Abstract
Objectives:
The purpose was to assess the cost effectiveness from a societal perspective of the recombinant human parathyroid hormones: PTH(1-34) (teriparatide) and PTH(1-84) for patients with osteoporosis with similar characteristics to patients treated in normal clinical practice in Sweden.
Methods:
A Markov model of osteoporosis in postmenopausal women was developed using 6-month cycles and a lifetime horizon. The model was populated with patients similar to the Swedish cohort of the European Forsteo Observational Study (postmenopausal women; mean age: 70 years, total hip T-score: −2.7 and 3.3 previous fractures). The cost effectiveness of both teriparatide and PTH(1-84) was estimated compared to no treatment and each other. Relative effectiveness assumptions were based on efficacy estimates from two phase III clinical trials.
Results:
The cost per QALY gained of teriparatide vs. no treatment was estimated at €43,473 and PTH(1-84) was estimated at €104,396. Teriparatide was indicated to be less costly and associated with more life-years and QALYs than PTH(1-84). When assuming no treatment effect on hip fractures the cost per QALY gained was €88,379. In the sensitivity analysis the cost effectiveness did not alter substantially with changes in the majority of the model parameters except for the residual effect of the treatment after stopping therapy.
Conclusions:
Based on the efficacy estimates from pivotal clinical trials and characteristics of patients treated in clinical practice in Sweden, teriparatide seems to be a more cost-effective option than PTH(1-84) when compared to no treatment. The relative efficacy between the two PTH compounds was based on an indirect comparison from two separate clinical trials which has to be considered when interpreting the results.
Introduction
The weakening of bones and decline in neuromuscular function occurs after menopause in women, leading to an increased risk of fractures. Osteoporosis has clinical and public health importance, as osteoporotic fractures are one of the most common causes of disability and a major contributor to medical care costs in many regions of the worldCitation1. The major complications of osteoporosis include vertebral and hip fractures, which are associated with pronounced morbidity and excess mortality. Therefore, the aim of treatment of postmenopausal osteoporosis is to reduce the frequency of vertebral and non-vertebral fractures, which are responsible for the morbidity associated with the diseaseCitation2,Citation3.
Treatment of osteoporosis with intermittent subcutaneous injections of parathyroid hormone (PTH) stimulates new bone formation that permits the restoration of bone microarchitecture, including improved trabecular connectivity and increased cortical thickness, and reduces fracture risk in postmenopausal women with osteoporosisCitation4. Currently, there are two approved preparations of PTH in Europe: the human recombinant (1-34) N-terminal fragment of PTH (teriparatide; Forsteo, Eli Lilly) and the full-length human recombinant molecule (rhPTH(1-84); Preotact, Nycomed). Teriparatide and PTH 1-84 are both administered by daily subcutaneous injection.
In pivotal phase III trials, both drugs significantly reduced the risk of vertebral fractures in postmenopausal women with osteoporosis compared with placeboCitation5,Citation6. Teriparatide has also been shown to significantly decrease the risk of non-vertebral fracturesCitation5. No head-to-head clinical trials have been performed to compare the two drugs.
The cost effectiveness of teriparatide has previously been estimated on women with characteristics similar to those of the patients in the Fracture Prevention TrialCitation7. This analysis did not consider PTH(1-84), which was not approved at the time of the study, and utilised a simulated patient population designed to follow the strict inclusion and ongoing protocol conditions of a clinical trial. By contrast, the present study aims to establish the cost effectiveness of both teriparatide and PTH(1-84) in postmenopausal women with postmenopausal osteoporosis representative of those prescribed teriparatide as administered in routine clinical practice.
Methods
Model overview
A seven health state Markov cohort simulation model was developed to investigate the cost effectiveness of teriparatide and PTH(1-84) at licensed doses in the treatment of postmenopausal osteoporosis relative to each other and placebo (). Due to a lack of comparative head-to-head clinical trials comparing the two forms of PTH, the clinical efficacy and safety data were extracted from two global, multi-centre placebo-controlled trials of teriparatide and PTH(1-84). The key outcomes of the model were the lifetime cost, measured under a societal perspective with a cost year of 2007, and the quality-adjusted life-years (QALYs) associated with teriparatide, PTH(1-84) and no treatment. Discounting was applied to costs and outcomes at 3% per year in the base case and varied in the sensitivity analysisCitation8.
Figure 1. Health state structure of the Markov model. Note: From each of the six health states above, transition to the seventh state (Dead) was possible.
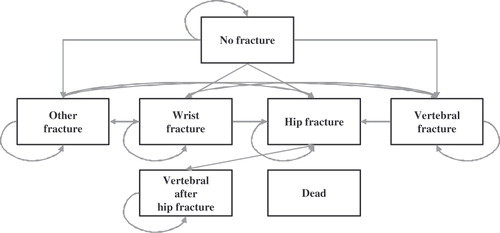
The cost-effectiveness model was a development of models that have previously been used for estimating the cost-effectiveness of other osteoporosis treatmentsCitation7,Citation9,Citation10 with modifications to include the results of PTH treatments and cost data for 2007. The model was programmed in TreeAge Pro 2007, with short-term (1st year) and long-term (2nd and subsequent years) outcomes estimated using a tunnelling techniqueCitation11. The model also included a health state which relates to patients sustaining a vertebral fracture after a hip fracture. The cycle length was for 6 months, and all patients were followed through the model from age at treatment initiation until they were 100 years old or dead. Patients began the simulation in the no-fracture health state. The patient cohort was adjusted according to their baseline characteristics, i.e. if the patients had a prevalent fracture before intervention; their quality of life, costs and mortality risk would be adjusted accordingly.
Target patient population
The population simulated in the model had the characteristics of the Swedish cohort of patients from the European Forsteo Observational Study prescribed teriparatide in routine clinical practiceCitation12,Citation13. This study covered eight European countries and included a cohort of 219 postmenopausal women from Sweden. At baseline, the Swedish cohort had a mean (SD) age of 69.9 (8.40) years, a mean (SD) T-score of −2.7 (1.08) and −3.5 (1.21) at the total hip and lumbar spine, respectively, and 3.3 (2.0) prevalent fractures (SD).
Clinical data
The model requires assumptions as to the clinical effectiveness of each modelled treatment and the risk of relevant adverse events. The two pivotal phase III trials on which the efficacy assumptions were based were the teriparatide Fracture Prevention Trial (FPT)Citation5 and the Treatment of Osteoporosis with PTH(1-84) (TOP) studyCitation6. Effectiveness was initially assumed to be equal to the efficacy results obtained from these two global, multi-centre trials.
Patients were assumed to be given treatment for 18 months, in line with Swedish treatment guidelines, and the effectiveness was assumed to apply at its maximum initial level for the whole treatment periodCitation14. Thereafter the effect was assumed to taper to zero over 24 and 30 months (i.e., the offset time of treatment effect) for vertebral and non-vertebral fractures, respectively, based on a follow-up of the FPTCitation7,Citation15,Citation16. In a sensitivity analysis, treatment periods of 12 and 24 months and scenarios assuming no and doubled offset time were explored.
Fracture efficacy
The FPT included 1,637 postmenopausal women with prior vertebral fracture to receive placebo or daily injections of 20 µg or 40 µg teriparatide. Treatment is currently licensed at 20 µg. The primary outcome was the occurrence of new vertebral fractures; the secondary outcome measures were the occurrence of non-vertebral fractures and changes in bone mineral density of the hip and lumbar spine. The risk of new vertebral fractures in the teriparatide arm was reduced by 65% compared with the placebo arm (95% CI: 45–78%) and the risk reduction of non-vertebral fragility was 53% (95% CI: 25–88%)Citation5. These point estimates were used as the base-case model assumptions. To acknowledge the fact that hip fracture reduction alone did not reach statistical significance in the FPT, since only five fragility hip fractures were observed (one case in teriparatide 20 µg/day and four cases in placebo) a sensitivity analysis was conducted assuming no effect on hip fracture risk with teriparatideCitation17.
The TOP study involved 2,532 postmenopausal women with low bone mineral density at the hip or lumbar spine treated with placebo or daily injections of 100 µg PTH(1-84), the licensed dose. The primary outcome measure was the occurrence of new vertebral fractures and the secondary outcome was the occurrence of non-vertebral fractures and changes in bone mineral density and markers of bone metabolism. A reduction in risk of vertebral fracture was observed in the active treatment arm relative to the placebo arm, but point estimates and confidence intervals for this varied according to assumptions made in relation to fracture incidence among those who discontinued the study. The relative risk reduction (RRR) of vertebral fractures was 40% (95% CI: 64–0%) if non-completers were assumed to have had the same incidence as completers in their respective treatment groups, 38% (95% CI: 63–4%) if non-completers were assumed to have had the same incidence as completers in the placebo group, and 58% (95% CI: 76–28%) if non-completers were assumed to have had zero fracture risk subsequent to leaving the trial. There was no statistically significant difference between treatment groups in the risk of non-vertebral fractures. The point estimates used for the base-case model assumptions were the 58% reduction in vertebral fractures (as this estimate is the closest to a standard ITT calculation) and no risk reduction in non-vertebral fractures. The lowest estimated efficacy (38%) was tested in a sensitivity analysis.
Adverse events
In the base-case analysis, no adverse events related to the treatments were considered. Data from the trials reported that the frequency of early post-injection (4–6 hours) hypercalcaemia in the 20 μg teriparatide group was 11 vs. 2% in the placebo armCitation5, while for full-length PTH(1-84) pre-injection (or 24 hours post previous injection) hypercalcaemia rates were 28% in the PTH(1-84) group with 4.5% in the placeboCitation6. Empirical data on the impact of hypercalcaemia on costs and quality of life is scarce but can be considered to be of low magnitude. In a sensitivity analysis, a cost and quality of life reduction related to hypercalcaemia based on the same frequencies as observed in the clinical trials was applied.
Compliance
Based on returned medications, the compliance for teriparatide in the FPT trial was estimated at approximately 80% in all trial arms (range: 79–83%)Citation5. In the TOP study, no such corresponding estimate was providedCitation6. In the base case, a compliance rate of 80% for both medications was used meaning that the drug costs were reduced by 20%. In a sensitivity analysis, 100% compliance without any adjustment of the treatment cost for both medications was explored.
Discontinuation/withdrawal rates
In the FPT study, after 18 months of treatment, 141 of the 541 (26%) patients in the 20 μg teriparatide arm at baseline had discontinued treatmentCitation5. The corresponding estimate in the TOP study was 36% of the patients receiving PTH(1-84)Citation6. In the analysis, these rates were used to model discontinuation of treatment for teriparatide and PTH(1-8). In studies of osteoporotic treatments, it has been shown that a large proportion of those patients withdrawing from treatment do so early in the intervention periodCitation18. Due to lack of data on discontinuation rates from long-term studies, it was assumed that patients who discontinued treatment did so during the first 6 months and that all remaining patients continued treatment for the full period of the model. Patients who discontinued did not receive any treatment benefit but was allocated 3 months of intervention costs. Discontinuation rates of 100% and 50% were tested in the sensitivity analysis.
Epidemiological data
Fracture risk
Age-differentiated fracture risks for hip, clinical vertebral and wrist fracture in a female Swedish population were derived from Kanis et al.Citation19. Incidences of other non-spine, non-hip osteoporotic fractures were derived from Kanis et al.Citation20. These risks were mainly based on Swedish data but some were imputed from fracture risk data from the US (Olmsted County; Rochester)Citation21. The population fracture risk needs to be adjusted to reflect the increased fracture risk in the target patient group. Based on an approach previously described in Kanis et al. and De Laet et al.Citation20,Citation22, the relative risk of fracture for the target patient group (i.e. the EFOS Swedish patient group) compared to the population risk was estimated based on their age, T-score and number of prevalent fractures. For the base case, the relative risk of fracture was estimated to be 5.33, 6.08, 3.65 and 3.48 for hip, vertebral, wrist and other osteoporotic fractures. These relative risks vary at different ages, T-scores and fracture prevalence.
A fracture is a strong risk factor for another fracture. The risk of a subsequent fracture is at its highest immediately after a fracture event, and subsequently decreases over time. Risk functions from a Swedish study that estimated the risk of subsequent fractures over a 5-year period after an initial fracture eventCitation23 was therefore incorporated into the model. In the model simulations, these risks were used up to 5 years after fracture or until the baseline fracture risk was higher. The same approach was used to calculate the risk of a subsequent fracture in the model by Lundkvist et al.Citation7.
Mortality
Mortality rates for the general female population in Sweden were based on the years 2001–2005Citation24. Hip and clinical vertebral fractures lead to an increased mortality compared to the general populationCitation25–29. The mortality after a hip fracture was estimated based on all hip fracture patients from the Swedish inpatient register and causes of death register between the years 1997 and 2001. Swedish age differentiated clinical vertebral mortality rates in the first and subsequent years after a fracture event were derived from a study by Johnell et al.Citation30.
The excess mortality after a fracture cannot entirely be related to the fracture event but also to co-morbidity factors among osteoporotic patientsCitation31–33. The proportion of deaths related to the actual hip fracture event has been estimated to lie between 15% and 50%Citation32,Citation33. Therefore, in this analysis it was assumed that 30% of the excess mortality (compared to mortality in the general population) after a hip and a vertebral fracture was associated with the fracture event itself. In the sensitivity analysis, the proportion of excess deaths related to fractures was varied from 0% (no excess mortality after fracture) to 100% (all deaths related to fractures). There are no indications that wrist fractures are associated with any excess mortalityCitation25,Citation26. Also, because of lack of data it was conservatively assumed that other osteoporotic fracture types were not associated with an increased mortality in the base-case scenario. In the sensitivity analysis, it was assumed that excess mortality after these fractures was half the excess mortality after a hip fracture. This is based on the assumption of the relative utility loss of other osteoporotic fractures relative to hip and wrist fractures made by Kanis et al.Citation34.
Costs
All costs were inflated to mid-2007 prices using the Swedish consumer price indexCitation24. Costs are given in euros (€) using the average exchange rate (9.22SEK/€) for the first 6 months of 2007Citation35.
Intervention costs
The price of teriparatide in Sweden is €404 (3,728.50 SEK) for 28 doses and for PTH(1-84) the price is €1,074.9 (9911 SEK) for 84 dosesCitation36. Therefore, on an annual basis the price for teriparatide will be €5275.2 (48,637 SEK) and €4674.1 (43,095 SEK) for PTH(1-84).
The monitoring of patients while on treatment was assumed to consist of one annual physician visit (€106) and a bone mineral density measurement (BMD) every second year (€126.60). Also, in the treatment guidelines and SPC for PTH(1-84) calcium monitoring visits are recommended at months 1, 3 and 6Citation36,Citation37. In a sensitivity analysis, the costs (€412 or 2800 SEK/visit) for these visits were added to the monitoring costs for PTH(1-84) and in another analysis both treatments were ascribed these extra monitoring costs.
Disease costs
Direct and indirect (productivity losses) fracture costs during the first year after a hip, clinical vertebral and wrist fracture were derived from the Swedish KOFOR study which collected resource use and quality of life related to hip, vertebral and wrist fractures during the 18 months after a fractureCitation38,Citation39. Downward adjustments were made to account for deaths, the proportion of patients living at home at the time of fracture and the proportion of vertebral fractures that are hospitalised in clinical practice. The costs of other osteoporotic fractures in the first year after a fracture event were not available for Sweden. To derive an estimate of these costs it was assumed that pelvic, humerus, tibia, fibula and other femoral fractures were associated with the same cost as a hip fracture and rib, clavicle, scapula and sternum were assumed to have the same costs as a wrist fracture. These assumptions are derived from Kanis et al.Citation34 in which this relationship was used for the quality of life loss related to all types of osteoporotic fractures. By weighting the assumed costs with the incidence of each fracture type an average cost of €6,455 was estimated.
Hip fracture costs relating to nursing home care in the second and following years were based on the age-differentiated proportion of patients that come from independent living before fracture and which reside in nursing home 1 year after fractureCitation17. These patients were assumed to remain in a nursing home for the rest of their livesCitation40 at an annual cost of €63,480Citation41. It is also likely that patients that sustain a hip fracture and move to a nursing home would eventually be institutionalised regardless of the fracture. Therefore, in a sensitivity analysis a down adjustment of the long-term costs of hip fracture was made for the annual population incidence of being institutionalisedCitation42.
Both hip and vertebral fracture was associated with considerable costs up to 18 months after fracture in the KOFOR study. In the analysis it was assumed that these costs were maintained for the whole second year after fracture. Although, it is likely that there are fracture-related costs beyond the second year after fracture the KOFOR estimated costs were not extrapolated for a longer time period. However, in the sensitivity analysis the costs were used for the remainder of a fractured patient’s lifetime.
The costs related to a wrist fracture in the KOFOR study in the 12–18-month period were minimal and would not impact on the cost effectiveness, therefore, wrist fractures were assumed to have related costs only in the first year after fracture. It was also assumed that other osteoporotic fractures were not associated with any costs beyond the first year after fracture. All fracture related costs are summarised in .
Table 1. Annual direct cost in different age groups, € (95% CI).
Cost related to hypercalcaemia as an adverse event was derived from a US-based analysisCitation43 that estimated the cost as $130 (2003 prices), which is €195 (2007 prices).
Costs in added life-years
The difference between consumption and production varies over the life cycle for any individual. It has been argued that this difference, called costs in added life-years, should be included in cost-effectiveness evaluationsCitation44. The base-case analysis is provided with and without costs in added life-years.
Quality of life
The loss in quality of life, measured by the EQ-5D instrument, after a hip fracture, vertebral fracture and wrist fracture was derived from the KOFOR studyCitation38,Citation39. The estimated quality of life loss in the second year after a hip and vertebral fracture was assumed to persist for all years beyond the first year after fracture. Wrist fracture was only assumed to have a reduction in quality of life in the first year after fracture. The proportionate quality of life loss in the first year after other osteoporotic fractures was calculated as 0.87 using the same method as described in the cost section above. Other osteoporotic fractures were assumed to have no reduction in quality of life beyond the first year after fracture event. By linking these fracture-related proportional quality of life losses to Swedish population utility valuesCitation45, age differentiated fracture specific quality of life weights were obtainedCitation34,Citation46. All fracture-related quality of life values used in the model are summarised in .
Table 2. Fracture related quality-of-life loss.
In the sensitivity analysis the cost effectiveness was estimated assuming no quality of life loss beyond the first year after a vertebral fracture. In another sensitivity analysis the fracture related utility multipliers were varied by ±50% from the mean estimates. Empirical data on the quality of life loss related to hypercalcaemia are not available. Therefore, we assumed a 2% quality of life reduction with hypercalcaemiaCitation43.
Univariate sensitivity analysis
The numeric assumptions for certain model parameters were each varied in turn so as to assess the sensitivity of the model results to each of the assumptions made. The sensitivity analyses performed are outlined in .
Table 3. Sensitivity analysis.
Probabilistic sensitivity analysis (PSA)
The collective sensitivity of the model results to assumptions relating to treatment effects, fracture related costs, quality of life loss, mortality after hip fracture and long-term institutionalisation after hip fracture were assessed through a probabilistic sensitivity analysis. The uncertainty for these variables was considered by randomly (using a uniform distribution) drawing bootstrapped mean values from the underlying individual dataCitation47. The effects of the drugs were assigned log normal distributions based on the reported confidence intervals in the clinical trials. The PSA was conducted by drawing 2000 samples and were presented in the form of acceptability curves, which show the proportion of samples that fall below different values of willingness to pay for a QALY gained. For the interpretation of the cost-effectiveness ratios a WTP of a QALY of €60,000 was used, which is in the vicinity of the threshold acknowledged by Swedish reimbursement authoritiesCitation48.
Results
Base-case analysis
The base-case cost-effectiveness results are presented in and . Teriparatide compared to no treatment was associated with a cost per QALY gained of €43,473 and €60,450 when excluding and including costs in added life-years, respectively. The corresponding cost per QALY gained for PTH(1-84) compared to no treatment were €104,396 and €124,897. Teriparatide was associated with less costs and more QALYs and life-years when compared to PTH(1-84).
Table 4. Simulated average costs and effects per patient in base-case analysis (€).
Table 5. Base-case incremental cost-effectiveness analysis.
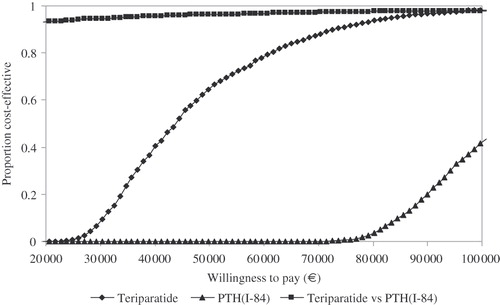
In , the results of the PSA for the base-case scenarios are shown in the form of acceptability curves. Given a willingness to pay (WTP) of €60,000, teriparatide was found to be cost effective in about 80% of the cost-effectiveness simulations compared to no treatment whereas PTH(1-84) was not below this WTP in any of the simulations. Compared to each other teriparatide was cost effective in 97% of the simulations at the assumed threshold.
Sensitivity analysis
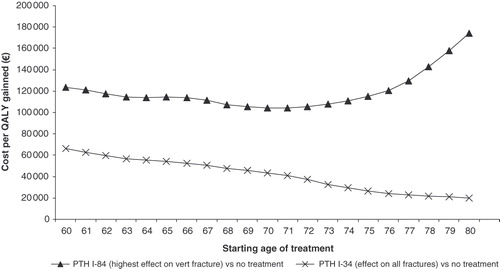
The cost per QALY gained of different starting ages of treatment is shown in . Although the risk of vertebral fracture increases with age, the gain in avoiding vertebral fracture decreases which is the reason why the cost effectiveness slightly improves up to an age of 71 years but declines thereafter for PTH(1-84) compared to no treatment. The reason for the steady decrease in the ICER for teriparatide is the applied treatment effect on all fractures, especially hip fracture for which the risk increases exponentially in ages above 70 years. In , the cost per QALY gained and the percentage change compared to the base case estimates for various different sensitivity analyses is shown. The impact on the ICER was moderate for changes in variables and parameters such as discount rate, exclusion of other osteoporotic fractures, treatment duration, persistence rates, increased mortality after other osteoporotic fractures, including consequences of hypercalcaemia-related events, calcium monitoring costs, extrapolating costs from the KOFOR study for hip and vertebral fracture beyond the 2nd year after fracture and no quality of life reduction related to vertebral fracture after the 1st year. Compared to the base-case estimates the ICER is fairly stable (±30% change) for most of the estimated sensitivity scenarios. A larger impact on the ICER was seen for changes in compliance, excess mortality after fracture and treatment effect on hip fracture risk. The parameter that was the most sensitive was assumptions concerning the offset time of the fracture risk reduction after the treatment period. When assuming that the effect stops immediately after the treatment period, the ICER increases by 149%, whereas by doubling the length of the offset time period the ICER is reduced by 51% for teriparatide. When assuming no effect on hip fractures with teriparatide, the cost-effectiveness ratio increased by 103%. Also, the cost per QALY gained of teriparatide vs. PTH(1-84) was estimated at €47,656 when no effect on hip fracture risk was assumed.
The sensitivity analysis over the variation of fracture related quality of life loss ±50% from the baseline quality of life estimates are presented in the form of a tornado diagram in .
Figure 4. Sensitivity tornado diagram over variation of fracture related quality of life loss ±50% from baseline estimates based on teriparatide (effect on all fractures) vs. no treatment.
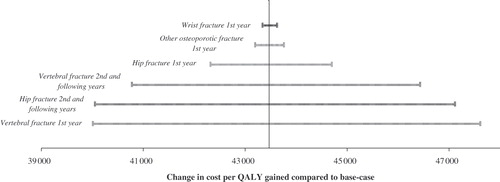
A 50% change in the utility loss after fracture does not lead to any major changes in the ICER compared to the base case. However, it is clearly the quality of life related to vertebral fracture in the 1st year and hip fracture in the 2nd and following years that are the most sensitive (about a 10% change compared to the base case), while changes in the quality of life related to other osteoporotic fractures and wrist fractures are marginal (about 1% compared to the base case).
Discussion
The cost effectiveness of teriparatide and PTH(1-84) in post-menopausal women in Sweden was estimated via a Markov model from a societal perspective using the fracture risk results from the pivotal clinical trials for each drugCitation5,Citation6. Compared to a no-treatment alternative, the cost per QALY gained was estimated as €43,473 for teriparatide and €104,396 for PTH(1-84). Treatment with teriparatide was less costly and associated with more life-years and QALYs than PTH(1-84). The main reason for this was a slightly better risk reduction for vertebral fractures for teriparatide, but more importantly the fact that there is no evidence on reduction of non-vertebral fractures with PTH(1-84). This is an essential part of the analysis. One might argue that there should be a class effect shared by these compounds; nonetheless this was not observed in the phase III trial. In the paper by Greenspan et al.Citation6, it is suggested that the lack of effect of PTH(1-84) is due to lack of power in the TOP study. The incidence of peripheral fractures in the placebo group was of 5.9% in TOP versus 9.7% in the FPT studyCitation5, which suggests a lower power. However the power is driven by the numbers of events, and given the fact that treatment groups were considerably larger in the PTH(1-84) trial (n = 1246) versus the teriparatide trial (n = 544) there were a similar number of non-vertebral fractures in the two trials. This was also the case for the number of some of the typical osteoporotic fractures such as forearm and hip. Therefore, the fact that the groups are larger would compensate for the fact that the incidence was lower in the PTH(1-84) trial, and thus the power for showing this effect is similar.
As these two clinical trials estimate the treatment effect compared to placebo, the relative efficacy between the compounds had to be derived by adjusted indirect comparison. That is, the relative effect of the treatments of interest are linked through a common comparator, i.e. placeboCitation49. The preferable approach would have been to derive evidence from a direct head-to-head trial, since an indirect comparison does not consider differences in, for example, study design, patient characteristics or factors that might have impacted the results in the clinical trials. However, based on the current available data the indirect comparison approach is the sole option to derive an estimate of relative efficacy for the use in a cost-effectiveness analysis. Therefore it is important that the results of the comparative cost-effectiveness analysis have to be interpreted in the light of this.
In the FPT, teriparatide was shown to significantly reduce the risk of non-vertebral fractures, which was a secondary endpoint in the study. Separated, the fracture types would not show a significant risk reduction, mainly because the trial was not powered to detect such significant changes. Using non-vertebral fracture as an aggregated outcome has become more common in recent clinical trials due to the difficulty in including patients at sufficiently high risk of fracture. The difficulty of applying a non-vertebral fracture risk reduction in a cost-effectiveness analysis is that the fracture types differ substantially in terms of costs and morbidity, e.g. hip fracture vs. wrist fracture. Using an aggregated measure of efficacy in the cost-effectiveness analysis might lead to underestimations of the potential benefits for some fracture types and overestimations for others. One approach could be to exclude all fracture types that have not shown significant risk reduction independently. This approach would most likely cause all fracture types except vertebral fractures to be excluded from the cost-effectiveness analysis and lead to an apparent underestimation of the fractures avoided with treatment. The other approach is to assume the non-vertebral fracture efficacy for all fracture types (except vertebral fractures). In this study we chose the latter alternative in the base-case, however, we conducted sensitivity analysis excluding a risk reduction of hip fractures with treatment.
Although the effect of treatment is an important parameter in a cost-effectiveness model, there are other variables which might impact on results. In the sensitivity analysis the ICER, however did not alter substantially with changes in the majority of the model parameters. For teriparatide, the cost per QALY gained compared to no treatment was below €70,000 in all sensitivity analyses except when assuming no residual effect on fracture risk after the intervention period. The model was particularly sensitive to the time over which the treatment effect is assumed to act. It must be assumed that the effect on fracture risk ceasing immediately after treatment is stopped is unrealistic, as is assuming that the effect will last indefinitely. Therefore, the offset time periods used in this study (24 and 30 months for vertebral and non-vertebral fractures, respectively) were derived from the follow-up studies of the FPT trialCitation15,Citation16 and should be seen as reasonable assumptions.
A common approach when estimating the cost effectiveness of osteoporotic treatments has been to use the patient characteristics of the clinical trial from which the treatment effect was derived when comparing one treatment with placeboCitation5,Citation6. The advantage with this approach is that the link between the treatment effect in the clinical trial and the patient group in the cost-effectiveness analysis is maintained even though the cost effectiveness is assessed in different geographical settings (internal validity). The disadvantage is that the patients in clinical trials often do not reflect the patients that will be given the treatment in clinical practice making the interpretation of the estimated cost effectiveness and patients in clinical practice unclear (external validity).
The analysis in this study was based on patient characteristics of the subjects in the Swedish cohort of the multinational EFOS study, resulting in an advantage in external validity. Although the link between the fracture-risk efficacy from the clinical trials and the patient group might be weaker, the interpretability of the cost effectiveness for the indication of PTH in Sweden is stronger.
The model also accounted for poor compliance. However, the compliance data used in the cost-effectiveness simulations were derived from the clinical trials. In a clinical setting, compliance is more likely to be poorer than what is observed in a controlled clinical trial, although studies have shown that in clinical settings patient education and follow-up programmes for teriparatide can result in high persistence and compliance ratesCitation50–52.
In this study PTH was not compared to other available osteoporotic treatments. This is because in Sweden, PTH is currently indicated for second- and third-line treatment in patients with osteoporosis (T-score < −2.5) with at least one prevalent clinical vertebral fracture and it should be documented that the patient cannot take any other osteoporosis drug due to safety and tolerability reasons. PTH could also be considered as a first-line treatment option for patients with a T-score of less than −3 and have at least two clinical vertebral fractures. Thus, the usage of PTH drugs for the treatment of osteoporosis is restricted to a fairly small segment of the osteoporosis population where no other osteoporotic treatment is a relevant option.
There are no other published cost-effectiveness estimates of PTH(1-84) treatment in Sweden that the authors are aware of. The cost effectiveness of teriparatide in a Swedish setting has previously been assessed by Lundkvist et al.Citation7, who found a cost per QALY gained for treatment of a population of 69-year-old women with a T-score at the femoral neck of −3.0 SD of €20,000–64,000 for patients with a recent or historic vertebral fracture. Although the analysis was not based on the same patient groups, the results from the previous study are similar to the results from this current study. The Lundkvist et al.Citation7 study was conducted before the introduction of PTH(1-84) in Sweden yet suggested that teriparatide might provide clinical and economic benefits.
Conclusions
Based on the efficacy estimates from pivotal clinical trials and characteristics of patients treated in clinical practice in Sweden, teriparatide appears to be cost effective relative to PTH(1-84) or no treatment. Randomised, head-to-head clinical trials of teriparatide and PTH(1-84) would provide further insight into the relative efficacy of these treatments, and thereby the validity of the current study results and conclusions.
Transparency
Declaration of funding
This study was supported by Lilly Europe.
Declaration of financial/other relationships
F.B. and O.S. have disclosed that they are have undertaken health economic analyses in the field of osteoporosis for several agencies and pharmaceutical companies, including Lilly. F.M. has disclosed that he is an employee of Lilly, and A.K. has disclosed that he is a former employee of Lilly. Ö.L. has disclosed that he has been a scientific advisor for Lilly.
Acknowledgements
The authors would like to thank Annabel Barrett, Lilly Europe for her help in preparing this manuscript.
Notes
*Forsteo, Eli Lilly AB, Sweden.
†Preotact, Nycomed AB, Sweden.
References
- Kanis JA, Burlet N, Cooper C, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 2008;19:399-428
- Delmas PD, Marin F, Marcus R, et al. Beyond hip: importance of other nonspinal fractures. Am J Med 2007;120:381-387
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761-1767
- Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev 2005;26:688-703
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001;344:1434-1441
- Greenspan SL, Bone HG, Ettinger MP, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 2007;146:326-339
- Lundkvist J, Johnell O, Cooper C, et al. Economic evaluation of parathyroid hormone (PTH) in the treatment of osteoporosis in postmenopausal women. Osteoporos Int 2006;17:201-211
- General guidelines for economic evaluations from the Pharmaceutical Benefits Board. (LFNAR 2003:2) [cited 2007 2007-05-12]. 2003; Available from http://www.lfn.se/LFNTemplates/Page____442.aspx
- Borgstrom F, Jonsson B, Strom O, et al. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int 2006;17:1781-1793
- Johnell O, Jonsson B, Jonsson L, et al. Cost effectiveness of alendronate (fosamax) for the treatment of osteoporosis and prevention of fractures. Pharmacoeconomics 2003;21:305-314
- TreeAge Pro 2007. User Manual TreeAge software inc 2007
- Rajzbaum G, Jakob F, Karras D, et al. Characterization of patients in the European Forsteo Observational Study (EFOS): postmenopausal women entering teriparatide treatment in a community setting. Curr Med Res Opin 2008;24:377-384
- Ljunggren O, Toll A, Myren KJ, et al. Prescription pattern of human recombinant 1-34 parathyroid hormone (Teriparatide, Forsteosup>/sup>) in Sweden. Osteoporos Int 2006;17:S71-72
- Behandling av Osteoporos - Rekommendationer. Läkemedelsverket (Medical Product Agency) [cited 2007-07-03]. 2007; Available from: http://www.lakemedelsverket.se/upload/Hälso-%20och%20sjukvård/behandlingsrek/osteoporos2007.pdf
- Lindsay R, Scheele WH, Neer R, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med 2004;164:2024-2030
- Prince R, Sipos A, Hossain A, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res 2005;20:1507-1513
- Zethraeus N, Strom O, Borgstrom F. What is the risk of institutionalization after hip fracture? Osteoporos Int 2006;17:57-58
- Weycker D, Macarios D, Edelsberg J, et al. Compliance with drug therapy for postmenopausal osteoporosis. Osteoporos Int 2006;17:1645-1652
- Kanis JA, Johnell O, Oden A, et al. Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 2000;11:669-674
- Kanis JA, Oden A, Johnell O, et al. The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 2001;12:417-427
- Melton III LJ, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos Int 1999;9:29-37
- De Laet CE, van Hout BA, Burger H, et al. Bone density and risk of hip fracture in men and women: cross sectional analysis. BMJ 1997;315:221-225
- Johnell O, Kanis JA, Oden A, et al. Fracture risk following an osteoporotic fracture. Osteoporos Int 2004;15:175-179
- Statistics Sweden. Swedeńs Statistical Databases [cited 2007-05-30]. 2007; Available from: http://www.scb.se/eng/databaser/ssd.asp
- Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-561
- Center JR, Nguyen TV, Schneider D, et al. after all major types of osteoporotic fracture in men and women: an observational study. Lancet 1999;353:878-882
- Cooper C, Atkinson EJ, Jacobsen SJ, et al. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-1005
- Jalava T, Sarna S, Pylkkanen L, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003;18:1254-1260
- Kanis JA, Oden A, Johnell O, et al. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108-112
- Johnell O, Kanis JA, Oden A, et al. Mortality after osteoporotic fractures. Osteoporos Int 2004;15:38-42
- Poor G, Atkinson EJ, O'Fallon WM, et al. Determinants of reduced survival following hip fractures in men. Clin Orthop 1995;319:260-265
- Parker MJ, Anand JK. What is the true mortality of hip fractures? Public Health 1991;105:443-446
- Kanis JA, Oden A, Johnell O, et al. The components of excess mortality after hip fracture. Bone 2003;32:468-473
- Kanis JA, Johnell O, Oden A, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int 2004;15:20-26
- Average Exchange Rates. Sveriges Riksbank [cited 2007-09-15]. 2007; Available from: http://www.riksbanken.com
- Farmaceutiska Specialiteter i Sverige (FASS). [cited 2007-10-31]. 2009; Available from: www.fass.se
- Summary of Product Characteristics. [cited 2007-12-01]. 2007; Available from: www.emea.europa.eu/humandocs/PDFs/EPAR/preotact/H-659-PI-en.pdf
- Borgstrom F, Zethraeus N, Johnell O, et al. Costs and quality of life associated with osteoporosis-related fractures in Sweden. Osteoporos Int 2006;17:637-650
- Strom O, Borgstrom F, Zethraeus N, et al. Long-term cost and effect on quality of life of osteoporosis-related fractures in Sweden. Acta Orthop 2008;79:269-280
- Jonsson B, Christiansen C, Johnell O, et al. Cost-effectiveness of fracture prevention in established osteoporosis. Scand J Rheumatol Suppl 1996;103:30-38
- Stockholms stads budgetavräkning 2003 [online]. [cited 2004-12-20]. 2009; Available from: www.stockholm.se/files/71600-71699/file_71645.pdf
- Borgstrom F, Strom O. Long-term hip fracture costs related to institutionalization. Osteoporos Int 2007;18(Suppl 1):S1-192. Osteoporos Int 2007;18:S1-192
- Liu H, Michaud K, Nayak S, et al. The cost-effectiveness of therapy with teriparatide and alendronate in women with severe osteoporosis. Arch Intern Med 2006;166:1209-1217
- Meltzer D. Accounting for future costs in medical cost-effectiveness analysis. J Health Econ 1997;16:33-64
- Burstrom K, Johannesson M, Diderichsen F. population health-related quality of life results using the EQ-5D. Qual Life Res 2001;10:621-635
- Kind P, Dolan P, Gudex C, et al. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-741
- Briggs AH, Wonderling DE, Mooney CZ. Pulling cost-effectiveness analysis up by its bootstraps: a non-parametric approach to confidence interval estimation. Health Econ 1997;6:327-340
- The National Centre for Priority Setting in Health Care (PrioriteringsCentrum). Nationell model for öppna vertikala prioriteringar inom svensk hälso- och sjukvård. PrioriteringsCentrum 2007;1
- Sutton A, Ades AE, Cooper N, et al. Use of indirect and mixed treatment comparisons for technology assessment. Pharmacoeconomics 2008;26:753-767
- Briot K, Ravaud P, Dargent-Molina P, et al. Persistence with teriparatide in postmenopausal osteoporosis; impact of a patient education and follow-up program: the French experience. Osteoporos Int 2009;20:625-630
- Adachi JD, Hanley DA, Lorraine JK, et al. Assessing compliance, acceptance, and tolerability of teriparatide in patients with osteoporosis who fractured while on antiresorptive treatment or were intolerant to previous antiresorptive treatment: an 18-month, multicenter, open-label, prospective study. Clin Ther 2007;29:2055-2067
- Arden NK, Earl S, Fisher DJ, et al. Persistence with teriparatide in patients with osteoporosis: the UK experience. Osteoporos Int 2006;17:1626-1629