Abstract
Objective:
Palivizumab is a prophylactic therapy shown to reduce the number of respiratory syncytial virus (RSV)-related hospitalizations but has a high acquisition cost. The objective was to systematically examine the cost effectiveness of palivizumab in defined infant groups and identify important cost and outcome determinants.
Methods:
Literature searches of MedLine, the Cost-Effectiveness Analysis registry and the UK NHS Economic Evaluation Database (NHS EED) were conducted to identify economic evaluations of palivizumab compared to no prophylactic treatment for RSV prevention in any infant population. Study quality was evaluated using Quality of Health Economic Studies (QHES) criteria and results converted to 2009 CAN$ for comparison.
Results:
A total of 23 articles meeting inclusion criteria were identified, including 11 cost-utility analyses (CUAs) and 12 cost-effectiveness analyses (CEAs). Quality of individual analyses was fairly high (range 60–100, median 86). Results ranged from cost dominance for prophylaxis to $3,365,769/QALY depending on population, outcome measures, and input parameters. Base-case and sensitivity-analysis mortality rates varied between studies and influenced results.
Conclusions:
RSV prophylaxis with palivizumab is cost effective in specific groups of high-risk infants, especially those with multiple environmental risk factors. Cost-effectiveness estimates vary between populations and settings and are more positive in those at highest risk for RSV hospitalization.
Limitations:
Direct comparison of the published reports was limited by restriction to English language articles and the varied methodologies, input measures, and populations across the studies reviewed. Although reported currencies were converted to a common unit for comparison, this does not completely account for monetary and inflation differences.
Introduction
Respiratory syncytial virus (RSV) infection occurs in nearly 50% of infants during the first year of life and almost all children have had RSV-related upper or lower respiratory tract disease (LRTI) by 2 years of ageCitation1,Citation2. Approximately half the children who develop RSV infection during their first year become re-infectedCitation1. In the majority of cases, affected infants are healthy but 7.7% require subsequent medical attention from family practitioners and 2.6% require assistance in the emergency departmentCitation3. A total of 0.5–2% of all infants with RSV infection are hospitalized with bronchiolitis and pneumonia and the severity of illness is often determined by preterm gestational age (GA), low birth weight and existing underlying medical disorders such as airway anomalies, cardiac and chronic lung disease and neuromuscular impairmentsCitation4–7. Environmental risk factors such as number of siblings, crowding in the household and day care attendance have also been found to increase the risk of RSV infection and hospitalizationCitation8. In Canada, RSV hospitalizations number 5800–12,000 annually while in the US between 1997–2000 there were 311,000 admissions for bronchiolitisCitation4,Citation9,Citation10. The burden of illness is substantial world-wide. In Kenya, RSV accounts for 85,000 LRTI cases annuallyCitation11, in the Netherlands there are 2000 annual RSV hospitalizationsCitation12 while in Liverpool UK, the RSV hospitalization rate was 19.7% over the 1998–2000 RSV seasonsCitation13. The overall RSV related mortality rate is 1–4% and has decreased significantly because of state of the art pediatric intensive care managementCitation6,Citation7,Citation14–16. However, the burden of illness has a significant impact on health care resources both within the hospital and in the community following patient dischargeCitation17–19. Of equal importance are family-related costs relative to the care of these children and the potential impact on the child’s health related quality of life and long term respiratory sequelae such as wheezing, asthma and pulmonary functionCitation20–24.
The cost incurred for RSV hospitalization in Canadian children 1–4 years of age between 1993 and 1994 was CAN$18.5 million, with 62% of the direct costs being for hospital servicesCitation4. Similarly, in the US from 1997–2000, the average annual direct costs for hospital care totaled US$750 million and in 2002 were estimated at US$1.1 billionCitation10,Citation25. Importantly, costs vary significantly in infants with risk factors and those with pre-existing medical disorders. In one study, costs increased from US$3777 for infants without risk factors to $7376 for prematurity, $11,335 for those with congenital heart disease (CHD) and $13,241 for infants with congenital anomaliesCitation18. Apart from direct costs, RSV illness imposes a significant financial burden on families. The average out of pocket expenses range from $214–295 per child to $644 for premature infants and encompass costs for travel, lost work days and family doctor consultation visitsCitation26,Citation27.
Palivizumab, a genetically engineered, humanized monoclonal antibody initially became available through the special access program and was approved for RSV prophylaxis in Canada in 2002. The efficacy and safety of the product was proven in infants less than 35 weeks’ gestation and those with chronic lung disease <2 years of age through a large scale, multicenter, randomized, double-blind, placebo-controlled trialCitation15. A similar randomized trial conducted in infants with hemodynamically significant CHD demonstrated an overall 45% relative reduction in RSV hospitalizations (p = 0.003)Citation16. This set the stage for universal position statements by pediatric committees on the use of palivizumab prophylaxis in high-risk populations and the strategy rapidly gained approvalCitation28–30.
As with all biologics, the acquisition costs for palivizumab are substantial. To address value for money, there have been several cost-effectiveness and cost-utility analyses on the use of palivizumab prophylaxis in high-risk populations based on societal and payers’ perspectives. However, there has been a wide variability in results and conclusions across studies. The primary objective of this study was to examine the cost effectiveness of palivizumab prophylaxis and the factors that affect it by conducting a systematic review of the scientific literature and existing databases and critically evaluating the results and determinants of outcomes.
Methods
Literature search
A systematic literature search was conducted to identify studies examining the cost effectiveness of palivizumab versus no prophylaxis for RSV in children. A MEDLINE (Ovid) search was performed from 1950 to February 2010 using the following criteria: (‘respiratory syncytial virus’ OR ‘rsv’) AND (palivizumab OR monoclonal antibody$) AND (‘costs and cost analysis’ OR econom$ OR ‘cost-benefit analysis’ OR cost effective$ OR cost$ OR pharmacoeconomic$ OR budget$). Searches of the Cost-Effectiveness Analysis registry and the UK National Health Service (NHS) Economic Evaluation Database (NHS EED) were also performed, as well as examinations of reference lists of the review articles retrieved. The search was limited to English language studies.
Cost-utility analyses (CUAs), cost-effectiveness analyses (CEAs), and cost-benefit analyses (CBAs) were considered for inclusion where palivizumab prophylaxis was compared to no prophylactic treatment for RSV prevention in any infant population. Cost comparison studies and cost analyses were excluded.
Analysis
Studies were assessed for quality using the guidelines for economic submissions to the British Medical JournalCitation31 and the Consensus Health Economic Criteria (CHEC) listCitation32 and given a quality score (QS) on a scale of 0–100 based on the Quality of Health Economic Studies (QHES) InstrumentCitation33. Results were converted to 2009 CAN$ to facilitate comparison using the Bank of Canada’s online currency converterCitation34 and inflation calculatorCitation35. Where currency or price year was not stated, country and year of study publication were used.
Results
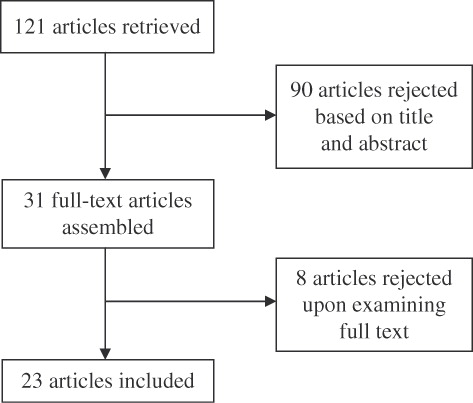
A total of 23 articles meeting inclusion criteria were identified for review (); 11 of these were CUAs measuring outcomes in incremental cost-effectiveness ratios (ICERs) based on cost per quality-adjusted life year (QALY) gained and 12 were CEAs measuring outcomes in ICERs per life-year gained (LYG) or hospital admission prevented (HAP). Characteristics of these studies are found in . compiles the base-case results of each study. QHES scores are given in .
Table 1. Characteristics of cost-effectiveness analyses of palivizumab versus no prophylaxis.
Table 2. Results of palivizumab cost-effectiveness analyses.
Table 3. Quality of health economic studies (QHES) scores.
Cost-utility analysis (CUAs)
The quality of CUAs was fairly high (from 84 to 100 with a median score of 94). The studies clearly described their study population, perspective, and time horizon. Efficacy of palivizumab was comprehensively established, and both results and consequences were succinctly documented. All but one studyCitation36 used a lifetime time horizon, and all discounted future costs and benefits and performed sensitivity analyses. Only three studiesCitation36–38 explicitly discussed magnitude and direction of potential biases. There was variability in input parameters and costs included in each analysis.
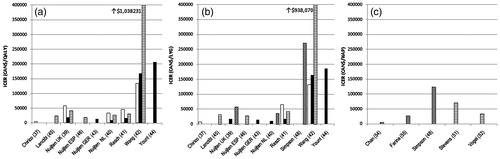
Mortality rate and inclusion of sequelae such as asthma and wheezing were notable sources of variability in the cost-effectiveness results. Three analyses – two by Nuijten et al. (UK 2008Citation39, Netherlands 2009Citation40) and one by Resch et al.Citation41 – were conducted using similar decision analytical models and input parameters examining preterm infants ≤35 weeks’ GA, infants with bronchopulmonary dysplasia (BPD), and infants with hemodynamically significant congenital heart disease (CHD). The UK analysis (QS 94) reported ICERs of $41,658/QALY, $58,648/QALY, and $18,653/QALY, respectively. Resch et al. (QS 91) calculated similar values of $30,114/QALY for preterm infants, $46,351/QALY for infants with BPD, and $16,566/QALY for infants with CHD. The Netherlands analysis (QS 94) determined ICERs of $26,862/QALY for the preterm group, $33,962/QALY for BPD infants, and $10,279/QALY for subjects with CHD. A fourth study by Chirico et al.Citation37 (QS 86) also included a subgroup of infants with BPD and calculated an ICER of $4331/QALY for that population. However, a UK cost-utility analysis performed by Wang et al. (QS 94) for the same three groups found different resultsCitation42. ICERs were $1,038,231/QALY for preterm infants without CLD, $134,160/QALY for preterm infants with CLD, and $168,808/QALY for infants with CHD.
Wang et al.Citation42 used mortality rates of 0.43% for preterm infants without BPD and 4.0% for those with BPD after RSV hospitalization compared to the Nuijten et al. and Resch et al. analyses that utilized a mortality rate of 8.11% in both groups, based on the Sampalis studyCitation19. However, the lowest ICER for the BPD group was calculated by Chirico et al. using the more conservative 4.0% rateCitation37. In the CHD group, Nuijten et al.Citation39,Citation40 and Resch et al.Citation41 applied different mortality rates in the palivizumab and no prophylaxis groups, ranging from 2.3 to 3.3% and 4.0 to 4.2% respectively, although trials had not been powered to show a significant difference in mortality between the two groups. This variation reflects diversity found in the literature and represents different measures, with the lower values including early mortality rates only during hospitalization, while the higher value comprises longer term, all-cause mortality, in confirmed and probable RSV positive hospitalizations versus matched control groups who were not hospitalized with RSV illnessCitation19. The effect of mortality on model output was demonstrated in sensitivity analyses performed by Wang et al. and Nuijten et al. (UK). Wang et al. reported ICERs ranging from $47,933/QALY for an 8.11% mortality rate to $7,768,528/QALY for a 0.05% rate, while Nuijten et al. estimated a range of ICERs from $25,846/QALY for a mortality rate of 0 in the palivizumab group to $163,588/QALY if rates are assumed to be equal in the palivizumab and control groups. Similarly, Wang et al. did not account for the potential increased risks and downstream costs of asthma in children following RSV infections in infancy. Unlike Chirico et al., both articles by Nuijten et al., and Resch et al. included asthma only in a secondary analysis, finding slightly lowered ICERs ($14,187–31,522/QALY). The impact of RSV infections in early infancy on subsequent wheezing and asthma is still contentious. However a meta-analysis of 12 clinical trials demonstrated that the association between RSV illness at a young age and subsequent asthma exists, but the effect steadily declines with increasing age into adolescenceCitation20.
CHD was determined to be the most cost-effective indication in the previously mentioned studies. An additional CHD analysis conducted by Nuijten et al.Citation43 in Germany (QS 94) calculated an ICER of $13,860/QALY from the societal perspective. Yount and MahleCitation44 (QS 84) studied a similar population and calculated an ICER of $207,458/QALY for infants with CHD, significantly higher than those found in the other analyses. The low estimate of benefit from palivizumab prophylaxis was ascertained on the basis of a relatively low RSV mortality rate of 3% coupled with quality of life estimates that were derived solely from CHD rather than RSV-related effects.
Specific gestational age was also shown to affect cost-effectiveness results. This is a reflection of the variability in effect size by gestational age found in the clinical trials. Chirico et al.Citation37 calculated an ICER of $14,881/QALY for infants <35 weeks’ GA compared to $23,967/QALY for infants 33–35 weeks’ GA. ElHassan et al. (QS 84) reported on a cohort of preterm infants (GA 26–32 weeks) without CLD, over an 8-year time horizonCitation36. ICERs were stratified by GA and ranged from $1,223,165/QALY to $3,365,769/QALY. That analysis limited the outcome benefit of palivizumab to a conservative estimate of the risk of developing asthma, with consequent health-utility increases and hospital cost decreases; RSV mortality was not included due to limited evidence from clinical trials that it is affected by palivizumab. These assumptions largely account for the higher ICER values, but both studies indicate that cost effectiveness is higher in infants of lower GA.
The impact of indirect costs, asthma, and risk scoring was demonstrated clearly by Lanctôt et al. (QS 94). This study analyzed costs for preterm infants of GA 32–35 weeksCitation45 and calculated ICERs of $31,383/QALY for direct medical costs not accounting for asthma, and $21,447 including asthma costs, comparable to the estimate from Chirico et al. for the 33–35-week GA group. With the inclusion of indirect costs the ICERs decreased substantially to $23,292/QALY and $13,354/QALY, respectively. Again, the higher 8.11% mortality rateCitation19 was employed in the base case since the Sampalis et al. study is the only nested case-control trial that examined RSV-related mortality in a cohort of 33–35 GA infants post-hospitalization, over a mean follow-up duration of 2.1 years. Importantly, patient groups were further stratified by risk scores, with results showing strong evidence for cost effectiveness in infants with two or more risk factors and those who were at moderate-to-high risk of RSV hospitalization. This was also shown by Wang et al., with cost effectiveness increasing considerably in infants with siblings in day care or schoolCitation42. Nuijten and WittenbergCitation46 (QS 91) utilized their original model and local data from Spain to study a population of preterm infants ≤32 weeks’ GA and <6 months of age at the start of the RSV season. ICERs of $18,638/QALY were determined for direct medical costs. This decreased to $9451/QALY with accounting for sequelae, and dominant values were achieved when indirect costs were included. This study further emphasizes the effects of sequelae and indirect costs. Notably, the Spanish data provided a higher effectiveness value for palivizumab using a lower mortality rate, though the higher 8.11% rateCitation19 was also incorporated in a scenario analysis.
Tam et al.Citation38 (QS 100) studied a specific population of term Inuit infants in the Canadian Eastern Arctic, stratified by rural or urban geographic location, reflecting distance from accessible medical care. An ICER of $40,421/QALY was determined for the whole population, ranging from cost savings for rural infants <6 months of age and high-risk rural infants <1 year of age to $153,527/QALY for infants <1 year of age in urban areas. This analysis distinctly illustrates the need for localized cost analyses in order to develop constructive guidelines for RSV prophylaxis.
The ICERs found in the CUAs range from cost dominance with palivizumab prophylaxis to $3,365,789/QALY for diverse high-risk populations based on cost, input, and outcome parameters examined. CHD is generally found to be the most cost-effective indication for palivizumab prophylaxis.
Cost-effectiveness analysis (CEAs)
The quality of CEAs varied from 60 to 93 with a median score of 75.5. The majority of publications clearly described their population and research question, but few used a lifetime time horizon and only twoCitation47,Citation48 applied discounting to future costs and outcomes. There was some variability in costs accounted for in each study. Fewer than half (42%) used sensitivity analyses to assess the robustness of input parameters.
Two studies assessed cost effectiveness using life-years gained as an outcome measureCitation47,Citation48. Simpson et al. (QS 76) examined infants ≤35 weeks’ GA and under 6 months of age at the start of the RSV season and infants under 2 years of age with BPD, estimating an ICER of $270,916/LYG for the combined group. An analysis of infants ≤36 weeks’ GA by Joffe et al.Citation47 (QS 90) examined risk groups stratified by GA, length of oxygen therapy in a neonatal intensive care unit (NICU), and month of NICU discharge. ICERs ranged from $60,495/LYG for infants 23–32 weeks’ GA who were oxygen dependent for ≥28 days and discharged between September and November to $2,198,512/LYG for infants 33–36 weeks’ GA who required oxygen for <28 days and were discharged between December and August of the following year. Both studies concluded that palivizumab was not cost effective in their study populations, though each stressed the importance of examining risk factors beyond the indications discussed. Joffe et al. used a low 1% mortality rate for infants hospitalized for RSV, and Simpson et al. assumed a 6-month course of prophylaxis rather than the more common 4–5-month course which significantly increased cost estimates.
Nine of the 11 CUA analyses also reported results in terms of LYGs; for most, these values were higher by approximately 50% than ICERs/QALY. Wang et al. found that the values were similar while Yount and Mahle calculated a slightly decreased ICER/LYG. Among all studies evaluating LYGs, results ranged from cost savingsCitation40,Citation46 to an ICER of $2,198,512/LYGCitation47 dependent on populations and input assumptions.
Of the ten cost-effectiveness analyses identified that measured outcomes in hospital admissions prevented (HAPs), four examined premature infants without BPDCitation49–52. Values ranged from $15,758/HAPCitation49 to $371,569/HAPCitation50 and reinforced the importance of specific gestational age as well as environmental risk factors for RSV hospitalization and determinants of cost effectiveness. For infants ≤35 weeks’ GA, Roeckl-Wiedmann et al. (QS 89) found ICERS of $29,152/HAP for male infants with siblings in day care discharged between October and December and up to $235,962/HAP for male infants with no other risk factorsCitation50. Vogel et al. (QS 93) determined cost per HAP to be $19,743 for infants ≤28 weeks’ GA, $60,463 for infants 29–31 weeks’ GA, and $17,707 for infants discharged home on oxygenCitation52. Rodriguez et al. (QS 60) calculated ICERs of $15,758/HAP for premature infants without siblings and $107,345/HAP for those with siblingsCitation49. Stevens et al. found ICERs ranging from $21,586/HAP for infants ≤28 weeks’ GA to $125,340/HAP for infants 30–32 weeks’ GACitation51. Each of these studies varies extensively by subgroups within the population, demonstrating the positive effect of risk factors such as home discharge on supplemental oxygen, presence of siblings in or out of day care and discharge date during the RSV season on cost effectiveness.
Two analyses also encompassed preterm infants with BPD. Roeckl-Wiedmann et al. included a subgroup of male, premature infants ≤35 weeks’ GA with BPD and siblings for which the ICER was $12,052/HAP. Vogel et al. studied infants ≤28 weeks’ GA and 29–31 weeks’ GA with BPD and found ICERs of $40,103/HAP and $149,337/HAP respectively. BPD enhanced cost effectiveness in Roeckl-Wiedmann et al. but decreased it in Vogel et al., largely because the former used an average palivizumab effectiveness rate rather than one stratified by indication. In the IMpact trial, infants with BPD had a 39% reduction in hospitalization versus 55% overall for patients in the studyCitation15. Only one CEA examined infants with CHD. Rackham et al.Citation53 (QS 68) calculated an ICER of $61,872/HAP for infants with severe CHD, slightly higher than estimates from comparable CUAs.
Cost effectiveness was limited in studies examining cost per HAP in all high-risk groups together, further supporting the need for refined risk characterization. Chan et al.Citation54 found a combined ICER of $4987/HAP for infants <2 years of age with BPD, infants ≤32 weeks’ GA, and infants of GA 32–35 weeks with multiple risk factorsCitation54. In Argentina, Fariña et al.Citation55 (QS 67) focused on infants ≤35 weeks’ GA who were <6 months chronological age, infants ≤28 weeks’ GA and <1 year of age, and infants <2 years of age with BPD and calculated an ICER of $26,474/HAPCitation55. Both of these studies concluded that palivizumab is not cost effective in these settings. Lofland et al.Citation56 (QS 76) studied a high-risk population comprising premature infants, infants with BPD, CHD, or other congenital anomalies, and infants with compromised immune function. ICERs were reported as a function of incidence rate at two palivizumab price points, with a range from cost savings to $121,445/HAP. The higher cost and lower incidence scenarios are very similar to the other analyses and suggest limited cost effectiveness.
Banerji et al. and Reeve et al. studied unique at-risk populationsCitation57,Citation58. Banerji et al. (QS 78) examined Inuit infants of all GAs in the Canadian Eastern Arctic. ICERs ranged from cost savings of $8321/HAP to a cost of $166,615/HAP depending on age and location (urban versus rural)Citation57. Reeve et al. (QS 67) reviewed a population of infants with birth weights <2500 g in three risk groups: all infants, Indigenous infants, and those from multiple births. ICERs were $79,333/HAP, $65,667/HAP, and $62,592/HAP respectivelyCitation58.
Cost per HAP varied from cost savings with palivizumab use to $371,569. Most concluded that palivizumab is not cost effective in large risk groups based on HAP, though none explicitly specified a threshold for acceptable results. Many further reinforced the importance of environmental risk factorsCitation49,Citation50,Citation54,Citation57 in determining palivizumab cost effectiveness. summarizes the ICERs for outcomes in QALYs, LYGs, and HAPs in the most commonly investigated populations.
Figure 2. Cost-effectiveness results for studies reporting outcomes for the most commonly investigated populations (white = BPD, black = CHD, horizontal lines = premature, diagonal lines = premature or BPD). Panel A: outcomes in quality-adjusted life-years (QALYs); Panel B: outcomes in life-years gained (LYGs); Panel C: outcomes in hospital admissions prevented (HAPs). ‘Premature’ population encompasses all subgroups of gestational ages ≤35 weeks studied. BPD, bronchopulmonary dysplasia; CHD, congenital heart disease; ICER, incremental cost-effectiveness ratio.
Discussion
Prophylaxis with palivizumab effectively reduces RSV hospitalization rates in high-risk infantsCitation15. Many cost analyses have been performed to quantify the economic burden of this strategy and assess cost effectiveness. Cost-effectiveness results varied widely between studies for all outcomes assessed. Input measures and costs accounted for in individual studies had a strong impact on overall costs and outcomes. Cost effectiveness was shown to be highest in a variety of high-risk subpopulations and from the perspective of society rather than the payer.
There was no significant difference in the quality of the studies that concluded palivizumab is or is not cost effective (t = 1.5, df = 19, p = 0.153). CUAs were generally more positive and of higher quality (t = 4.5, df = 19, p < 0.001) than CEAs. Three of the highest quality studies (QS ≥ 90) reported negative findingsCitation42,Citation47,Citation52 (). However, the majority demonstrated positive cost effectiveness and in this subset of 23 rigorous studies, the effects of discrepancies in key input assumptions strongly influenced the conclusions.
Several studies across all outcome measures reinforced the importance of environmental risk factors in the determination of palivizumab cost effectivenessCitation38,Citation45,Citation48,Citation50,Citation55. The stronger an infant’s risk profile, the higher the probability that palivizumab will be a cost-effective option. This indicates that risk assessment and stringent application of country-specific guidelines for prophylaxis may prove beneficial, by targeting healthcare resources appropriately at predetermined high-risk infant populations.
Mortality rate was a significant source of variability between studies. The randomized placebo-controlled efficacy trials have not been powered to either provide an estimate of the mortality rate in the small number of infants hospitalized with RSV, or find a difference in mortality rate with and without palivizumab prophylaxis. The overall mortality rates in the treatment and placebo arms of the clinical trials ranged from 0.4 to 4.2 % over 5 monthsCitation15,Citation16. Only one nested cohort study is currently available that followed infants for 2 years after a confirmed or probable RSV hospitalization and found a relatively high mortality rate of 8.1%Citation19. As a result, the mortality rates utilized in the analyses across all of the studies differed widely. Many of the studies in our review considered the effect of varying mortality rates in scenario analyses and showed that the models are sensitive to changes in this parameter. Although better data on the RSV specific mortality following palivizumab versus no palivizumab prophylaxis is essential to refining cost-effectiveness analyses, this end point may prove difficult to determine due to prohibitive sample sizes. Additionally, the dubious link between RSV infection and asthma is also variably accounted for in these analyses based on the current evidence.
With some exceptionsCitation42,Citation44, cost effectiveness was generally higher when outcomes were measured in QALYs rather than LYGs. This indicates that the benefits of palivizumab are largely in quality of life effects rather than mortality or hospitalization costs, emphasizing the relevance of health-utility estimates in assessing palivizumab cost effectiveness. Assessing quality of life effects also helps to mitigate the uncertainty caused by lack of consensus on mortality rates by measuring outcomes differently.
The primary limitation of this review is the heterogeneity of the methodologies, costs and outcomes in the included analyses. Major cost drivers such as sequelae, as well as minor input parameters such as administrative costs, were variably accounted for across the reports. Discrepancies in the primary research on which the assumptions are based (e.g., mortality) may have also contributed to the variations identified among the studies. Although reported currencies were converted to a common unit for comparison, this does not completely account for monetary and inflation differences. As such, it is difficult to clearly determine specific reasons for overall cost disparities between countries. Finally, several non-English language articles were excluded from this review, further limiting international comparison.
Conclusion
In summary, RSV prophylaxis with palivizumab is cost effective within certain subgroups of high-risk infants. These subgroups include children with CHD, where cost effectiveness has been shown to be higher than that in preterm infants with or without BPD. Also, judicious use in the relatively small proportion of moderate-to-high risk 33–35 weeks’ GA infants based on the presence of risk factors is supported by scientific evidenceCitation59–61. In populations such as the aboriginal children where the risk of RSV hospitalization is extremely high, prophylaxis is a dominant strategy with cost savings. Environmental risk factors are also key indicators that can be used to target the most cost-effective patient groups. Country-specific studies based on local risk factors and models that accurately determine RSV hospitalization rates in defined populations will likely yield the best evidence for the cost effectiveness of palivizumab.
Transparency
Declaration of funding
No funding for this study was provided.
Declaration of financial/other interests
K.S. has disclosed that she has no relevant financial relationships. B.P. and K.L. are currently Co-Principal Investigators of a multicenter investigator initiated research study entitled ‘CARESS’ (Canadian Registry of Synagis). The Principal Investigators are supported by a grant in aid of research by Abbott Laboratories Ltd. The investigators for the CARESS study maintain control over any abstracts and presentations arising from the data and the rights to publish. B.P. and K.L. are also co-investigators on a research study funded by Abbott International entitled ‘Surveillance and cost analysis for Respiratory Syncytial Virus hospital admissions in the Arctic communities in Canada’.
References
- Glezen W, Taber L, Frank A, et al. Risk of primary infection and re-infection with respiratory syncytial virus. Am J Dis Child 1986;140:543-546
- Parrott R, Kim H, Brandt C, et al. Respiratory syncytial virus in infants and children. Prev Med 1974;3:473-480
- Hall C, Weinberg G, Iwane M, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med 2009;360:588-598
- Langley J, Wang E, Law B, et al. Economic evaluation of respiratory syncytial virus infection in Canadian children: a Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. J Pediatr 1997;131:113-117
- Shay D, Holman R, Newman R, et al. Bronchiolitis-associated hospitalizations among US children 1980-1996. JAMA 1999;282:1440-1446
- Arnold S, Wang E, Law B, et al. Variable morbidity of respiratory syncytial virus infection in patients with underlying lung disease: a review of the PICNIC RSV database. Pediatr Infect Dis J 1999;18:866-869
- Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child 2009;94:99-103
- Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, et al. FLIP-2 study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J 2008;27:788-793
- Health Canada, PPHB. Respiratory virus detections/isolations in Canada. http://www.hc-sc.gc.ca/pphb-dgspsp/bid-bmi/dsd-dsm/rvdi-divr/2003pdf/rvdi1103.pdf
- Leader S, Kohlase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003;143:S127-132
- Nokas J, Okiro E, Ngama M, et al. Respiratory syncytial virus infection and disease in infants and young children studied from birth in Kilifi district, Kenya. Clin Infect Dis 2008;46:50-57
- Rothbarth P, Kimpen J, Roord J, et al. Respiratory syncytial virus infections and preventive options. Ned Tijdschr Geneeskd 2000;144:15-19
- Clark S, Beresford M, Subhedar N, et al. Respiratory syncytial virus infection in high risk infants and the potential impact of prophylaxis in a United Kingdom cohort. Arch Dis Child 2000;83:313-316
- Navas L, Wang E, de Carvalho V, et al. Improved outcome of respiratory syncytial virus infection in a high-risk hospitalized population of Canadian children. Pediatric Investigators Collaborative Network on Infections in Canada. J Pediatr 1992;121:348-354
- IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from syncytial virus infection in high-risk infants. Pediatrics 1998;102:531-537
- Feltes T, Cabalka A, Meissner H, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. Cardiac Synagis Study Group. J Pediatr 2003;143:532-540
- Horn S, Smout R. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr. 2003;143:S133-141
- Willson D, Landrigan C, Horn S, et al. Complications in infants hospitalized for bronchiolitis or respiratory syncytial virus pneumonia. J Pediatr 2003;143:S142-149
- Sampalis J. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr 2003;143:S150-156
- Pérez-Yarza E, Moreno A, Lázaro P, et al. The association between respiratory syncytial virus infection and the development of childhood asthma: a systematic review of the literature. Pediatr Infect Dis J 2007;26:733-739
- Bont L, Steijn M, van Aalderen W, et al. Impact of wheezing after respiratory syncytial virus infection on health-related quality of life. Pediatr Infect Dis J. 2004;23:414-417
- Leidy N, Margolis M, Marcin J, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics 2005;115:1536-1546
- Sigurs N, Gustafsson P, Bjarnason R, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med 2005;171:137-141
- Stein R, Sherrill D, Morgan W, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541-545
- McLaurin K, Leader S. Growing impact of RSV hospitalizations among infants in the US, 1197-2002. Pediatric Academic Societies Annual Meeting. Washington, 2005
- Miedema C, Kors A, Tjon A, et al. Medical consumption and socioeconomic effects of infection with respiratory syncytial virus in The Netherlands. Pediatr Infect Dis J 2001;20:160-163
- Leader S, Yang H, DeVincenzo J, et al. Time and out of pocket costs associated with respiratory syncytial virus hospitalization of infants. Value Health 2003;6:100-106
- Samson L. Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Paediatr Child Health 2009;14:521-526
- Committee on Infectious Diseases. From the American Academy of Pediatrics: Policy statements – modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. 2009;14:521-526
- Figueras Aloy J, Quero J, Doménech E, et al. Comité de Estándares de la Sociedad Española de Neonatología. [Recommendations for the prevention of respiratory syncytial virus infection]. An Pediatr (Barc) 2005;63:357-362
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:238-275
- Evers S, Goossens M, de Vet H, et al. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-245
- Ofman JJ, Sullivan SD, Neumann PJ, et al. Examining the value and quality of health economic analyses: implications of utilizing the QHES. J Manage Care Pharm 2003;9:53-61
- Bank of Canada. 10-year currency converter. http://www.bankofcanada.ca/en/rates/exchform.html. Accessed 5 Feb 2010
- Bank of Canada. Inflation calculator. http://www.banqueducanada.ca/en/rates/inflation_calc.html. Accessed 5 Feb 2010
- ElHassan NO, Sorbero ME, Hall CB, et al. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med 2006;160:1070-1076
- Chirico G, Ravasio R, Sbarigia U. Cost-utility analysis of palivizumab in Italy: results from a simulation model in the prophylaxis of respiratory syncytial virus infection (RSV) among high-risk preterm infants. Ital J Pediatr 2009;35:4
- Tam DY, Banerji A, Paes BA, et al. The cost-effectiveness of palivizumab for term Inuit infants in the Eastern Canadian Arctic. J Med Econ 2009;12:361-370
- Nuijten MJC, Wittenberg W, Lebmeier M. Cost effectiveness of palivizumab for respiratory syncytial virus prophylaxis in high-risk children. Pharmacoeconomics 2007;25:55-71
- Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab for RSV prevention in high-risk children in the Netherlands. J Med Econ 2009;12:291-300
- Resch B, Gusenleitner W, Nuijten MJC, et al. Cost-effectiveness of palivizumab against respiratory syncytial viral infection in high-risk children in Austria. Clin Ther 2008;30:749-760
- Wang D, Cummins C, Bayliss S, et al. Immunoprophylaxis against respiratory syncytial virus (RSV) with palivizumab in children: a systematic review and economic evaluation. Health Technol Assess 2008;12.2008;12:iii, ix-x, 1-86
- Nuijten M, Lebmeier M, Wittenberg W. Cost effectiveness of palivizumab in children with congenital heart disease in Germany. J Med Econ 2009;12:301-308
- Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004;114:1606-1611
- Lanctôt KL, Masoud S, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin 2008;24:3223-3237
- Nuijten MJ, Wittenberg W. Cost effectiveness of palivizumab in Spain: an analysis using observational data. Eur J Health Econ 2010;11:105-115
- Joffe S, Ray GT, Escobar GJ, et al. Cost-effectiveness of respiratory syncytial virus prophylaxis among preterm infants. Pediatrics 1999;107:419-427
- Simpson S, Burls A. A systematic review of the effectiveness and cost-effectiveness of palivizumab (Synagis) in the prevention of respiratory syncytial virus (RSV) infection in infants at high risk of infection. Birmingham, UK: Department of Public Health & Epidemiology, University of Birmingham; 2001. Available at: http://www.rep.bham.ac.uk/reports_list.shtml [Last accessed 5 July 2010]
- Rodriguez SP, Fariña D. Respiratory syncytial virus prophylaxis in a high-risk population in Argentina: a cost-effectiveness analysis. Pediatr Infect Dis J 2008;27:660-661
- Roeckl-Wiedmann I, Liese JG, Grill E, et al. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003;162:237-244
- Stevens TP, Sinkin RA, Hall CB, et al. Respiratory syncytial virus and premature infants born at 32 weeks' gestation or earlier. Arch Pediatr Adolesc Med 2000;154:55-61
- Vogel AM, McKinlay MJ, Ashton T, et al. Cost-effectiveness of palivizumab in New Zealand. J Paediatr Child Health 2002;38:352-357
- Rackham OJ, Thorburn K, Kerr SJ. The potential impact of prophylaxis against bronchiolitis dues to the respiratory syncytial virus in children with congenital cardiac malformations. Cardiol Young 2005;15:251-255
- Chan PW-K, Abdel-Latif ME-A. Cost of hospitalization for respiratory syncytial virus chest infection and implications for passive immunization strategies in a developing nation. Acta Paediatrica 2003;92:481-485
- Fariña D, Rodriguez SP, Bauer G, et al. Respiratory syncytial virus prophylaxis: cost-effective analysis in Argentina. Pediatr Infect Dis J 2002;21:287-291
- Lofland JH, Touch SM, O'Connor JP, et al. Palivizumab for respiratory syncytial virus prophylaxis in high-risk infants: a cost-effectiveness analysis. Clin Ther 2000;22:1357-1369
- Banerji A, Lanctôt KL, Paes BA, et al. Comparison of the cost of hospitalization for respiratory syncytial virus disease versus palivizumab prophylaxis in Canadian Inuit infants. Pediatr Infect Dis J 2009;28:702-706
- Reeve CA, Whitehall JS, Buetnner PG, et al. Cost-effectiveness of respiratory syncytial virus prophylaxis with palivizumab. J Paediatr Child Health 2006;42:253-258
- Sampalis J, Langley J, Carbonell-Estrany X, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making 2008;28:471-480
- Simões EA, Carbonell-Estrany X, Fullarton JR, et al. and European RSV Risk Factor Study Group. A predictive model for respiratory syncytial virus (RSV) hospitalisation of premature infants born at 33-35 weeks of gestational age, based on data from the Spanish FLIP study. Respir Res 2008;9:78
- Paes BA, Cole M, Latchman A, et al. Predictive value of the respiratory syncytial virus risk-scoring tool in the term infant in Canada. Curr Med Res Opin 2009;25:2191-296