Abstract
Background:
Tiotropium has been shown to reduce exacerbations and improve quality of life for patients with chronic obstructive pulmonary disease (COPD), a lung disease characterized by a persistent and progressive airflow limitation.
Objectives:
To present a systematic literature review of the cost effectiveness of treatment with tiotropium compared with other currently used treatments for COPD.
Methods:
A systematic search was performed via PubMed, the Cochrane database, and EMBASE from 2002 to 2009. Methods and results by study design and by country were compared.
Results:
Seventeen studies were included in the review. Study designs were characterized as follows: modeling based on clinical trial data, and empirical analysis based on either clinical trial or observational data. Comparing monotherapy regimens (12 studies), all study designs found that treatment with tiotropium was associated with lower costs for hospitalisation and other non-drug services. Total costs, including the costs of maintenance drugs, were lower with tiotropium in some, but not all, of the studies. Tiotropium was shown to be cost effective based on commonly accepted benchmark values. Limitations of the review included the wide variety of outcome measures used in different studies, the limited number of observational database studies for monotherapy, and limited data for combination therapy regimens.
Conclusions:
The main conclusions of the economic evaluations derived from clinical trial data at the time of product approval and from later observational data reflecting clinical use are similar: use of tiotropium monotherapy is associated with lower hospital and other non-drug costs and better health outcomes and is either cost saving or cost effective compared with other maintenance monotherapies.
Introduction
Chronic obstructive pulmonary disease (COPD) is a lung disease characterized by a persistent airflow limitation that interferes with normal breathing, is not fully reversible, and can be life threatening when severeCitation1. Interference with breathing generally grows worse, breathing becomes more difficult, and exercise tolerance deteriorates over time, thus limiting patients in their usual activities and thereby prompting further deteriorationCitation1,Citation2. In addition, exacerbations, defined as ‘a sustained worsening of the patient’s condition, from the stable state and beyond normal day-to-day variations that is acute in onset and may warrant additional treatment in a patient with underlying COPD,’Citation3 become more frequent and more severe as the disease progressesCitation4.
COPD is associated with a large health and economic burden worldwide. The World Health Organization estimates that 210 million people have COPD worldwide and approximately 5% of all deaths in 2005 were attributable to the diseaseCitation1. Most of these deaths occur in low- or middle-income countriesCitation1. The costs associated with COPD are primarily related to the need for maintenance treatment, disease monitoring, and treatment of exacerbations. For example, in the United Kingdom, the annual costs to the National Health Service due to COPD have been estimated to be between £486 million and £848 millionCitation5. Costs increase as disease severity increases. The increase in cost is small when moving from mild to moderate disease but is much larger when moving from moderate to severe diseaseCitation5. The primary cost driver for COPD has been shown to be the need for hospital care for severe exacerbationsCitation6.
Several different types of pharmacologic treatment are used in the management of COPD, with the treatment goal to alleviate symptoms and prevent or reduce the severity of exacerbations. To date, no pharmacologic treatment has been shown to reduce the rate of disease progression. International guidelines for the management of COPD have been developed: the American Thoracic Society and the European Respiratory Society Treatment AlgorithmCitation7 and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Treatment AlgorithmCitation8. In addition, many national guidelines have been developed including CanadaCitation9, the UKCitation10, the USCitation11, and GermanyCitation12. The recommended pharmacologic treatments from these guidelines include short-acting beta-agonists or short-acting anticholinergic drugs for individuals with intermittent COPD symptoms or for those with Stage 1 mild disease (forced expiratory volume in 1 second [FEV1] ≥ 80% predicted). Monotherapy treatment with long-acting beta agonists (LABAs) or long-acting anticholinergic (LAAC) drugs is recommended as first-line treatment for individuals with more persistent symptoms or for those with Stage II moderate to Stage III severe disease in the GOLD classification. However, all guidelines recommend combination therapy for those in whom monotherapy is not effective or for those with multiple exacerbations or more severe disease. The combinations recommended include use of a LABA plus a LAAC as well as the addition of an inhaled corticosteroid for individuals whose disease is not controlled with long-acting bronchodilators or when there are repeated exacerbations or severe respiratory impairment. The most commonly recommended long-acting bronchodilators are tiotropium and salmeterol, but most guidelines state that there is insufficient evidence to distinguish between approved long-acting bronchodilators as monotherapy or in combination therapy regimens.
Tiotropium is a long-acting anticholinergic bronchodilator drug that was first approved as monotherapy for the treatment of COPD in Europe in 2002. Both 1-year and 6-month clinical trials conducted in patients with moderate to very severe COPD have shown that tiotropium provides sustained bronchodilation, reduces exacerbation rate, and improves dyspnea and health-related quality of life, as measured by the St. George’s Respiratory Questionnaire (SGRQ), when compared with placebo or ipratropiumCitation13,Citation14. Improvements in lung function also have been shown to be significantly better with tiotropium than with salmeterolCitation15. The 4-year UPLIFT trial compared tiotropium versus placebo on a background of usual care that allowed the use of LABA and/or inhaled corticosteroids; the trial demonstrated that the improvement in exacerbation rate and quality of life with tiotropium compared with placebo was maintained over the full 4-year trial period, although the rate of decline in FEV1 was not affected by tiotropiumCitation16.
The goal of this paper is to present a systematic literature review and evaluation of the data that have been generated to estimate the cost effectiveness of treatment with tiotropium monotherapy and with tiotropium as part of a combination therapy compared with other currently used treatments. Some of the reviewed studies are based on efficacy data for tiotropium derived from clinical trials, while others are based on data from observational studies of the effectiveness of tiotropium in standard clinical practice. Some of the reviewed studies use a modeling approach based on a structural model of disease progression, and some performed empirical analyses of the trial or observational data directly. The extent to which the results of the cost-effectiveness analyses are consistent across countries and methodologies also is discussed.
Patients and methods
A systematic electronic literature search from 2002 (the first launch of tiotropium) to December 2009 was performed using three databases: PubMedCitation17, a service of the United States (US) National Library of Medicine; Cochrane CollaborationCitation18, an international not-for-profit independent organization; and EMBASECitation19, a privately supported database with papers from a broader range of journals than those included in PubMed. The literature search strategies targeted papers that included economic analyses of tiotropium for COPD. As each database follows different syntax rules, a librarian experienced in the syntax of each database developed parallel literature search strategies for each (see appendix: PubMed, Table A-1; Cochrane Collaboration, Table A-2; EMBASE, Table A-3). The exclusion criteria and the number of papers identified by the electronic searches also are presented in the appendix (in Table A-4 and Table A-5, respectively). From an initial review of the abstracts identified in the electronic searches and by applying the prespecified inclusion and exclusion criteria, papers were selected for full review. Further, the reference lists of all full papers reviewed were searched for additional papers that might meet the inclusion criteria for an abstract and possible full review. Reviews and papers that presented economic analyses of treatments for COPD but did not include tiotropium in any treatment option were excluded.
Once the full set of published studies comparing treatment regimens, including tiotropium, to other current treatments were identified, the next step was to create a set of detailed evidence tables that included the following items abstracted from the published studies: the methodology used to estimate the cost effectiveness of tiotropium, the data sources and input values used, the base-case results, and the results of the sensitivity analyses. These tables were organized by the methodology used in the study as follows: modeling studies based on clinical trial data, empirical studies directly based on clinical trial data, and empirical studies directly based on observational data. In addition, detailed tables were prepared for the unit or event costs and the utility weights (where applicable) used in each study.
The detailed evidence tables then were used to create two text tables presenting the study characteristics and the results of the studies, respectively. The first table also was organized by the methodology used to generate the cost-effectiveness estimates and included information about the country of the study, the study design, the COPD population included, the study comparators, the cost and health outcome measures, the cost-effectiveness measures, and the sponsorship of the study. Because different journals have different rules about disclosure of study sponsorship, when sponsorship information was not provided we obtained additional information from Boehringer Ingelheim, the manufacturer of tiotropium. The second table was organized by country so that the results using different study methods could be compared both within and across countries. The results of the studies were reported in the country-specific currency to enable the reader to make a comparison of the incremental cost-effectiveness ratios with the willingness-to-pay threshold for each country. This table presented the following data comparing the tiotropium regimen with the comparator regimen for each study: differences in drug, hospital, and other costs; the differences in the number of exacerbations in patients, the percentage of patients with a gain in SGRQ score of 4 or more, and quality-adjusted life-years (QALYs); the incremental cost-effectiveness ratios (ICER = difference in costs ÷ difference in outcomes); and the key results of the sensitivity analyses. In studies where the differences in costs or health outcomes or the incremental cost-effectiveness ratios were not presented in the publication, simple deterministic estimates were calculated if sufficient data were presented in the paper. For example, many papers presented the costs by type of costs for the different study drug regimens but did not present the differences between each type of cost for the tiotropium regimen versus other regimens. The results text table uses italics to present costs, health outcomes, and ICERs calculated using estimates from the published paper to distinguish them from the values that were presented directly in the published paper.
These text tables form the basis for a description of the range of study characteristics and the differences in estimates depending on the methodology used to derive the estimates, as well as on the costs and outcomes measured and the country-specific costs. The broad range of outcomes from these studies and the implication of such diversity for healthcare decision making also was evaluated. Additionally, the methods and results for the tiotropium studies were compared with those methods and results used to estimate the cost effectiveness of comparisons of other treatments not including tiotropium, based on recent review papers.
Results
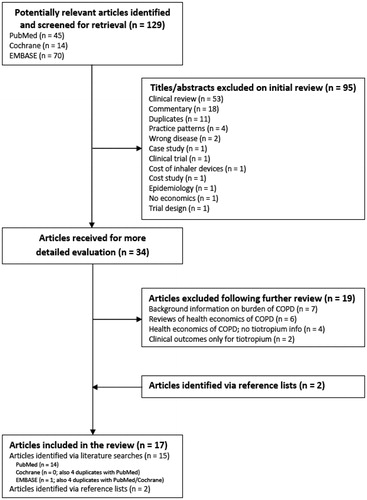
presents a flow diagram illustrating the search strategy and the number of papers identified and/or excluded at each step in the search process. A total of 129 unique abstracts were retrieved from the three electronic database searches. A review of these abstracts identified 34 papers that could meet the inclusion criteria for our review. After reviewing the full text for these papers, 19 were excluded because they were review papers, did not include tiotropium in the economic analyses presented in the paper, or presented only clinical outcomes for tiotropium. An additional two papers were identified from the reference lists of the 34 papers. The final number of papers included in this literature review was therefore 17.
Figure 1 Flow diagram of the literature search and review process. COPD, chronic obstructive pulmonary disease.
presents a summary of the different study characteristics, organized by study design. There were five studies that used modeling based on clinical trial dataCitation20–24, seven studies that used empirical analysis based on clinical trial dataCitation25–31, and five studies that used empirical analysis based on observational dataCitation32–36.
Table 1 Study characteristics.
All the modeling studies used adaptations of the initial model developed by Oostenbrink and colleaguesCitation20. This model was a Markov model that included three health states describing disease severity (moderate, severe, and very severe) following the current GOLD definitions and three health states describing exacerbation status in each cycle (no exacerbation, mild exacerbation, and severe exacerbation). A death state was not included in this 1-year model. An initial 8-day cycle to capture the rapid initial impact of all bronchodilators in improving disease symptoms and lung function was followed by monthly cycles to capture exacerbations and the transitions to more severe disease stages over time. The transition probabilities for the model were derived by pooling data from six tiotropium clinical trials, two compared with placebo (1-year studies), two compared to ipratropium (1-year studies), and two compared to salmeterol (6-month studies). The population included in the model matched that in the tiotropium clinical trials; all had a prebronchodilator FEV1 of 65% or less (≤60% for the salmeterol trials) of predicted normal and an FEV1 of 70% or less of forced vital capacity.
The model was adapted to estimate the cost effectiveness of tiotropium compared with other treatments in the Netherlands, Canada, Switzerland, Greece, and Spain, using the same model structure and clinical trial data but country-specific treatment patterns and costs. All model adaptations were sponsored by Boehringer Ingelheim, the manufacturer of tiotropium, to inform early reimbursement decisions for coverage. The death state was not included in modeling study adaptations that used a 1-year time horizonCitation21,Citation22, but this state was added to the model when adapted to run for a 5-year time horizon for the Netherlands and SpainCitation23,Citation24.
Although all studies were based on the same Markov model and used data from the same clinical trials, different studies presented different health outcome measures, including total number of exacerbations, number of severe exacerbations, number of non-severe exacerbations, exacerbation-free months, quality-adjusted months or years, percentage of patients in different disease stages at 1 year, time in different disease stages over the model time period, and life-years. The costs included in the models generally were shown as subdivided into study drug costs, costs for disease monitoring, and costs for treating exacerbations. All of the studies performed a one-way sensitivity analysis, and three studies included a probabilistic sensitivity analysisCitation20,Citation22,Citation23. One study performed an expected value of perfect information analysis to estimate the impact of alternative estimates of utility weights in the different health statesCitation24.
The seven empirical analyses based on clinical trials, presented in , take data either from one or two specific trialsCitation25–28,Citation31. or from a systematic literature review of all relevant trialsCitation29,Citation30. The analyses are either for the trial time period only or, for two studies comparing tiotropium with salmeterol, extrapolated from 6-month trials to a 1-year time horizonCitation28,Citation30. Analyses have been done for the US, the Netherlands, Belgium, Spain, and Canada. Depending on the clinical trials used for the analysis, tiotropium monotherapy was compared with placebo, ipratropium, or salmeterol in all the studies except the Najafzadeh studyCitation31, in which tiotropium monotherapy was compared with combination therapy—either tiotropium plus salmeterol or tiotropium plus salmeterol and fluticasone given as a fixed-dose combination (salmeterol/fluticasone). Four out of the seven studies were sponsored by Boehringer Ingelheim.
The health outcomes included in the empirical analyses based on clinical trials were the same as those outcomes used in the clinical studies and varied from study to study. Costs included study drugs, hospital costs, and other costs in most studies. One studyCitation29 included only study drug costs. Five of the studies included a one-way sensitivity analysisCitation26–28,Citation30,Citation31; and three studies also included multivariate sensitivity analysesCitation26,Citation28,Citation30. Two of these studies based their multivariate sensitivity analyses on different confidence intervals for the data from the clinical trialsCitation26,Citation30.
The five empirical studies based on observational data, presented in , are all more recent than the studies based on the clinical trial data, likely because of the time lag from drug approval until sufficient observational data become available. The observational database studies have a greater variability in the patient populations studied as follows: those individuals with FEV1 of 80% or less, anyone with a diagnosis of COPD, and patients admitted to hospital with a COPD exacerbation. Because individuals are not randomized to alternative treatments in observational databases, it is important to use an analysis method that adjusts for possible confounding due to differences in the populations being treated with the different drugs. The Reynoso studyCitation32 was based on data from a randomized, open-label, naturalistic trial, so confounding by indication should not be present. The Sicras-Mainar et al.Citation33 and the Dalal et al.Citation36 studies used multivariate regression analyses to control for observed confounding variables, and Onukwugha et al.Citation35 used a matched cohort design. Drescher et al.Citation34 used a pre-post design to estimate the impact of adding tiotropium to the protocol for treating patients in hospital with COPD exacerbations. The observational data studies included a variety of health outcomes and costs, depending on availability in the database. Hospital stay or days in hospital were the most common health outcome measures, and total costs were the most common cost presented. Only one of the studies attempted any sensitivity analysisCitation35. Two out of five of these studies were sponsored by Boehringer Ingelheim. One of the studiesCitation36 was sponsored by GlaxoSmithKline, the manufacturer of salmeterol/fluticasone.
The results of the different studies are presented in , grouped by country. The five Spanish studies included a modeling studyCitation23 as well as three empirical studies based on trial dataCitation27–29 and one empirical study based on observational dataCitation33. All five studies gave similar results, with increased maintenance-drug costs associated with treatment with tiotropium, offset to some extent by savings in hospital and other costs. The four Spanish studies based on clinical trial data included health outcomes measures and showed that tiotropium was more effective at improving symptoms than monotherapy with other drugs. Only the modeling studyCitation23 presented ICERs using QALYs gained as the outcome measure. These were €4118 per QALY for tiotropium compared with salmeterol and €8287 per QALY for tiotropium compared with ipratropium over a 5-year time period. The empirical studies based on trial data presented ICERs using incremental cost per exacerbation avoided and incremental cost per change in SGRQ score. Three of the studies compared tiotropium to ipratropium, and two of the studies also compared tiotropium to salmeterol. In one of these two studiesCitation29, the ICERs were more favorable when tiotropium was compared with salmeterol than for the comparison with ipratropium; while in the other studyCitation28, the comparison with ipratropium gave more favorable ICERs.
Table 2 Results of the studies by country.
The US studies included two empirical studies based on clinical trialsCitation25,Citation30 and three empirical studies based on observational dataCitation34–36. Two of the studies compared tiotropium monotherapy with ipratropium monotherapy for maintenanceCitation30,Citation35; two studies compared tiotropium monotherapy with placeboCitation25,Citation30; one study looked at drug expenses for hospital treatment only, with and without tiotropium on the protocolCitation34; and one study compared combination therapy with salmeterol/fluticasone with tiotropium monotherapy, ipratropium monotherapy, and ipratropium plus albuterol combination therapyCitation36. The Oba studyCitation30 found that tiotropium was dominant compared with ipratropium, with lower costs and greater health benefits. Friedman et al.Citation25 estimated hospital cost savings with tiotropium compared with placebo but did not include a cost for tiotropium, so the net costs were not available. Onukwugha et al.Citation35 did not present the net cost estimates, but the ICER measured as the incremental cost per exacerbation avoided indicated that the net cost with tiotropium compared with ipratropium was positive (approximately US$212). The Drescher et al.Citation34 study found that including tiotropium in a hospital’s treatment protocol both decreased COPD drug costs and reduced the average hospital length of stay. Finally, the Dalal et al.Citation36 study, which compared combination therapy with salmeterol/fluticasone to ipratropium plus albuterol, ipratropium monotherapy, or tiotropium monotherapy, found that, for all three comparisons, combination therapy with salmeterol/fluticasone resulted in lower total costs and better health outcomes than the comparators. However, the comparison of the combination of salmeterol/fluticasone with tiotropium monotherapy showed almost identical clinical outcomes with only small overall cost savings with the salmeterol/fluticasone combination treatment.
Three papers presented economic evaluations for the Netherlands, two using a modeling approach and one using empirical analysis based on trial dataCitation20,Citation24,Citation26. The results from the modeling approach indicated that tiotropium was cost saving compared with ipratropium or salmeterol. The empirical analysis found that, although there were offsetting hospital and other healthcare costs with tiotropium compared with ipratropium, the net costs with tiotropium were still positive.
The modeling approach initially used for the Netherlands was applied to Canada and estimated a small increased cost with tiotropium compared with ipratropium or salmeterol that generated an ICER of under Can$250 per QALY gained (when Can$1.00 = €0.62)Citation20. A second Canadian study, an empirical analysis of a trial comparing monotherapy with tiotropium to combination therapy with tiotropium plus salmeterol or with tiotropium plus salmeterol/fluticasone, found that tiotropium alone was less costly and more effective than the combination of tiotropium plus salmeterol but was less costly and less effective than the combination of tiotropium plus salmeterol/fluticasoneCitation31. However, the ICER for the triple combination compared with tiotropium monotherapy was Can$243,180 per QALY gained.
Studies were identified for three other countries, Greece, Mexico, and Switzerland. The studies in Greece and Switzerland used a Markov model. The study in Greece showed that tiotropium was less costly and had better health outcomes than salmeterolCitation22. The study in Switzerland showed that tiotropium was less costly and had better health outcomes than salmeterol, ipratropium, or standard careCitation21. Finally, the study in MexicoCitation32 was based on a trial of combination therapies and found that tiotropium plus salbutamol had lower costs and better health outcomes than ipratropium plus salbutamol.
Discussion
The results of the review of the economic evaluations of tiotropium compared with either no maintenance treatment or monotherapy with salmeterol or ipratropium found that all published studies, including the studies based on observational data, estimated superior health outcomes with tiotropium monotherapy as well as reduced hospital and other costs (where these were presented). Some published studies also found that the net annual total costs with tiotropium were lower than with the comparators, while other studies found that the reductions in hospital and other costs were not sufficient to completely offset the increased drug costs with tiotropium. When the annual costs were higher with tiotropium, ICERs were calculated for a number of outcomes. When incremental cost per QALY was estimated, the ICERs were generally below commonly accepted benchmark values for willingness to pay. Incremental cost per exacerbation avoided or per person with a 4-point or greater increase in the SGRQ was variable, ranging from tiotropium being dominant to €7100 in one Spanish study. There are no established benchmarks for these ratios. The results of the economic evaluations of studies with combination therapy found that fluticasone/salmeterol and tiotropium plus fluticasone/salmeterol were more effective than tiotropium monotherapy, and use of fluticasone/salmeterol may result in net cost savings compared with monotherapy.
Three different study designs were included in this systematic review: a Markov model, empirical analysis directly based on clinical trial data, and empirical analysis directly based on observational data. In general, the results from all three designs for monotherapy were similar, showing health benefits with tiotropium and reduced hospital and other costs. The designs differed as to whether or not total annual costs would be lower with tiotropium monotherapy, with the Markov models indicating total cost savings or very similar costs. The exception to this was the 5-year Markov model for SpainCitation23, which estimated increased total costs with tiotropium. The empirical analyses generally estimated net annual total costs that were higher with tiotropium. These differences between the model-based studies and the empirical studies based on trial data may result from the latter not being as reflective of routine clinical practice because they used, for the most part, the resource use data collected during the clinical trial and imputation was necessary for patients who withdrew from the trial early. The modeling studies used alternative data sources more reflective of routine clinical practice to estimate the costs associated with exacerbations and followed all patients for the same time period. Of importance for decision makers is that the two studies based on observational dataCitation33,Citation35 found similar offsetting hospital and other non-drug cost savings with tiotropium, indicating that the results found in clinical trials also were observed in routine clinical practice.
The results were presented by country in order to determine whether there were systematic differences in the results either within or among countries. As expected, there were differences in the results from different studies within a single country because of different study methods and input values used. However, the general result of better health outcomes and lower hospital and other non-drug costs with tiotropium monotherapy compared with other monotherapy was shown consistently in all studies in the same country. There was a similar consistency in results for monotherapy across different countries.
Comparisons of combination therapy with other combination therapy or with monotherapy within or across countries were not possible because of the limited number of studies available. There were only three studies included in the current review that compared a specific combination therapy regimen either with alternative monotherapy regimens or with another combination therapy. These were an observational database study in the US, comparing fluticasone/salmeterol with tiotropium, ipratropium monotherapy, and ipratropium plus albuterol, and two empirical studies based on clinical trials in Canada and Mexico, comparing combinations including tiotropium with tiotropium monotherapy or with a combination including ipratropium, respectively.
Another way of ‘validating’ the findings of the cost-effectiveness analyses would be to compare the effect estimates used in the economic analyses with the evidence of the clinical benefits, as available from other sources. A recently published systematic mixed-treatment comparison meta-analysis of COPD trialsCitation37 has shown that tiotropium monotherapy was statistically significantly superior to LABA monotherapy for exacerbations and trial withdrawal rates and showed a trend to superiority for mortality. The differences between tiotropium monotherapy and combination treatment with inhaled corticosteroids and LABAs were not statistically significant for exacerbations, mortality, and trial withdrawal rates. The publication did not provide any estimates for a comparison of tiotropium with ipratropium and did not include the 4-year tiotropium study UPLIFTCitation16 as the analyses predated the UPLIFT study publication. These findings indicate the robustness of the cost-effectiveness analyses for the monotherapy comparison with LABA and demonstrate remaining uncertainties when comparing tiotropium monotherapy with combination therapies.
The costs for each unit of the different healthcare services and/or health outcomes and the utility weights (for those studies that included an estimate of QALYs gained) included in the analysis affected the results of the different studies. For example, in the Oostenbrink et al.Citation20 modeling study that presented results for both the Netherlands and Canada, tiotropium was found to be cost saving with better health outcomes in the Netherlands, while net costs were slightly higher with tiotropium compared with salmeterol or ipratropium in Canada. This difference in the results was most likely due to the difference in the estimated cost of a severe (non-severe) exacerbation in the two countries, which was approximately €3600 (€328) in the Netherlands and €2900 (€42) in Canada.
A frequent concern about economic evaluations is that they may be biased when sponsored by the company that manufactures and markets the product being evaluated. Some of the empirical analysis papers in this review were not sponsored by the manufacturer of tiotropium, yet the results from those studies generally were similar to the results from the studies that were industry sponsored.
One of the problems encountered in comparing the results from the different studies included in this review was the wide variety of health outcomes measured in the clinical trials and included in the economic evaluation and the variations in what was presented in the economic evaluation publicationsCitation5. Health outcomes used in the economic evaluations included measures relating to the frequency of exacerbations; measures relating to symptom improvement, such as changes in the SGRQ score and the FEV1; and measures relating to improvement in QALYs. The advantage of using QALYs as the outcome measure for a cost-effectiveness analysis is that it allows comparison of the results not only across alternative treatment options for the same disease but also across results from treatments for different diseases. This informs decisions at a health-system levelCitation38–40. On the other hand, the Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (German: Institute for Quality and Efficiency in Health Care) in GermanyCitation41 recently proposed that the focus of healthcare decisions should be on alternative treatments within a disease area; therefore, QALYs are not necessarily the best health outcome measure, especially if there are other cardinal measures that are well accepted within the disease area. Thus, outcome measures, such as exacerbations avoided, 12% gain in FEV1, or 4-point gain in SGRQ score, might be more acceptable for decision makers who are primarily interested in comparing treatments for COPD and who prefer measures used in clinical trials and routine medical practiceCitation39. Another advantage of estimating an ICER measured as the incremental cost per QALY gained is that there are generally accepted benchmark values that can be used to define ‘good value for money’, while ICERs such as incremental cost per exacerbation avoided or incremental cost per number of individuals experiencing more than a 12% gain in FEV1 or a 4-point gain in the SGRQ generally do not have such accepted benchmarks.
In this paper, the authors have focused on the cost effectiveness of tiotropium compared with other maintenance treatments for COPD. Two recent reviews presented cost-effectiveness estimates both for treatment comparisons that did not include tiotropium as well as for comparisons that included tiotropium in one comparator regimen. Rutten-van Molken and LeeCitation42 and Starkie et al.Citation5 found that all published models of COPD disease progression were similar in the disease-state descriptions for COPD but differed in the input parameter values, including time horizon, transition rates and utility weights, and costs. These differences in input parameter values drove model results, which varied from one treatment being cost saving to cost-effectiveness ratios that were above US$50,000. In particular, Starkie et al.Citation5 pointed out that differences in the magnitude of the decrease in utility at more severe disease stages or the magnitude or duration of the utility decrease during an exacerbation could have a major impact on the estimated cost per QALY gained. Thus, for incremental cost per QALY to become a more useful measure of cost effectiveness for treatments for COPD, Starkie et al.Citation5 recommended further research to generate generally accepted utility weight decrements by disease stage and exacerbation.
When a new drug is introduced, estimates of its value have to be based on clinical trial data, either through modeled or empirical analyses. The advantage of the modeling approach at this early stage is that the results of the clinical trials can be extended to non-trial populations in routine clinical practice and to longer time horizons. After the drug has been in clinical use for a few years, estimates of its value can be revisited and based on observational studies of costs and health outcomes in routine practice. In this systematic literature review of the cost effectiveness of tiotropium, the authors were able to compare the results of studies performed using data from randomized clinical trials at the time of product approval with results of studies performed using observational data after several years of clinical use.
Conclusion
The present review has demonstrated that the main conclusions of the economic evaluations derived from clinical trial data at the time of product approval and from observational data reflecting clinical use after product approval are similar and that use of tiotropium monotherapy is associated with lower hospital and other non-drug costs and better health outcomes and is either cost saving or cost effective compared with other maintenance monotherapies. Because of the many possible drug combinations and the limited number of clinical trials or observational studies providing outcomes data for these combinations, the cost effectiveness of combination therapy regimens should be an area of further research.
Declaration of interest
J.M. and M.J. have disclosed that they are employees of RTI Health Solutions, a company that received funding to prepare this paper. C.L.B. and B.U.M. have disclosed that they are employees of Pfizer Inc. and Boehringer Ingelheim International GmbH.
Transparency
Declaration of funding
The preparation of this manuscript was funded by Boehringer Ingelheim International GmbH and Pfizer Inc.
Supplementary Material
Download PDF (28 KB)Supplementary Material
Download MP3 Audio (5.2 MB)References
- World Health Organization (WHO). Chronic obstructive pulmonary disease. Fact sheet #315, 2009. Available at: http://www.who.int/mediacentre/factsheets/fs315/en/. Accessed February 11, 2010
- Wise RA. The value of forced expiratory volume in 1 second decline in the assessment of chronic obstructive pulmonary disease progression. Am J Med 2006;119:S4-11
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J Suppl 2003;41:46-53s
- Donaldson GC, Seemungal TAR, Patel IS, et al. Longitudinal changes in the nature, severity and frequency of COPD exacerbations. Eur Respir J 2003;22:931-936
- Starkie HJ, Briggs AH, Chambers MG. Pharmacoeconomics in COPD: lessons for the future. Int J COPD 2008;3:71-88
- Ramsay SD, Sullivan SD. The burden of illness and economic evaluation for COPD. Eur Respir J Suppl 2003;41:29s-35s
- Celli BR, MacNee W, and committee members. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23:932-946
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respire Crit Care Med 2007;176:532-555
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease—2007 update. Can Respir J 2007;14 (Suppl B):5B-32B
- National Collaborating Centre for Chronic Conditions. NICE guideline: chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease for adults in primary and secondary care. National Institute for Clinical Excellence, NHS, Clinical Guideline 12, February 2004
- Qaseem A, Snow V, Shekelle P, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2007;147:633-638
- Programm fur Nationale VersoorgungsLeitlinie. COPD Langfassung, Version 1.7. February 2010. Available at: http://www.versorgungsleitlinien.de/themen/copd/pdf/nvl_copd_lang.pdf. Accessed June 1, 2010
- Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002;19:217-224
- Vincken W, van Noord JA, Greefhorst AP, et al. Improved health outcomes in patients with COPD during 1 year’s treatment with tiotropium. Eur Respir J 2002;19:209-216
- Brusasco V, Hodder R, Miravitlles M, et al. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003;58:399-404
- Tashkin DP, Celli B, Kesten S, et al. Long-term efficacy of tiotropium in relation to smoking status in the UPLIFT trial. Eur Respir J 2010;35:287-294
- PubMed [Web site]. Available at: http://www.ncbi.nlm.nih.gov/pubmed/. Accessed May 26, 2010
- The Cochrane Library [Web site]. Available at: http://www.thecochranelibrary.com. Accessed May 26, 2010
- EMBASE [Web site]. Available at: http://library.dialog.com/bluesheets/html/bl0072.html. Accessed May 26, 2010
- Oostenbrink JB, Rutten-van Molken M, Monz BU, et al. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005;8:32-46
- Schramm W, Haake D, Brandt A. Economic value of tiotropium (Spiriva®) in the treatment of chronic obstructive pulmonary disease. Praxis 2005;94:1803-1810
- Maniadakis N, Tzanakis N, Fragoulakis V, et al. Economic evaluation of tiotropium and salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) in Greece. Curr Med Res Opin 2006;22:1599-1607
- Rutten-van Molken MP, Oostenbrink JB, Miravitlles M, et al. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ 2007;8:123-135
- Oostenbrink JB, Maiwenn J, Oppe M, et al. Expected value of perfect information: an empirical example of reducing decision uncertainty by conducting additional research. Value Health 2008;11:1070-1080
- Friedman M, Menjoge SS, Anton SF, et al. Healthcare costs with tiotropium plus usual care versus usual care alone following 1 year of treatment in patients with chronic obstructive pulmonary disorder (COPD). Pharmacoeconomics 2004;22:741-749
- Oostenbrink JB, Rutten-van Molken MP, van Noord JA, et al. One-year cost-effectiveness of tiotropium versus ipratropium to treat chronic obstructive pulmonary disease. Eur Respir J 2004;23:241-249
- Ramos P de Lucas, Miravitlles M, Gonzalez-Moro JM, et al. Cost-effectiveness analysis of the use of tiotropium compared with ipratropium in the treatment of patients with chronic obstructive pulmonary disease. PharmacoEconomics Spanish Research Articles 2004;1:121-128
- Sanz-Martinez H, Perez-Maroto MT. Cost-efficacy analysis of tiotropium versus ipratropium and salmeterol in the treatment of chronic obstructive pulmonary disease. Farmacia Aten Primaria 2004;2:72-82
- Garcia Ruiz AJ, Leiva Fernandez F, Martos Crespo F. Cost-effectiveness analysis of tiotropium compared to ipratropium and salmeterol. Arch Bronconeumol 2005;41:242-248
- Oba Y. Cost-effectiveness of long-acting bronchodilators for chronic obstructive pulmonary disease. Mayo Clin Proc 2007;82:575-582
- Najafzadeh M, Marra CA, Sadatsafavi M, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax 2008;63:962-967
- Reynoso FN. Effect of inhaled salbutamol-ipratropium and salbutamol-tiotropium combinations and oral theophylline in patients with chronic obstructive pulmonary disease: a cost-effectiveness analysis. Rev Inst Nal Enf Resp Mex 2006;19:122-126
- Sicras-Mainar A, Velasco-Velasco S, Llopart-Lopez JR, et al. Calculation of morbidity, use of resources and costs of patients treated with tiotropium bromide for COPD in a Spanish population. Aten Primaria 2007;39:547-555
- Drescher GS, Carnathan BJ, Imus S, et al. Incorporating tiotropium into a respiratory therapist-directed bronchodilator protocol for managing in-patients with COPD exacerbations decreases bronchodilator costs. Respir Care 2008;53:1678-1684
- Onukwugha E, Mullins CD, DeLisle S. Using cost-effectiveness analysis to sharpen formulary decision-making: the example of tiotropium at the Veterans Affairs Health Care System. Value Health 2008;11:980-988
- Dalal A, Petersen H, Simoni-Wastila L, et al. Healthcare costs associated with initial maintenance therapy with fluticasone propionate 250 µg/salmeterol 50 µg combination versus anticholinergic bronchodilators in elderly US Medicare-eligible beneficiaries with COPD. J Med Econ 2009;12:339-347
- Baker WL, Baker EL, Coleman CI. Pharmacologic treatments for chronic obstructive pulmonary disease: a mixed-treatment comparison meta-analysis. Pharmacotherapy 2009;29:891-905
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes, 3rd edn. Oxford, UK: Oxford University Press, 2005:211–245
- Sculpher M. Using economic evaluations to reduce the burden of asthma and chronic obstructive pulmonary disease. Pharmacoeconomics 2001;19(Suppl 2):21-25
- Gold MR, Siegel JE, Russell LB, et al. Cost-effectiveness in Health and Medicine. New York: Oxford University Press, 1996:82–134
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (Institute for Quality and Efficiency in Health Care). IQWiG methods for assessment of the relation of benefits to costs in the German statutory health care system, version 1.1, 2008
- Rutten-van Molken MP, Lee TA. Economic modeling in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2003;3:630-634