previous research has indicated that the indirect costs of MS are largeCitation41,Citation42. A richer dataset would be necessary to explore the relative impacts of lost work time, unemployment, the increased use of other medical care services, and other indirect costs of multiple sclerosis. Furthermore, the data available on total costs does not allow for an examination of the role of patient co-payments, coinsurance, and deductibles has on patient outcomes. Additionally, publication of the recent head-to-head studies are needed to further elucidate the relative effectiveness, as well as cost effectiveness, of the available disease-modifying medications. Given these limitations, it is believed that these results are generally reliable, as the data for this study comprise one of the largest and most robust healthcare databasesCitation43.
Conclusion
In conclusion, this retrospective, multivariate comparison of the outcomes of MS patients using either glatiramer acetate or interferon beta-1a for intramuscular administration indicates that patients with relapsing-remitting multiple sclerosis who are treated with glatiramer acetate have significantly lower chances of relapse, and that the differences in the risk of relapse are more pronounced among patients who use these therapies persistently over 24 months. In addition, this study indicates that direct medical costs are significantly lower for those treated with GA than for those treated with IFN beta-1a-IM.
Transparency
Declaration of funding
Funding for this study was provided by Teva Neuroscience.
Teva Neuroscience paid for the purchase of the data.
Declaration of financial/other relationships
J.C.-H. and MK.A.O-B. Have disclosed that they are employees of Teva Neuroscience. M.J.L. has disclosed that she is an employee of HealthMetrics Outcomes Research, a company that received compensation from Teva Neuroscience. K.P.J. has disclosed that he is on the Speakers’ bureau and is a consultant for Teva Neuroscience.
Acknowledgements
The authors would like to thank Patricia Platt for assistance in the drafting of the manuscript.
Notes
*Teva Pharmaceutical Industries, Inc.
†Biogen Idec.
‡EMD Serano Inc.
§Bayer HealthCare Pharmaceuticals.
References
- Frohman EM, Frohman TC, Zee DS, et al. The neuro-ophthalmology of multiple sclerosis. Lancet Neurol 2005;4:111-121
- Frohman EM. Multiple sclerosis. Med Clin North Am 2003;87:867-897, viii-ix
- Multiple Sclerosis International Federation. About MS: what is MS? Accessed October 6, 2008. http://www.msif.org/en/about_ms/what_is_ms.html
- National Multiple Sclerosis Society. About MS: who gets MS? Accessed October 6, 2008. Available at http://www.nationalmssociety.org/about-multiple-sclerosis/who-gets-ms/index.aspx
- Noonan CW, Kathman SJ, White MC. Prevalence estimates for MS in the United States and evidence of an increasing trend for women. Neurology 2002;58:136-138
- Richards RG, Sampson FC, Beard SM, et al. A review of the natural history and epidemiology of multiple sclerosis: implications for resource allocation and health economic models. Health Technol Assess 2002;6:1-73
- Multiple Sclerosis International Federation. About MS: introduction. Accessed October 6, 2008. http://www.msif.org/en/about_ms/index.html
- Parkin D, Jacoby A, McNamee P, et al. Treatment of multiple sclerosis with interferon beta: an appraisal of cost-effectiveness and quality of life. J Neurol Neurosurg Psychiatry 2000;68:144-149
- Murphy N, Confavreux C, Haas J, et al. Quality of life in multiple sclerosis in France, Germany and the United Kingdom. Cost of Multiple Sclerosis Study Group. J Neurol Neurosurg Psychiatry 1998;65:460-466
- Multiple Sclerosis International Federation. About MS: recognised treatment for MS. Accessed October 6, 2008. http://www.msif.org/en/about_ms/recognised_treat.html
- Tullman MJ, Lublin FD, Miller AE. Immunotherapy of multiple sclerosis—current practice and future directions. J Rehabil Res Dev 2002;39:273-286
- Pohlman CH, Uitdehaag BM. Drug treatment of multiple sclerosis. BMJ 2000;321:490-494
- Calabresi P. Investigating glatiramer acetate for relapsing-remitting multiple sclerosis at the double dose—is more better? Nat Clin Pract Neurol 2007;3:540-541
- Ford CC, Johnson KP, Lisak RP, et al. A prospective open-label study of glatiramer acetate: over a decade of continuous use in multiple sclerosis patients. Mult Scler 2006;12:309-320
- Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology 1998;50:701-708
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-294
- Sela M, Teitelbaum D. Glatiramer acetate in the treatment of multiple sclerosis. Expert Opin Pharmacother 2001;2:1149-1165
- Davis WM. Multiple sclerosis: continuing mysteries and current management. Drug Topics 2000;144:93-102
- Vallittu AM, Peltoniemi J, Elovaara I, et al. The efficacy of glatiramer acetate in beta-interferon-intolerant MS patients. Acta Neurol Scand 2005;112:234-237
- Prosser LA, Kuntz KM, Bar-Or A, et al. Cost-effectiveness of interferon beta-1a, interferon beta-1b, and glatiramer acetate in newly diagnosed non-primary progressive multiple sclerosis. Value Health 2004;7:554-568
- Chilcott J, McCabe C, Tappenden P, et al. Modeling the cost effectiveness of interferon beta and glatiramer acetate in the management of multiple sclerosis. Commentary: evaluating disease modifying treatments in multiple sclerosis. BMJ 2003;326:522-525
- Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm 2009;15:543-555
- Bell C, Graham J, Earnshaw S, et al. Cost-effectiveness of 4 immunomodulatory therapies for relapsing-remitting multiple sclerosis: a Markov model based on long-term clinical data. J Manag Care Pharm 2007;13:245-261
- Phillips CJ. The cost of multiple sclerosis and the cost effectiveness of disease-modifying agents in its treatment. Curr Opin CNS Drugs 2004;18:561-574
- Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3-12
- Morrow T. The costs and consequences of multiple sclerosis relapses: a managed care perspective. J Neurol Sci 2007;256:S39-44
- Ollendorf D, Jilinskaia E, Oleen-Burkey M. Clinical and economic impact of glatiramer acetate versus beta interferon therapy among patients with multiple sclerosis in a managed care population. J Manag Care Pharm 2002;8:469-476
- Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988;75:800-808
- Alonso A, Hernán MA. Temporal trends in the incidence of multiple sclerosis: a systematic review. Neurology 2008;71:129-135
- National Multiple Sclerosis Society. About MS: epidemiology of MS. Accessed October 8, 2008. Available at http://www.nationalmssociety.org/about-multiple-sclerosis/who-gets-ms/epidemiology-of-ms/index.aspx
- Marrie R, Horwitz R, Cutter G, et al. Comorbidity, socioeconomic status and multiple sclerosis. Mult Scler 2008;14:1091-1098
- Miller A. Paroxysmal disorders. In Burkes JS, Johnson KP, eds. Multiple Sclerosis: Diagnosis, Medical Management, and Rehabilitation. New York: Demos Medical Publishing, 382
- Pöllmann W, Feneberg W. Current management of pain associated with multiple sclerosis. CNS Drugs 2008;22:291-324
- Patten SB, Francis G, Metz LM, et al. The relationship between depression and interferon beta-1a therapy in patients with multiple sclerosis. Mult Scler 2005;11:175-181
- Biogen, Inc. Medication guide: Avonex – interferon beta-1a. Published January 31, 2003. Available at http://www.fda.gov/cder/foi/label/2003/ifnbbio013103mg.pdf
- Haas J, Firzlaff M. Twenty-four-month comparison of immunomodulatory treatments - a retrospective open label study in 308 RRMS patients treated with beta interferons or glatiramer acetate (Copaxone®). Eur J Neurol 2005;12:425-431
- Carra A, Onaha P, Sinay V, et al. A retrospective, observational study comparing the four available immunomodulatory treatments for relapsing-remitting multiple sclerosis. Eur J Neurol 2003;10:671-676
- Johnson KP, Brooks BR, Ford CC, et al. Sustained clinical benefits of glatiramer acetate in relapsing multiple sclerosis patients observed for 6 years. Copolymer 1 Multiple Sclerosis Study Group. Mult Scler 2000;6:255-266
- Clanet M, Kappos L, Hartung HP, et al. Interferon beta-1a in relapsing multiple sclerosis: four-year extension of the European IFNbeta-1a Dose Comparison Study. Mult Scler 2004;10:139-144
- Prescott JD, Factor S, Pill M, et al. Descriptive analysis of the direct medical costs of multiple sclerosis in 2004 using administrative claims in a large nationwide database. J Manag Care Pharm 2007;13:44-52
- Kobelt G, Berg J, Atherley D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the USA. Neurology 2006;66:1696-1702
- Whetten-Goldenstein K, Sloan FA, Goldenstein LB, et al. A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult Scler 1998;4:419-425
- i3. Data assets: better data matters. Accessed October 8, 2008. http://www.i3global.com/DataAssets/