Abstract
Objective:
To explore treatment patterns and resource utilization and cost for subjects with pulmonary arterial hypertension (PAH).
Research design:
Retrospective claims database analysis of 706 patients with PAH enrolled in a large, geographically diverse US managed-care organization.
Results:
In the final sample of PAH patients treated with bosentan (n = 251) or sildenafil (n = 455), average age was 57 years, 86% of patients were commercially insured, and 52% of patients were male. Gender distribution varied significantly across subgroups, with a lower proportion of males in the bosentan (30%) subgroup compared with the sildenafil group (64%) (p < 0.001). Average baseline Charlson comorbidity score was 2.4. Average numbers of fills per month were 0.8 and 0.4 for bosentan and sildenafil patients, respectively (p < 0.001). Over 80% of patients received only one PAH treatment in the first 90 days following the index date, with 28% of bosentan and 13% of sildenafil patients receiving combination therapy (p < 0.001). Over one-third of bosentan patients and one-quarter of sildenafil patients experienced a dose increase in the follow-up period (p = 0.009). Sixteen percent of sildenafil patients experienced a dose decrease in the follow-up period, while a smaller proportion of patients receiving bosentan (4%) experienced a dose decrease (p < 0.001). On average, number of PAH-related per subject per month (PSPM) inpatient stays and emergency department visits and PSPM length of inpatient stays were statistically similar between the subgroups. PAH-related PSPM healthcare costs were high for both subgroups, with average monthly costs of $5,332 and $3,632 among bosentan and sildenafil patients, respectively (p = 0.003). Differences in total costs were driven mainly by differences in pharmacy expenditures.
Conclusions:
Of the oral agents approved for treating PAH at the time of this study, sildenafil was most commonly prescribed as index therapy and was also associated with the lowest costs, largely due to significantly lower pharmacy costs. This study is characterized by limitations inherent to claims database analyses, such as the potential for coding errors and lack of information on whether a drug was taken as prescribed. Furthermore, PAH severity (WHO functional class) was not assessed.
Introduction
Pulmonary arterial hypertension (PAH) is a rare but life-threatening condition characterized by chronic and progressive elevation of pulmonary artery pressure and pulmonary vascular resistance that leads to right-sided heart failure and deathCitation1–3. The pulmonary hypertension classification system has been revised several times, with the most recent revision occurring in 2008 during the 4th World Symposium on Pulmonary HypertensionCitation4. PAH is designated as Group 1 of 5 different pulmonary hypertension groupsCitation1. Group 1 encompasses idiopathic PAH, heritable PAH, drug- and toxin-induced PAH, and PAH associated with other diseases and conditions such as connective tissue disease, HIV infection, portal hypertension, congenital heart disease, schistosomiasis and chronic hemolytic anemiaCitation4.
The symptoms of PAH are nonspecific in nature and include shortness of breath (dyspnea), fainting/loss of consciousness (syncope), swelling (edema) in the ankles or legs, fatigue, and chest pain. PAH is defined by a mean pulmonary artery pressure of greater than 25 mmHg at rest, mean pulmonary-capillary wedge or left ventricular end-diastolic pressure ≤15 mmHg, and pulmonary vascular resistance greater than 3 Wood units (≥240 dyn s cm−5)Citation5.
PAH is a condition that can affect men and women of all ages, but primarily afflicts middle-aged women. The Centers for Disease Control and Prevention (CDC) reported that of the 807,000 PAH patients hospitalized between 2000 and 2002, 61% were women and 66% were ≥65 years of ageCitation6. In contrast, the US Registry to Evaluate Early And Long-Term PAH Disease Management (REVEAL) study found that about 80% of their PAH patients were female (which, according to the study authors, signifies an over-representation of the female preponderance observed in earlier studies), with a mean age of 53 ± 14 yearsCitation7,Citation8. The gender and age differences between the two estimates may reflect differences in the proportions of patients who had primary versus secondary PAH. Primary PAH is rare, not always linked to a specific cause, and occurs mainly in younger to middle-aged women. In contrast, secondary PAH is more common than primary PAH, although still rare, and occurs in conjunction with a primary disease (e.g., lungs, liver, or heart and blood vessel disease)Citation6. Almost half of the patients from the REVEAL study had primary PAH, while the CDC PAH surveillance sample consisted of hospitalized patients that had PAH as an ‘any-listed’ diagnosis.
Despite the progress made in the pharmacological treatment of PAH over the past decade, the prognosis for PAH patients is still poor, with diagnosis often delayed because of the nonspecific nature of the symptoms. Among the REVEAL Registry patients, the mean time until diagnosis from symptom onset was 2.8 yearsCitation8. Even with earlier diagnosis and the advent of new therapies, median survival averages 3.6–5.0 years with an estimated 58% 5-year survival rateCitation9.
Understandably, PAH imparts a tremendous burden of illness on both the patient and healthcare system. Wilkens and colleaguesCitation10 recently reported on the burden of illness for PAH patients in Germany. They found that treatment costs on average were €47,400 per patient per year, primarily due-to-drug costs, where analyses were conducted from the patient and third-party payer perspective. The authors were unable to find comparable studies examining the economic burden of PAH for a managed-care population of patients in the US at the beginning of this study, and also at the time of writing this manuscript.
The objective of the present study was to explore treatment patterns, resource utilization, and costs for PAH patients in the US, in order to better understand the burden of illness and costs associated with PAH. Only three oral pharmacotherapies were available on the US market that were approved for the treatment of PAH at the time this study was conducted (January 2006 – December 2008): sildenafil, ambrisentan, and bosentan. Oral PAH treatments are the primary focus of this paper. To the authors’ knowledge, this is the first retrospective claims database analysis to explore PAH treatment patterns and associated costs in a managed-care population in the US.
Methods
Data source
The present study is a retrospective database analysis using medical and pharmacy claims data (January 2006 – December 2008) and enrollment information from a large managed-healthcare plan. The health plan comprises discounted fee-for-service independent practice association plans spanning the United States. During the timeframe of this study, the administrative claims database included data for approximately 23 million commercial and Medicare Advantage health plan enrollees with both medical and pharmacy benefits (pharmacy benefits for Medicare Advantage patients were provided through the Medicare Part D benefit), approximately 97% of these enrollees were enrolled in a commercial plan. Among the commercial population available in the database during the identification period, males and females were equally represented (i.e., half were male and half were female); 10% of patients were in the Northeast health plan region, 27% in the Midwest, 47% in the South, and 16% in the West. The commercial population is younger in general, with approximately 97% of patients under the age of 65. The Medicare Advantage population was slightly different; 41% of patients were male and 59% were female, and 13% of patients were in the Northeast, 27% in the Midwest, 52% in the South, and 8% in the West. Approximately 14% of the Medicare population was under 65 years old.
Medical claims data are collected from healthcare sites (inpatient hospital, outpatient hospital, emergency room, physician's office, surgery center, etc.) for specialty, preventive, and office-based treatments. Claims for ambulatory services submitted by individual providers (e.g., physicians) use the HCFA-1500 format. Claims for facility services submitted by institutions (e.g., hospitals) use the UB-82 or UB-92 format. Medical claims include multiple diagnosis codes recorded with the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes; procedures recorded with ICD-9-CM procedure codes, Current Procedural Terminology (CPT), or Health Care Financing Agency (HCFA) Common Procedure Coding System (HCPCS) codes; site of service codes; provider specialty codes; revenue codes (for facilities); and paid amounts. Claims for pharmacy services are typically submitted electronically by the pharmacy at the time prescriptions are filled. The claims history is a profile of all outpatient prescription pharmacy services provided and covered by the health plan. All study data were de-identified and accessed with protocols compliant with the Health Insurance Portability and Accountability ActCitation11. Institutional review board approval was therefore not required for this study.
Subject identification
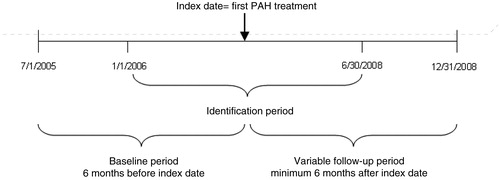
This study included commercial and Medicare Advantage health plan members with medical claims for pulmonary hypertension and/or evidence of PAH treatments. To be included in the PAH population, patients were required to have at least two medical claims with pulmonary hypertension as the primary or secondary diagnosis (ICD-9-CM codes 416.0, 416.8, 416.9) appearing 14 or more days apart, or at least one claim for endothelin receptor antagonists (ERAs) or prostanoid analogues (PAs) between January 1, 2006 and December 31, 2008. Because ERAs and PAs are primarily indicated for the treatment of PAH, patients with claims for these treatments were not required to have diagnoses for pulmonary hypertension to be included in the PAH sample. Use of PAH treatments including ERAs, PAs, phosphodiesterase type 5 (PDE5) inhibitors, and calcium channel blockers (CCBs) were identified in this population. Patients whose first claim for a PAH treatment was for an approved oral treatment including ambrisentan, bosentan, or sildenafil between January 1, 2006 and June 30, 2008 were identified as the final study sample. For these patients, the date of the first PAH treatment claim during the identification period was defined as the index date, and the type of treatment filled on the index date became the index PAH treatment. Because of the more recent approval of ambrisentan, the sample of patients with index ambrisentan use was small (n = 21). To allow for a more robust comparison of outcomes among patients on oral treatments, patients receiving ambrisentan were excluded from analysis. To be included in the final study sample, patients were required to have continuous enrollment in the health plan 6 months prior to the index date (baseline period) and for a minimum of 6 months following the index date (follow-up period). Patient characteristics and comorbidities were assessed during the baseline period, and outcomes were assessed during the follow-up period (). Patients were followed until the earlier of disenrollment or December 31, 2008.
Patient characteristics
Age, gender, geographic region of health plan enrollment, and type of insurance plan (commercial or Medicare Advantage) were captured from the enrollment data. Age was defined as of the index year. Presence of comorbid conditions during the 6-month baseline period was identified from the medical claims data using the algorithm maintained by the Agency for Healthcare Research and Quality (AHRQ)Citation12. In addition, the Charlson comorbidity score was calculated for each patient using the claims algorithm developed by Quan et al.Citation13,Citation14. The Charlson comorbidity score is a sum of weights for the following conditions: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, and diabetes (these are assigned a weight of 1); hemiplegia, moderate or severe renal disease, diabetes with end organ damage, and any tumor, leukemia, or lymphoma (these have a weight of 2), moderate or severe liver disease (this has a weight of 3), and metastatic solid tumor and AIDS (these have a weight of 6)Citation13. Healthy patients without diagnoses for any of these conditions in the medical claims have a Charlson score of 0.
Outcomes
Treatment patterns
Patients’ treatments for PAH were assessed during the follow-up period, and the initial PAH treatment received on the index date is also reported. The PAH treatments used during the follow-up period were categorized as oral, IV/subcutaneous infusion, or inhaled. Other PAH-related treatments (including anticoagulants, diuretics, digoxin, and oxygen therapy) used during the follow-up period were also noted. In addition, patients were defined as monotherapy users if they were treated with only one class of PAH medication (ERAs, PAs, PDE5 inhibitors, or CCBs) during the first 90 days of the follow-up period. Patients who were treated with more than one class of PAH medication during the first 90 days of the follow-up period were defined as combination therapy users.
Treatment dose
The total average daily dose of the index medication (bosentan or sildenafil) during the follow-up period was calculated as:
(quantity of index therapy × drug strength of index therapy (in mg)) ÷ total days supply of all pharmacy claims for the index medication during the follow-up period.
Dose changes were defined as a change in dose from the index fill to a subsequent fill. Dose increases or decreases of 62.5 mg or more per day for bosentan or 20 mg or more per day for sildenafil were evaluated. Patients with only one fill for the index medication were not included in the analysis of index therapy dose changes. The quantity supplied per day (number of pills) of the index medication was determined using all index therapy fills in the follow-up period. This was calculated as the total quantity supplied divided by the total days’ supply as determined from pharmacy claims for the index medication during the follow-up period.
Health care utilization and costs
All-cause healthcare resource utilization
Healthcare resource utilization was calculated for ambulatory visits, emergency department visits, inpatient admissions, and total length of inpatient stay during the follow-up period. Ambulatory visits included physician office visits and visits at an outpatient facility. Inpatient visits were identified based on American Medical Association (AMA) place of service codes in combination with revenue and provider specialty codes. Emergency department, physician office visit, and outpatient visits were identified based on AMA place of service codes. The count for each type of visit and the total length of inpatient stay are reported as per subject per month (PSPM).
PAH-related healthcare resource utilization
PAH-related healthcare resource utilization was calculated for ambulatory visits, emergency department visits, and inpatient admissions, and total length of inpatient stay during the follow-up period was recorded. Visits with a primary or secondary diagnosis of pulmonary hypertension (determined by ICD-9-CM codes 416.0, 416.8, 416.9) were considered PAH-related. The count of each type of visit and total length of inpatient stay are reported as PSPM.
All-cause healthcare costs
Healthcare cost estimates were based on the combined health plan- and patient-paid amounts in the follow-up period and include: total costs, medical costs, pharmacy costs, ambulatory costs, emergency services costs, inpatient costs, and other costs. Costs for ambulatory costs, emergency services costs, and inpatient costs were calculated using claims occurring at each site of services based on AMA place of service codes, revenue codes, and provider specialty codes as described in the all-cause healthcare resource utilization description above. Costs are reported as PSPM and were adjusted using the annual medical care component of the Consumer Price Index (CPI)Citation15 to reflect inflation between 2006 and 2008. Payments from Medicare (and other payers) were estimated for commercial patients based on coordination of benefits information obtained by the health plan in its usual course of business. This study incorporates the amounts estimated to be paid by other payers for a total paid or allowable amountCitation16.
PAH-related healthcare costs
PAH-related healthcare costs include: CPI-adjusted total costs; medical costs; pharmacy costs; ambulatory visit costs; emergency services costs; inpatient costs; and other costs during the follow-up period. Costs are reported as PSPM. Medical costs for claims with a primary or secondary diagnosis of pulmonary hypertension (determined by ICD-9-CM codes 416.0, 416.8, 416.9) are considered PAH-related. Pharmacy costs for PAH-related medications (pharmacy-filled ERAs, PAs, PDE5 inhibitors, and CCBs) are included in the PAH-related pharmacy and total costs.
Analytic strategy
All study variables, including baseline and outcome measures, were analyzed descriptively. Numbers and percents are provided for dichotomous and polychotomous variables. Means and standard deviations are provided for continuous variables. Incidence rates per person-year and 95% confidence intervals are presented for dichotomous utilization outcomes. Continuous outcomes are presented as PSPM amounts. All measures were compared among index users of bosentan and sildenafil using t-tests and chi-squared tests. Due to non-normality of data, median costs are also presented, and cost results were compared using Wilcoxon rank sum tests. Incidence rates of inpatient stays and emergency department visits were compared using exact significance tests of the incidence rate ratio. P-values less than 0.05 were considered statistically significant. All analyses were conducted using SAS, version 9.1 [SAS Institute, Inc., Cary, NC, USA].
Results
Prior to application of exclusion criteria, 21,960 patients were identified from the database with either ≥2 medical claims with a diagnosis of pulmonary hypertension appearing 14 or more days apart or claims for ERAs or PAs during the study identification period. A total of 1,295 of these patients had an index claim for ambrisentan, bosentan, or sildenafil. An additional 568 patients were excluded because they were not continuously enrolled in the health plan with a medical and pharmacy benefit during the baseline and follow-up periods, resulting in a sample of 727. Of these, 21 patients were identified with ambrisentan use on the index date, 251 were identified with bosentan use, and 455 were identified with index sildenafil use. Due to the small proportion of patients with index ambrisentan use, the final sample included patients with index bosentan or sildenafil use (706 patients).
In the final sample of PAH patients treated with bosentan or sildenafil, the average age was 57 years, 86% of patients were commercially insured, and 52% of patients were male (). Average age was similar regardless of the index treatment received, with average ages of 56 and 57 for bosentan and sildenafil patients, respectively (p = 0.353). The distribution of insurance type was statistically similar in the subgroups (p = 0.382). The gender distribution varied significantly across the subgroups with a lower proportion of male patients in the bosentan (30%) subgroup compared to sildenafil (64%; p < 0.001). A majority of patients came from either the Midwest (31%) or South (46%) health plan regions, with a smaller proportion of patients from the Northeast (8%) or West (15%) regions. Patients were required to be continuously enrolled for a minimum of 6 months following the index date, and the average length of follow-up in the PAH sample was 1.74 years; length of follow-up was slightly longer for bosentan patients as compared to sildenafil patients (p = 0.023).
Table 1. Patient demographics among patients with index bosentan or sildenafil use.
Patients identified with PAH had multiple comorbidities, with an average baseline Charlson comorbidity score of 2.4. The Charlson comorbidity score was similar among bosentan and sildenafil patients, with average scores of 2.3 and 2.5, respectively (p = 0.445). Common AHRQ comorbidities included: diseases of the heart (99.2%); other lower respiratory disease (93.1%); hypertension (80.9 %); respiratory infections (70.8%); diseases of the arteries, arterioles, and capillaries (66.6%); other connective tissue disease (63.7%); diseases of the urinary system (63.2%); disorders of lipid metabolism (58.6%); chronic obstructive pulmonary disease and bronchiectasis (57.9%); and eye disorders (55.8%).
describes the PAH treatments received during the follow-up period. Patients received about 0.5 fills for their index medication per month on average during the follow-up period, and this differed among the cohorts with average number of fills per month of 0.8 and 0.4 for bosentan and sildenafil patients, respectively (p < 0.001). Approximately 31% of bosentan patients filled a prescription for a PDE5 inhibitor at some time during the post-index period, while 12% of sildenafil patients filled a prescription for an ERA. Over 80% of patients received only one PAH treatment in the first 90 days following the index date, with 28% of bosentan and 13% of sildenafil patients receiving combination therapy (p < 0.001). A small proportion of patients filled a prescription for a PA during the follow-up period, with 5% of patients receiving an IV or subcutaneous infusion PA and 3% receiving an inhaled PA. Over 85% of patients received at least one other PAH-related treatment during the follow-up period, including anticoagulants (41%), diuretics (71%), digoxin (15%), and/or oxygen therapy (53%). Use of diuretics was slightly higher among sildenafil patients (73%) as compared to bosentan patients (66%; p = 0.032), while oxygen therapy was more prevalent among bosentan patients (61%) as compared to sildenafil patients (48%; p = 0.001).
Table 2. PAH treatments among patients with index bosentan or sildenafil use.
Dosing outcomes are presented in . On average, bosentan patients received two pills per day and sildenafil patients received between two and three pills per day. The average daily dose of each medication was similar to the recommended dose for PAH. The average daily dose among bosentan patients was 222 mg; the recommended daily dose is 125–250 mg. The average daily dose among sildenafil patients was 61 mg; the recommended daily dose of sildenafil for PAH is 60 mg. Of the 455 patients who initiated treatment with sildenafil, 365 (80%) started at a dose of ≤60 mg per day and had an average daily dose of 50 mg during the follow-up period. The remaining 90 sildenafil patients started at a dose >60 mg per day and had an average daily dose of 107 mg per day during the follow-up period. Patients who received >1 fill of their index medication during the follow-up period were assessed for dose changes (n = 597). Over a third of bosentan patients and a quarter of sildenafil patients experienced a dose increase in the follow-up period (p = 0.009). Alternatively, 16% of sildenafil patients experienced a dose decrease in the follow-up period, while 4% of bosentan patients experienced a dose decrease (p < 0.001). When comparing patients who started on sildenafil ≤60 mg per day versus patients who started on sildenafil >60 mg per day, a larger proportion of patients who started on a lower dose were more likely to experience a dose increase (28%) compared with those who started on a higher sildenafil dose (15%; p = 0.028). Similarly, a larger proportion of sildenafil patients who started on a higher dose experienced a dose decrease (38%) compared with those who started on a lower dose (10%; p < 0.001).
Table 3. Dosing patterns among patients with index bosentan or sildenafil use.
presents the PAH-related healthcare utilization patterns of patients with index bosentan or sildenafil use. The per person-year incidence of PAH-related inpatient stays was statistically similar among bosentan (0.31) and sildenafil (0.35) patients (p = 0.306). However, the per person-year incidence of PAH-related emergency department visits was significantly higher in bosentan patients (0.09) compared with sildenafil patients (0.05; p = 0.017). On average, the count of PAH-related PSPM inpatient stays and emergency department visits and the PSPM length of inpatient stays were statistically similar among the subgroups. However, the average count of PAH-related PSPM ambulatory visits differed among the subgroups, with average PSPM counts of 0.8 and 0.5 among bosentan, and sildenafil patients, respectively (p < 0.001). A large proportion of all-cause inpatient utilization was PAH-related, while a smaller proportion of emergency department and ambulatory utilization was PAH-related (data not shown).
Table 4. PAH-related follow-up healthcare resource utilization among patients with index bosentan or sildenafil use.
PAH-related PSPM healthcare costs are presented in . PAH-related PSPM healthcare costs were high among the subgroups, with average (median) monthly costs of $5332 ($4317) and $3632 ($1024) among bosentan and sildenafil patients, respectively (p < 0.001). This was a large portion of average all-cause PSPM healthcare costs for bosentan ($7224) patients and a large, but slightly smaller portion of average all-cause PSPM costs for sildenafil patients ($5987). This was also true of average (median) PAH-related PSPM pharmacy costs, which were $3478 ($3899) and $734 ($189) for bosentan and sildenafil patients, respectively (p < 0.001), while average (median) all-cause PSPM pharmacy costs were $3870 ($4146), and $1180 ($709) in the subgroups. Pharmacy costs were significantly different between the subgroups, while medical costs were similar (p = 0.123), indicating that differences in total costs were driven mainly by differences in pharmacy expenditures. PAH-related PSPM costs specific to emergency department visits and ambulatory visits were significantly different among the subgroups, but made up a small proportion of the total costs. Costs for inpatient stays were high on average, but heavily right skewed, indicating that a majority of inpatient costs were incurred by a small proportion of the study sample. A majority of average all-cause inpatient costs were PAH-related for both of the subgroups, while much smaller proportions of average all-cause emergency department and ambulatory costs were PAH-related (data not shown).
Table 5. PAH-related follow-up healthcare costs among patients with index bosentan or sildenafil use.
Discussion
This study explored treatment patterns, healthcare utilization, and costs associated with PAH in a group of insured US patients being treated with oral therapies. The authors chose to focus on patients who received one of two oral pharmacotherapies (sildenafil and bosentan) approved in the US for treatment of PAH at the time of the study. Sildenafil was the oral agent most commonly prescribed as an index medication (455 patients) and an additional 251 patients received bosentan as an index medication. Over 80% of patients who initiated treatment with sildenafil started at a dose of 60 mg per day or lower, while the remaining 20% of sildenafil patients were receiving doses over 60 mg per day. The low number of patients prescribed ambrisentan (n = 21) was likely due to the fact that this medication received FDA approval more recently than sildenafil or bosentan, and for this reason, patients receiving ambrisentan were not analyzed as part of this study.
Many patients who received bosentan or sildenafil as index therapy received other PAH-related treatments at some point during the follow-up period, and increases in the dosage of oral agents were common. This latter observation is not surprising because the labels for both ERAs recommend a dose escalation strategy. Patients in the sildenafil subgroup were less likely to be combination therapy users in the first 90 days of the follow-up period as compared to bosentan patients.
Although this study did not directly evaluate efficacy of treatments, these observations suggest that monotherapy with currently available oral agents does not adequately control PAH symptoms in all patients, and suggest the need for development of new therapies and/or use of combinations of existing therapies. Clinical studies have demonstrated that combination therapy with two oral agents from different classes may be more effective than monotherapyCitation17,Citation18. In the present study, 31% of patients taking bosentan as an index therapy received follow-up treatment with a PDE5 inhibitor. Dose increases were common, with 29% of patients experiencing a dose increase. Dose decreases were less prevalent, with approximately 11% of patients experiencing a dose decrease. Sildenafil patients were less likely to experience a dose increase and more likely to receive a dose decrease, with approximately 28% of sildenafil patients who started on lower sildenafil doses having a dose increase and over 38% of patients who started on higher doses having a dose decrease. This latter observation is difficult to explain clinically.
Healthcare utilization and costs were determined for patients receiving bosentan or sildenafil as the index medication. The average number of PAH-related ambulatory visits during the follow-up period was significantly different between the subgroups, and patients receiving sildenafil had a lower average number of PAH-related ambulatory visits compared with patients receiving bosentan. The number and length of PAH-related inpatient stays and the number of PAH-related emergency department visits was similar between the subgroups. However, overall PAH-related costs differed significantly among the subgroups; the sildenafil subgroup had lower PAH-related costs. Sildenafil patients had PAH-related costs of $3632 PSPM, compared with $5332 PSPM for bosentan patients. Although PAH-related ambulatory costs and emergency department costs did not statistically significantly differ between the subgroups, sildenafil patients had significantly lower PAH-related pharmacy costs and significantly higher PAH-related medical costs than bosentan patients. Pharmacy costs accounted for nearly two-thirds of overall PAH-related costs for bosentan patients, but only for about 20% of PAH-related costs for sildenafil patients. However, in both subgroups, PAH-related costs made up a high proportion (60% or more) of total all-cause healthcare expenditures, demonstrating that PAH imposes a substantial economic burden on the healthcare system. Furthermore, these findings are in line with what Wilkens and colleaguesCitation10 recently found when examining PAH costs in Germany.
Several previous pharmacoeconomic studies have evaluated the costs associated with oral PAH treatments and showed a similar trend for lower costs associated with sildenafil than bosentan. A recent modeling study using a hypothetical population of US patients with PAH compared the cost effectiveness of several oral agents and found that yearly costs for sildenafil ($11,088 per patient) were lower than those for bosentan ($42,804 per patient)Citation19. However, sildenafil and bosentan resulted in the same gain in quality-adjusted life-years. An earlier modeling study estimated the yearly costs of treating PAH patients with bosentan to be $36,208; however, this study did not compare bosentan costs to other oral agentsCitation20. Another modeling study performed in Canada also found that total costs over a 3-year period were lower for PAH patients treated with sildenafil (CAN$48,351 per patient) than for patients treated with bosentan (CAN$164,745 per patient)Citation21. However, unlike these previous studies, which relied on modeling, the present study was based on ‘real-world’ patient data. For a modeling study to capture a healthcare utilization event, the event must already be assumed by the model, but in a real-world study no prior assumptions about the type or frequency of healthcare utilization events are needed. Therefore, modeling studies may not fully account for healthcare resources utilized by patients, and real-world studies may have higher estimates for the amount of healthcare utilization (and subsequently the costs) associated with a certain treatment. Accordingly, when extrapolated out to a year, per-patient costs reported in the present study are higher than those reported in previous modeling studies. However, the results of the present study are consistent with those of previous studies, as they demonstrate that treatment with sildenafil is associated with lower overall costs than treatment with bosentan.
It should be noted that males and females were equally represented in the present study, which is not reflective of the preponderance of females with PAH in the general populationCitation7,Citation8. However, the gender distribution differed significantly between the subgroups, with a lower proportion of male patients in the bosentan (30%) subgroup as compared to the sildenafil subgroup (64%). Thus, the male : female ratio in the bosentan group is more representative of the ratio seen in the general PAH population. In addition, the mean age of the patients in the present study (57 years) is higher than that cited in earlier studies, but is consistent with that observed in the REVEAL Registry (53 ± 14)Citation7,Citation8. The older age of the present study population may be attributable to the demographic composition of the health plan and the particular inclusion and exclusion criteria applied, which may have yielded a patient population that is not precisely representative of the broader PAH population.
A limitation of this study is the ability to attribute use of sildenafil to a true diagnosis of PAH. All patients with an index treatment of sildenafil were required to have diagnoses for pulmonary hypertension and/or have additional claims for ERA or PA treatments. However, because ICD-9-CM diagnosis codes specific to PAH do not exist, it is possible that some proportion of the sildenafil sample may not truly have PAH. Sildenafil is also indicated for the treatment of erectile dysfunction (ED). Approximately 11% of the total sample had a medical claim with a diagnosis code for erectile dysfunction during the baseline or follow-up periods, and the rate was higher among sildenafil patients (16%) compared to bosentan (2%) patients. This indicates that some proportion of the sildenafil sample may have received sildenafil for the treatment of ED and not PAH. However, because these patients had evidence of both conditions, the reason for sildenafil treatment is unknown. It is important to note that the larger percentage of ED patients in the sildenafil group could have contributed to the lower costs in that group, compared to the bosentan group. However, medical utilization costs were higher for sildenafil patients indicating that they were more likely to be patients with PAH than ED.
Several limitations relating to the use of claims data should be considered when interpreting the results of the present study. Claims data are collected for payment and not research, and are subject to possible coding errors. A diagnosis code may be included as a rule-out criterion and does not necessarily indicate disease presence. In addition, ICD-9-CM diagnosis codes specific to PAH do not exist, so ICD-9-CM codes for pulmonary hypertension in combination with claims for PAH treatments were used to identify the study sample. Also, a prescription claim does not necessarily mean a drug was taken as prescribed, and some patients may receive drugs without a prescription claim (for example, by receiving samples). Claims data do not contain information on disease severity, and it is possible that patients in some subgroups had more severe disease than patients in other subgroups. Finally, there are limitations to the generalizability of this study. The data used for this study come from a managed-care population, and may not be applicable to the entire US population. However, the health plans used for analysis in this study include a wide geographic distribution of patients and should be generalizable to managed-care populations on a national level.
In conclusion, of the oral agents approved for treating PAH at the time of this study, sildenafil was most commonly prescribed as index therapy and was also associated with lower costs, largely due to significantly lower pharmacy costs. However, PAH-related costs made up a substantial proportion of all-cause healthcare costs for both sildenafil and bosentan users. Future research that includes a cohort of patients treated with ambrisentan should further investigate whether the suboptimal management of PAH drives medical, emergency department, inpatient, and ambulatory costs.
Transparency
Declaration of funding
Funding for this study was provided by Eli Lilly and Company.
Declaration of financial/other relationships
M.A., E.E. and A.B. have disclosed that they are employed by Eli Lilly & Company. E.B. and T.B. have disclosed that they are employed by i3 Innovus, a company that received support from Eli Lilly and Company to assist with the study design and analysis, and the manuscript preparation.
Acknowledgments
The authors would like to thank Leigh Borton and Randall Gerdes of i3 Innovus for their analytic and programming support for this study. The authors would also like to thank Jesse Potash and Victoria Porter, medical writers at i3 Innovus, for their assistance with the preparation of this manuscript.
References
- Chin KM, Rubin LJ. Pulmonary arterial hypertension. J Am Coll Cardiol 2008;51:1527-1538
- Humbert M, Sitbon O, Simmoneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425-1436
- Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655-1665
- Simonneau G, Robbins IM, Beghetti M, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2009;54:S43-54
- McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 Expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009;53:1573-1619
- Pulmonary Hypertension Fact Sheet. Centers for Disease Control and Prevention (http://www.cdc.gov/DHDSP/library/fs_pulmonary_hypertension.htm). Accessed on 04/22/2010
- Frost AE, Badesch DB, Barst RJ, et al. A comparison of REVEAL registry demographic data with other/prior registries of pulmonary arterial hypertension (PAH). Chest Meeting Abstracts 2008;134:p134001
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2009 Oct 16 [Epub ahead of print]
- Thenappan T, Shah SJ, Rich S, et al. A USA-based registry for pulmonary arterial hypertension: 1982-2006. Eur Respir J 2007;30:1103-1110
- Wilkens H, Grimminger F, Hoeper M, et al. Burden of pulmonary arterial hypertension in Germany. Respir Med. 2010 Feb 8. [Epub ahead of print]
- Health Insurance Portability and Accountability Act of 1996. Public Law 104--191, 104th Congress. Available at: http://www.cms.hhs.gov/HIPAAGenInfo/Downloads/HIPAALaw.pdf [Last accessed 10 November 2009]
- Agency for Healthcare Research and Quality, Healthcare Cost and Utilization Project. Comorbidity Software. Available at: http://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp [Last accessed 10 November 2009]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-383
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-1139
- US Department of Labor, Bureau of Labor Statistics. Consumer Price Index. Chained Consumer Price Index for all urban consumers (C-CPI-U) 1999-2008, Medical Care. Series ID: SUUR0000SAM. Washington, DC: U.S. Dept. of Labor, Bureau of Labor Statistics, 2008. Available at: http://data.bls.gov/cgi-bin/surveymost?su [Last accessed 10 November 2009]
- Frytak JF, Henk JH, Zhao Y, et al. Health service utilization among Alzheimer’s disease patients: evidence from managed care. Alzheimers Dement 2008;4:361-367
- Mathai SC, Girgis RE, Fisher MR, et al. Addition of sildenafil to bosentan monotherapy in pulmonary arterial hypertension. Eur Respir J 2007;29:469-475
- Hoeper MM, Faulenbach C, Golpon H, et al. Combination therapy with bosentan and sildenafil in idiopathic pulmonary arterial hypertension. Eur Respir J 2004;24:1007-1010
- Garin MC, Clark L, Chumney EC, et al. Cost-utility of treatments for pulmonary arterial hypertension: a Markov state-transition decision analysis model. Clin Drug Investig 2009;29:635-646
- Highland KB, Strange C, Mazur J, et al. Treatment of pulmonary arterial hypertension: a preliminary decision analysis. Chest 2003;124:2087-2092
- Dranitsaris G, Mehta S. Oral therapies for the treatment of pulmonary arterial hypertension: a population-based cost-minimization analysis. Appl Health Econ Health Policy 2009;7:43-59