Abstract
Objective:
To determine the cost-effectiveness of vaccination against herpes zoster (HZ) and post-herpetic neuralgia (PHN) in individuals aged 60 years and older in Belgium.
Methods:
A Markov model was developed to compare the cost-effectiveness of vaccination with that of a policy of no vaccination. The model estimated the lifetime incidence and consequences of HZ and PHN using inputs derived from Belgian data, literature sources, and expert opinion. Cost-effectiveness was measured by the incremental cost-effectiveness ratio (ICER), expressed as cost per quality-adjusted life-year (QALY) gained.
Results:
Vaccination in individuals aged 60 years and older resulted in ICERs of €6,799 (third party payer perspective), €7,168 (healthcare perspective), and €7,137 (societal perspective). The number needed to vaccinate to prevent one case was 12 for HZ, and 35 or 36 for PHN depending on the definition used. Univariate sensitivity analyses produced ICERs of €4,959–19,052/QALY; duration of vaccine efficacy had the greatest impact on cost-effectiveness. Probabilistic sensitivity analysis showed at least a 94% probability of ICERs remaining below the unofficial €30,000 threshold.
Discussion:
Key strengths of the model are the combination of efficacy data from a pivotal clinical trial with country-specific epidemiological data and complete sensitivity analysis performed. Main limitations are the use of non country-specific PHN proportion and non Belgian disease-specific utilities. Results are comparable with those recently published.
Conclusions:
HZ vaccination in individuals aged 60 years and older would represent a cost-effective strategy in Belgium.
Introduction
Herpes zoster (HZ; ‘shingles’) is caused by reactivation of the varicella zoster virus (VZV) that has remained latent in dorsal root ganglia after primary infection acquired in childhood (varicella or chickenpox). The lifetime risk of HZ has been estimated to be 25%Citation1, and the risk of having a recurrent HZ has been assessed between 1 and 5%Citation2 for those having had a first HZ episode. The incidence rises rapidly after the age of 50 years, resulting in a lifetime risk of 25–50% in individuals reaching their eighth decadeCitation3,Citation4. VZV reactivation and the resulting HZ is attributable to the progressive decrease in the VZV-specific cell-mediated immunity that occurs with aging (‘immunosenescence’) or other conditions or therapies that cause immune impairmentCitation5.
HZCitation6 is characterised by a painful, unilateral, vesicular rash localised to the dermatome corresponding to the affected ganglion. Rash eruption may be preceded by itching, numbness or pain during a prodromal phase. The pain and rash usually resolve spontaneously within 3–4 weeksCitation7, but the former may persist for months or years after the rash has healed in 20–25% of casesCitation8. This persistent pain is referred to as ‘post-herpetic neuralgia’ (PHN), and is the commonest complication of HZ, occurring in 25–50% of individuals over the age of 50 yearsCitation8. PHN is usually defined as significant pain that occurs or persists three months after rash onsetCitation9, although it may also be defined as pain that occurs or persists one month after rash onsetCitation10. PHN can cause severe physical, functional, social and psychological impairment as a consequence of the continuing painCitation11–16. As a result, this condition is associated with a marked reduction in health-related quality of lifeCitation17,Citation18.
A new live attenuated vaccine, Zostavax, has been developed for the prevention of HZ and PHN in older adults. The efficacy of Zostavax has been demonstrated in the Shingles Prevention Study (SPS)Citation19, a randomized, double-blind, placebo-controlled trial involving 38,546 immunocompetent participants aged 60 years and older. In this study, the incidence of HZ and PHN was reduced by 51.3% and 66.5%, respectively, and the burden of illness due to HZ (defined as the average severity of illness, which was measured as the area under the curve of HZ pain scores divided by time), reduced by 61.1%. Moreover, vaccinated patients who developed HZ had less severe illness than unvaccinated patients with HZ. The vaccine was approved by the Food and Drug Administration (FDA) in the USA in 2006 for use in individuals aged 60 years or older, and was recommended for use in this age group later the same yearCitation20,Citation21. The European Medicines Agency (EMEA) approved the vaccine in 2007 for individuals aged 50 years and older and it was recommended for use in this age group in Austria in 2008Citation22.
Ideally, vaccination programmes that aim to promote healthy ageing (including the prevention of HZ and PHN) should begin in middle age, before the age-related decline in cell-mediated immunity has begun. HZ vaccination at the age of 50 years may offer benefits from a public health and health economic perspective because productivity losses resulting from HZ in individuals of working age would be diminished. The incidence and severity of HZ increase with age, so vaccination later in life (e.g., 60 years) may be more cost-effective than earlier vaccination. Cost-effectiveness data are required for a medicinal product to be approved for reimbursement in BelgiumCitation23. A health economic model was therefore developed to evaluate the cost-effectiveness of HZ vaccination in Belgium.
Methods
A Markov model was developed to evaluate the cost-effectiveness of the HZ vaccine in Belgium. Two policies were compared in the model: adoption of an HZ vaccination policy with coverage of 20% among individuals aged 50 years and older in the Belgian population, and the current policy of no vaccination. The model was developed in accordance with guidelines for healthcare evaluations issued by the Belgian Health Care Knowledge Centre (KCE)Citation23, and with published guidelines for modelling studiesCitation24.
Perspectives
Three perspectives were considered: a third-party payer (TPP) perspective (includes only direct healthcare costs covered by the Belgian National Institute for Health and Disability Insurance; RIZIV-INAMI); a healthcare perspective (includes patient co-payments in addition to the costs considered in the TPP perspective); and a societal perspective (includes productivity losses in addition to the costs included in the healthcare perspective).
Model design
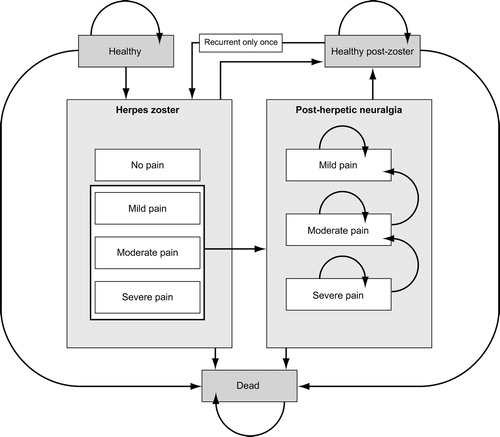
The Markov model was used to simulate the lifetime incidence and consequences of HZ and PHN in the current Belgian population aged 50 years and older. It was developed in Microsoft Office Excel 2003 (Microsoft, Seattle, WA, USA). Several health states were described in the model (). These included healthy (i.e., no HZ or PHN), dead, HZ and PHN. HZ was subdivided into four levels of pain severity: no pain, mild, moderate or severe and PHN was subdivided into three levels of pain severity: mild, moderate or severe. Single episodes of recurrent HZ and associated neuropathic pain were also allowed. The population was analysed over the lifetime of 5-year age groups (50–54 years, 55–59 years, etc.) The lifetime of the cohort was divided into 1-month Markov cycles. Within each cycle, cohort members could remain in their current health state, or move into one of the permitted states (); these transitions were governed by a matrix of probability values. During each successive cycle, an increasing proportion of the cohort would move through the HZ and PHN states and eventually to death. These cycles were repeated until the entire cohort had died.
Movement from the healthy state to HZ was based on monthly age-specific HZ incidence data. The Markov model applied a monthly cycle length and the 1-month definition of PHN, i.e. pain occurring or persisting 1 month after HZ onset was adopted in the model. PHN is more commonly defined as pain occurring within 3 months of HZ onsetCitation9, so the model was adjusted to produce results according to both definitions. In particular, the model was calibrated to ensure that the calculated proportion of PHN cases occurring 3 months after the onset of the HZ rash matched that observed in the SPSCitation19.
Because the likelihood of developing PHN is higher in patients with severe zoster-related pain than in those with milder pain, the proportion of HZ patients developing PHN was derived by applying odds ratios of 2.39 for severe pain and 0.88 for mild pain (both relative to an odds ratio of 1.0 for moderate pain)Citation25. The model assumed that PHN diminishes over time and eventually ceases, and hence all patients must pass through the mild PHN state to reach the healthy (post-zoster) state. As a result, the mean duration of PHN will depend on the initial split of the population (i.e., proportions of patients with mild, moderate or severe pain): patients with severe pain will take longer to return to health than patients with mild pain. It was therefore essential to calibrate the transition probabilities between PHN severity states and the healthy state to reflect the mean duration of PHN from the literature. The model assumed that the transition rates from severe to moderate, moderate to mild, and mild to healthy (post-zoster) are identical, although this constant rate differs between age groups: older patients experience a longer duration of pain than younger patientsCitation19.
Recurrent HZ was considered to be a distinct state in the model because it is uncommon. The characteristics of recurrent HZ, such as duration, initial pain split (severity), and occurrence of subsequent PHN, were assumed to be identical to those of first episodes. The monthly probability of an individual reaching the death state was derived from age-specific mortality data.
Model validation
The model was validated using clinical data from the SPSCitation19. For the observed number of cases of HZ and PHN over 3 years (duration of the SPS), the figures from the placebo arm of the SPS (n = 642 and n = 80, respectively were used, corresponding to incidence rates of 11.1 and 1.4 per 1000 person-years, respectively). Among vaccinated individuals, the number of HZ cases was the same as in the vaccine arm of the SPS (n = 315, corresponding to incidence rates of 5.4 per 1000 person-years), but the number of PHN cases was 15% lower (23 versus 27). Vaccine efficacy was therefore adjusted downwards according to the PHN pain state transition probabilities. Vaccination reduced the mean duration of PHN in the SPS by 2.2 months in patients aged 60–69 years and 3.3 months in older patientsCitation19, so the transition probability for moving to the next lowest pain state (severe-to-moderate; moderate-to-mild; mild-to-no pain) was calculated by modelling an initial cohort with the SPS pain split. A sufficient number of cycles were simulated until >99% of patients were no longer in pain, and the mean duration of pain calculated for this population. This constant probability was adjusted until the mean duration of pain was equal to the PHN duration observed in the SPS, to yield the PHN pain state transition probability. Among vaccinated individuals, the calculated transition probabilities were 23.0% in those aged 60–69 years, and 22.0% in older individuals; the corresponding figures in unvaccinated persons were 18.2% and 16.2%, respectively. Following this adjustment, the model results exactly matched those reported in the SPS, which suggests that the model provides a valid estimate of clinical benefit.
Model inputs
Data used in the model were derived from the SPSCitation19 and other published sources, in addition to expert opinion (). Expert opinion was obtained from a Delphi panel comprising four primary care physicians, three dermatologists, four ophthalmologists, and eight pain specialists or neurologists. The panel was conducted in two rounds using written questionnairesCitation26.
Table 1. Model inputs: epidemiology, vaccine characteristics and utilities.
Table 2. Model inputs: healthcare resource use (2007 prices). Monthly costs.
Table 3. Costs of lost productivity due to HZ and PHN.
Epidemiology of HZ and PHN
For the base-case analysis, data on the incidence of HZ in BelgiumCitation27 were derived from a 2006 survey conducted by the Belgian Sentinel of General PractitionersCitation28. This is an epidemiological surveillance system that involves approximately 180 physicians selected to be representative of Belgian general practitioners in terms of age, sex and geographical location. The survey covered 183,895 individuals; the reported age-specific HZ incidence rates ranged from 0.35% to 1.82% (). This survey did not provide data on the proportion of patients with HZ developing PHN, and hence data were derived from the General Practice Research Database (GPRD) in the UKCitation29. This yielded a PHN proportion between 10.3% and 28.9% using the 1-month definition of PHN; these numbers were adjusted by applying the pain split observed in the SPSCitation19 to ensure that GPRD data reflected only HZ cases with pain (i.e., the dominator used to calculate the conditional probability of PHN was the number of patients with painful HZ). As described above, the proportion of PHN cases obtained using the 1-month definition was calibrated to ensure that the proportion of patients calculated in the model matched that observed in the SPS (in which the 3-month definition was used). The mean durations of HZ and PHN were derived from the SPSCitation19. Data on mortality associated with HZ or PHN for Belgium were not available. Disease-specific mortality was therefore set at zero, based on data from the GPRDCitation29 and supported by expert opinion. The pain split used in the model, both for HZ and PHN, was that observed in the SPSCitation19. The incidence of recurrent HZ was calculated from Belgian life expectancy data for each 5-year age groupCitation30, applying a conditional lifetime risk of 3%, which is the midpoint of the 1–5% range reported by Cunningham et al.Citation2. It was assumed that the proportion of patients with PHN after recurrent HZ would be the same as that after a first episode.
Vaccine characteristics
Data on vaccine efficacy were derived from the SPSCitation19. This trial included individuals aged 60 years and older. Two clinical trials showing that the immunological response to the vaccine in the younger 50–59 years age group was not inferior to that observed in older persons suggest that efficacy data may be extrapolated to the entire population aged 50 years and olderCitation31,Citation32. Both direct and indirect effects of vaccination were included in the model. It was assumed that vaccination would have a direct effect in reducing the number of cases of HZ and PHN. As HZ precedes PHN, the number of PHN cases was assumed to be reduced indirectly through the reduction in the HZ cases; hence the indirect effects of vaccination were included implicitly as additional consequences of the direct effects.
It was assumed that vaccine efficacy would have a lifelong duration. Current available data do not show any decline of vaccine efficacy over timeCitation33. After having reviewed those data, EMEA confirmed that vaccine efficacy was maintained for up to 7 years and the labelling of Zostavax, one-dose vaccine, has not been changed. To anticipate a possible future decline of efficacy over time, a waning function was built into the model. This function was set at zero for the base-case analysis; alternative efficacy durations were examined in sensitivity analyses (see below). In the absence of literature to validate the choice of vaccine coverage rate the authors assumed a hypothetical rate of 20% in all age groups, but they also included other coverage rates in the sensitivity analyses. The unit cost of vaccination was set at €141.18 in the base-case analysis, including co-payments. Different vaccination costs were included in sensitivity analyses.
Utilities
The base-case analysis used applied an additive method to calculate utilities in which disease-specific decrements were subtracted from age-specific utilities. Age-specific Belgian utility data were derived from a report of the EuroQoL GroupCitation34 which used data from a 2004 survey of 2,754 individuals in the Flemish-speaking Belgian population ()Citation35. In the absence of data specific to Belgium, utility decrements associated with HZ and PHN were derived from a study by Oster et al. in the USACitation18. The applicability of these data to the Belgian population is supported by their similarity to reported data from patients with neuropathic pain in five European countriesCitation36.
Use and cost of healthcare resources
Data on the use and cost of healthcare resources associated with HZ and PHN () were obtained from a burden of illness study conducted by IMS Health (Brussels, Belgium)Citation26. This used the previously mentioned expert panel comprising four primary care physicians, three dermatologists, four ophthalmologists, and eight pain specialists. This study examined the use of primary and secondary care, oral and topical medications, non-pharmacological treatments (transcutaneous electrical nerve stimulation, acupuncture, infiltrations, hypnosis), diagnostic tests, and hospitalisations from societal and TPP perspectives. HZ was classified according to the localisation and number of dermatomes affected. For analyses of medications and non-pharmacological treatments, a distinction was made between HZ diagnosed within 72 hours of rash onset and HZ diagnosed later. This generated two sets of data; because >50% of patients contact their general practitioners within 72 hours of rash onsetCitation7, mean resource use and cost values were derived by applying a 50% weighting to the two datasets.
Costs of lost productivity
Productivity costs () were applied in the societal perspective and were calculated by combining data on work absenteeism (obtained from the IMS report) with national Belgian employment statisticsCitation37, with adjustment for the higher proportion of women affected by HZ and PHN compared with men (). It was assumed that nobody was employed after the age of 70 years, based on reported employment rates for individuals aged 65−69 years (the official retirement age in Belgium is 65 years). Costs resulting from absence from work were calculated by multiplying the number of workdays lost by the proportion of patients who missed work because of HZ or PHN in each pain category, and multiplying the result by the mean daily cost of absenteeism in Belgium in 2004 (€200)Citation38.
Model outputs
The model outputs were total costs, quality-adjusted life-years (QALYs), number of cases of HZ and PHN under vaccination and no-vaccination policies, and the number needed to vaccinate (NNV) to prevent one case of HZ or PHN. The cost-effectiveness of vaccination was assessed by comparing the total costs and effectiveness in terms of the number of cases of HZ or PHN avoided or QALYs gained under both policies. The incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in cost between the two policies by the difference in effectiveness. In Belgium there is no official threshold below which an intervention would be considered cost-effective, although ICERs below €30,000/QALY have previously been considered to be cost-effectiveCitation39. This threshold was therefore used as a criterion for cost-effectiveness in the present study.
Discount rates
Based on the KCE recommendationsCitation23, discount rates of 3% were applied for costs and 1.5% for outcomes (QALYs). Other discount rates were investigated in sensitivity analyses (see below).
Sensitivity analyses
Deterministic sensitivity analyses were carried out to assess the sensitivity of the ICER obtained in the base-case analysis to variations of several key input variables within feasible ranges. These analyses were univariate, i.e. only one variable was changed in each analysis.
The impact of a reduction in the incidence of HZ on the cost-effectiveness of vaccination was investigated by applying data from an epidemiological study carried out in the NetherlandsCitation7, in which the incidence of HZ was lower than that reported in Belgium (). In addition, the effect of varying durations of PHN and changes in the level of pain used in the base-case analysis were examined using GPRD dataCitation29. A further analysis examined the impact of potential HZ-related mortality because, although it is widely considered that there is no mortality risk associated with HZ, an epidemiological study in the UK reported evidence of a low riskCitation40. Data from this study were therefore included in the sensitivity analysis ().
As noted above, a waning function was incorporated in the model to account for uncertainty around vaccine efficacy decline over the time. In the sensitivity analysis, this function was set at 8.3% per year, which has been reported to be the upper limit of the waning rate from the first month after vaccinationCitation41. The authors also conducted analyses assuming that the efficacy of the vaccine was limited to 10 years with an additional vaccine dose, or 20 years with no additional dose. The former analysis assumed that a repeat dose of the vaccine would be given after 10 years to 50% of patients who had received the vaccine and who were healthy and without HZ. This enabled the impact of coverage rates of 10–75% to be determined. In addition, the sensitivity analysis allowed vaccine prices to be determined (€100.38–160.38).
The effects of differing utility decrements were analysed using HZ-specific data from the SPSCitation19, and a study by Bala et al.Citation42. (). As the Bala et al. study provided data for only two pain states, mild and severe, the sensitivity analysis used an extrapolated value of 0.6 for moderate pain. A further analysis explored the impact of the methodology adopted for the calculation of utilities. As noted above, the base case applied an additive method whereby disease-specific decrements were subtracted from age-specific utilities. The additive method of QALY calculation was selected on equity grounds as it treats all individuals as equal, irrespective of their age whereas a multiplicative approach values people with higher age-specific utilities more than those with lower age-specific utilities. To evaluate the impact of the choice of methodology, the sensitivity analysis used the multiplicative technique in which the final utility value was obtained by multiplying age-specific utilities with disease-specific utilities.
As noted above, the analysis of the use and cost of healthcare resources used a 50% weighting to distinguish between HZ diagnoses made within and later than 72 hours after rash onset. The uncertainty surrounding the assumption that most patients contact their physician within this time was tested by applying weightings of 25% and 75% in sensitivity analyses. Further analyses explored the impact of 0%, 3% and 5% discount rates for costs and benefits.
Probabilistic sensitivity analyses were carried out using Monte Carlo simulations in Microsoft Excel 2003. The model was run 1000 times using inputs randomly selected from their statistical distributions. In these analyses, vaccine efficacy was tested by varying the mean efficacy value of the base case using a beta distribution, and by applying a waning function ranging between 0% and 8.3% using a uniform distribution. HZ incidence and PHN distribution were also varied over a beta distribution. Resource use mean costs were varied across known maximum and minimum values by means of a triangular distribution, whereas utility decrements were tested by a log-normal distribution.
Results
Base-case results
The base-case analysis examined the cost-effectiveness of vaccination in members of the Belgian population aged 60 years and older from societal, healthcare, and TPP perspectives. Subgroup analyses included individuals aged ≥50 or ≥65 years, and those aged 60–64, 65–69 and 60–69 years. Discount rates of 3% for costs, and 1.5% for utilities, were applied in the base-case analysis.
The model estimated that vaccination against HZ in individuals aged 60 years and older would cost between €51,640,533 (TPP perspective) and €54,439,238 (healthcare perspective), and would prevent 39,480 cases of HZ, and 13,121 (1-month definition) or 13,442 (3-month definition) cases of PHN, resulting in a gain of 7,595 QALYs (assuming 20% coverage and a lifetime duration of efficacy). ICERs showed that vaccination was a cost-effective strategy from all three perspectives (): the cost per QALY was €6,799 from a TPP perspective, €7,168 from a healthcare perspective, and €7,137 from a societal perspective. The NNV to prevent one case of disease in this population was 12 for HZ, 36 for PHN defined using the 1-month criterion, and 35 for PHN defined using the 3-month criterion.
Table 4. Base-case analysis: lifetime cost-effectiveness in individuals aged 60 years and older.
Cost-effectiveness results for the subgroup analyses are summarized in . Vaccination was consistently found to be a cost-effective strategy for the prevention of HZ and PHN, with ICERs below the unofficial but widely accepted threshold of €30,000, and NNVs of 8–14 for HZ and 27–39 for PHN.
Table 5. Subgroup analyses: lifetime cost-effectiveness by age group.
Sensitivity analyses
The results of the deterministic sensitivity analyses are summarised in the tornado diagrams shown in , which present the cost per QALY associated with each intervention.
Figure 2. Tornado diagrams showing the outcome of deterministic sensitivity analyses from third party payer (a), healthcare (b) and societal (c) perspectives. The vertical line represents the base-case incremental cost-effectiveness ratio (ICER; cost per quality-adjusted life year [QALY]), as shown in . The bars represent the change in ICER resulting from alterations in the variable shown on the left of the figure. GPRD, General Practice Research Database; HZ, herpes zoster; PHN, post-herpetic neuralgia; SPS, Shingles Prevention Study.
![Figure 2. Tornado diagrams showing the outcome of deterministic sensitivity analyses from third party payer (a), healthcare (b) and societal (c) perspectives. The vertical line represents the base-case incremental cost-effectiveness ratio (ICER; cost per quality-adjusted life year [QALY]), as shown in Table 4. The bars represent the change in ICER resulting from alterations in the variable shown on the left of the figure. GPRD, General Practice Research Database; HZ, herpes zoster; PHN, post-herpetic neuralgia; SPS, Shingles Prevention Study.](/cms/asset/eb9a30e7-67ab-4689-8480-26ee6465163b/ijme_a_502854_f0002_b.jpg)
Epidemiology of HZ and PHN
Applying the lower HZ incidence rates and PHN proportion, and the lower proportion of women affected by HZ and PHN, reported by Opstelten et al.Citation7 resulted in an increase in the ICERs to €12,897, €13,721 and €13,669 from a TPP, healthcare and societal perspective, respectively. NNVs were also higher than in the base-case analysis (14 for HZ, and 69 and 67 for PHN defined using the 1-month and 3-month criteria, respectively). The use of HZ duration and pain split data from the GPRD had a marked impact on cost-effectiveness, mainly due to a reduction in the number of patients with moderate or severe pain. The resulting ICERs were €14,418 from a TPP perspective, €15,382 from a healthcare perspective, and €15,352 from a societal perspective.
Vaccine characteristics
The duration of vaccine efficacy had the greatest impact on cost-effectiveness results (). The use of a 20-year duration increased ICERs to €9,184 from a TPP perspective, €9,739 from a healthcare perspective, and €9,699 from a societal perspective. Use of the lower 95% limit of the confidence interval of vaccine efficacy reported in the SPSCitation19 increased ICERs to €7,741 (TPP perspective), €8,200 (healthcare perspective) and €8,170 (societal perspective). Conversely, use of the upper 95% limit decreased ICERs to €6,129, €6,433 and €6,401 from a TPP, healthcare and societal perspective, respectively. Inclusion of a repeat dose after 10 years, at an identical unit cost as the initial vaccine, resulted in lifetime ICERs of €13,511, €14,426 and €14,382 from a TPP, healthcare and societal perspective, respectively. Vaccine coverage rate had no effect on cost-effectiveness because outcomes and costs change proportionally at each coverage rate. Analysis of the impact of vaccine price on cost-effectiveness showed that each €10 increase in vaccine price would increase the cost per QALY by €613 from all three perspectives.
Utilities
The use of alternative utility values, derived from the study by Bala et al.Citation42, had minimal impact on the ICERs from all three perspectives (). Use of a multiplicative (rather than additive) method to calculate utilities resulted in a reduction in the number of QALYs gained from 7,595 to 5,416 (data not shown). As a result, ICERs increased to €9,535 (TPP perspective), €10,052 (healthcare perspective) and €10,009 (societal perspective).
Use of healthcare resources
Weighting resource use by 25% or 75% to allow for different proportions of diagnoses made within 72 hours of rash onset had little effect on ICERs ().
Discount rates
Applying a discount rate of 5% resulted in increased ICERs (), reflecting vaccination costs incurred during the first year that are not affected by discounting.
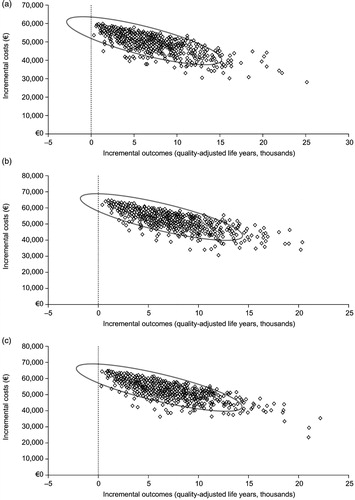
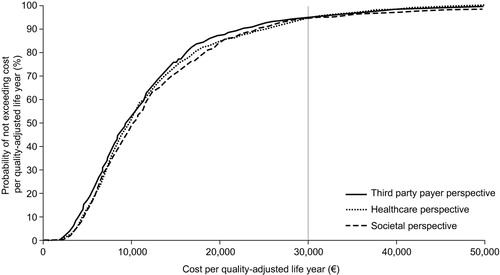
Probabilistic sensitivity analysis using Monte Carlo simulation methods showed that the cost-effectiveness of vaccination was maintained under various conditions. The mean (±SE) costs per QALY from TPP, healthcare and societal perspectives were €8,057 ± 324, €8,624 ± 347 and €8,853 ± 408, respectively. The higher ICERs obtained in these analyses, compared with the base-case analysis, are largely attributable to the inclusion of variations in vaccine efficacy rates and the HZ or PHN resource utilisation costs. As the maximum value for these variables was skewed far from the mean, the probability of a higher monthly cost of HZ or PHN was more likely. Thus the benefits of a vaccination that prevented these high costs associated with the disease were more pronounced. Scatter plots of the Monte Carlo simulations on the cost-effectiveness plane are shown in . A cost-effectiveness acceptability curve derived from these analyses () shows that the probability of not exceeding the unofficial Belgian cost-effectiveness threshold of €30,000 is 94.5% from a TPP perspective, 94.3% from a healthcare perspective, and 94.0% from a societal perspective.
Discussion
The results of this study indicate that vaccinating members of the Belgian population aged 60 years and older against HZ is likely to yield substantial benefits in terms of the number of cases of HZ and PHN prevented, and that this strategy is likely to be cost-effective. The costs per QALY gained were considerably lower than the threshold of €30,000 which is widely accepted to indicate cost-effectivenessCitation39, and the model was robust to changes in key epidemiological and vaccine-related variables. According to this model, it would be necessary to vaccinate only 12 people to prevent one case of HZ, whereas vaccination of 36 or 35 people would prevent one case of PHN defined according to 1-month or 3-month criteria, respectively.
The model has several strengths that support its validity as a measure of the clinical and economic benefits of HZ vaccination. It combines efficacy data from a pivotal clinical trialCitation19 with country-specific epidemiological data, and takes into account the ageing of the population over the duration of the model, allowing for mortality, likelihood of developing HZ or PHN, and vaccine efficacy. It also incorporates a high level of detail, taking into account the duration of HZ and PHN, and differing levels of pain severity for both conditions. This allowed HZ, PHN, and their treatment to be modelled accurately. The direct effect of vaccination on the incidence of HZ and PHN, and the indirect effect whereby the frequency of PHN decreases as a result of the reduced number of HZ cases, are included in the model.
A further strength of the model is that it was subjected to comprehensive and robust univariate sensitivity analysis in which ICERs consistently remained below the €30,000 threshold for cost-effectiveness. It is noteworthy that cost-effectiveness was maintained when utilities were calculated by either additive or multiplicative techniques. The additive method of calculating QALYs was chosen for the base-case analysis because it treats all members of the population as equal, irrespective of their age. The multiplicative approach would value people with higher age-specific utilities more highly than those with lower age-specific utilities (i.e., the elderly). The sensitivity analysis showed that vaccination remained cost-effective from the healthcare and societal perspectives if the multiplicative approach was used. The validity of these findings is further supported by the probabilistic sensitivity analysis, which showed that there was at least a 94% probability of the ICER remaining below the €30,000 threshold.
One limitation of this study was that, due to the lack of country-specific data for Belgium, the proportion of patients with HZ who developed PHN was derived from UK dataCitation29. This proportion is a major driver of the model results, and having country-specific data would clearly have been an advantage.
Another limitation was that the model did not take into account the effect of the vaccine in reducing pain in individuals who develop HZ despite vaccination. In the SPS, such patients reported a 22% reduction in HZ painCitation19, which may translate into a subsequent reduction in PHN. However, vaccine effects on PHN were included indirectly in the model by shortening PHN duration. A related issue is that the model used the pain split reported in the SPSCitation19 rather than that used in the GPRDCitation29, and sensitivity analysis showed that this had a marked impact on the observed ICERs. Nevertheless, pain assessments in the SPS are probably more reliable than those in the GPRD because the former measured pain by means of the Zoster Brief Pain Inventory (a specific tool for assessing zoster-related pain)Citation43, whereas the latter assessed pain retrospectively based on the level of analgesic drugs prescribedCitation29.
A further limitation of the study is that resource use and cost data were derived from a burden of disease studyCitation26 that was based on expert opinion, rather than population data. As a result, the predictive strength of the model and the generalisability of its results could be limited.
The utility weights for severe pain in the studies by Oster et al.Citation18 and Bala et al.Citation42 differ markedly (0.25 vs. 0.45, respectively). The weights used by Oster et al. were preferred for the base-case analysis because these included a specific weight for moderate pain, whereas those of Bala et al. covered only mild and severe pain. Moreover, Bala et al. assessed HZ pain, which is of shorter duration than PHN, and may therefore have a smaller impact on quality of life. Support for the use of the utility weights of Oster et al. comes from the finding that similar values were reported in a study of neuropathic pain in patients from five European countriesCitation36.
The results of the present analysis are comparable with those of a recent evaluation of the cost-effectiveness of HZ vaccination in England and WalesCitation44. This study reported an ICER of £20,400 (€22,843) per QALY gained, which is markedly higher than that obtained in the base-case analysis of the present study. However, there were several important differences between the two models. The model used in the UK study included clinically relevant pain, which incorporated moderate and severe pain, and assumed a limited duration of vaccine efficacy by incorporating a waning rate. The two models also differed in input variables such as HZ incidence rates, disease-specific utilities, and vaccine price. Direct comparison of the base-case results of the two analyses is not possible, but sensitivity analysis applying the maximum vaccine protection duration (100 years) for the cohorts aged 60, 65, 70, and 75 years in the UK study resulted in ICERs ranging between £5,660 and £9,891 (€6,017–10,515†) per QALY gained, which are of a similar order of magnitude to those obtained in the present study. In the Recommendations of the Advisory Committee on Immunization Practices for the Prevention of Herpes Zoster published in 2008 by Harpaz et al.Citation6, the cost-effectiveness of such a program has been discussed. The estimated cost per QALY ratios for a zoster vaccination program for people aged 60 and over was presented acceptable in comparison to established interventions or standard thresholds, ranging from US$27,000 to 112,000/QALY, under a societal perspective and at a $150 per dose. Two main limitations in this comparison are to be addressed such as the fact that models and most of data used to set-up this economic review were non-European and that vaccine efficacy against PHN was not always taken into account in the models, making any robust conclusion difficult.
Conclusion
Results from the present study suggest that vaccination against HZ and PHN in the Belgian population aged 60 years and older is cost-effective. This is an important finding because as the general population ages the number of cases of HZ and PHN may be expected to riseCitation45. HZ vaccination would be expected to offer important public health benefits in such an ageing population by preventing the deterioration in quality of life due to HZ and PHN in otherwise healthy individuals. Further clinical data are needed to determine the duration of vaccine efficacy and the potential impact of this on the cost-effectiveness of vaccination. Currently available data support the hypothesis that the duration of efficacy is lifelong. Indeed, sustainable vaccine efficacy has been demonstrated for at least 7 years with a single doseCitation33; this length of follow-up is one of the longest reported for a vaccine at European launch and an efficacy follow-up study remains ongoing. The deterministic sensitivity analysis in the present study provided an initial estimation of long-term cost-effectiveness by assessing the impact of a shorter duration of efficacy (10 years with a repeat dose, or 20 years). A budget impact analysis of a vaccination for the prevention of HZ and PHN in older adults will be required.
Transparency
Declaration of funding
This study was funded by Sanofi Pasteur MSD, France.
Declaration of financial/other relationships
L.A. has disclosed that he received grants from Sanofi Pasteur to conduct this study. X.B. and C.G. have disclosed that they are employees of Sanofi Pasteur. M.P. has disclosed that he is an employee of i3 Innovus, a company who was received funding to conduct this study.
Acknowledgements
The authors gratefully acknowledge the contribution of M. Lamotte and K. Caekelbergh from IMS Health, Belgium, and Communigen Limited, Oxford, UK for their assistance in preparing the manuscript.
Notes
*Zostavax is a registered trademark of Sanofi Pasteur MSD, Lyon, France.
†Calculated using the exchange rate in force on 15 November 2009 (£1 = €1.11974).
References
- Miller E, Marshall R, Vurdien JE. Epidemiology, outcome and control of varicella-zoster virus infection. Rev Med Microbiol 1993;4:222-230
- Cunningham AL, Dworkin RH. The management of post-herpetic neuralgia. Br Med J 2000;321:778-779
- Brisson M, Edmunds WJ, Law B, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect 2001;127:305-314
- Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc 2007;82:1341-1349
- Johnson R, McElhaney J, Pedalino B, et al. Prevention of herpes zoster and its painful and debilitating complications. Int J Infect Dis 2007;11:S43-48
- Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster - Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2008;57:1-40
- Opstelten W, Mauritz JW, de Wit NJ, et al. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract 2002;19:471-475
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002;18:350-354
- Johnson RW, Wasner G, Saddier P, et al. Postherpetic neuralgia: epidemiology, pathophysiology and management. Expert Rev Neurother 2007;7:1581-1595
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract 1975;25:571-575
- Schmader K, Gnann JW Jr, Watson CP. The epidemiological, clinical, and pathological rationale for the herpes zoster vaccine. J Infect Dis 2008;197:S207-215
- Schmader K. Herpes zoster in the elderly: issues related to geriatrics. Clin Infect Dis 1999;28:736-739
- Volpi A, Gatti A, Pica F, et al. Clinical and psychosocial correlates of post-herpetic neuralgia. J Med Virol 2008;80:1646-1652
- Goh CL, Khoo L. A retrospective study of the clinical presentation and outcome of herpes zoster in a tertiary dermatology outpatient referral clinic. Int J Dermatol 1997;36:667-672
- Chidiac C, Bruxelle J, Daures JP, et al. Characteristics of patients with herpes zoster on presentation to practitioners in France. Clin Infect Dis 2001;33:62-69
- Schmitt S, Weinke T, Edte A, et al. Herpes zoster and post-herpetic neuralgia seriously impact patients' quality of daily life. 19th IAGG World Congress of Gerontology and Geriatrics (IAGG). Paris, France, July 5-9, 2009 (Abstract)
- Katz J, Cooper EM, Walther RR, et al. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004;39:342-348
- Oster G, Harding G, Dukes E, et al. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005;6:356-363
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005;352:2271-2284
- Centers for Disease Control and Prevention. Prevention of Herpes Zoster. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2008;57
- Advisory Committee on Immunization Practices (ACIP). Recommended Adult Immunization Schedule – United States, October 2007-September 2008. 2010
- Austrian Superior Sanitary Council, Vaccination Committee March 2nd, 2010. http://www.bmg.gv.at/cms/site/attachments/1/4/0/CH0780/CMS1038913010412/impfplan_2010_korr_maerz.pdf
- Cleemput I, van Wilder P, Vrijens F. Guidelines for pharmacoeconomic evaluations in Belgium. Brussels: KCE reports 78 C, 2008
- Weinstein MC, O'Brien B, Hornberger J. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices – Modeling Studies. Value Health 2003;6:9-17
- Nagasako EM, Johnson RW, Griffin DR, et al. Rash severity in herpes zoster: Correlates and relationship to postherpetic neuralgia. J Am Acad Dermatol 2002;46:834-839
- Caekelbergh K, Lamotte M, Muchada JP. Management of herpes zoster and post-herpetic neuralgia in Belgium: a cost of illness study. 11th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Athens, Greece (Abstract)
- Sabbe M, Van Casteren V. Netwerk van Huisartsenpeilpraktijken, Wetenschappelijk Instituut voor Volksgezondheid, 2008. Persoonlijke mededeling, 2008
- Sentinel General Practitioners - Scientific Institute of Public Health of Belgium. http://www.iph.fgov.be/epidemio/epien/index10.htm. 2010
- Gauthier A, Breuer J, Carrington D, et al. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2008;137:38-47
- Statistics Belgium. (2008). Belgian population data 01/01/2006. 2010
- Gilderman LI, Lawless JF, Nolen TM, et al. A double-blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator-stable formulation of Zostavax®. Clin Vaccine Immunol 2008;15:314-319
- Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of Zostavax and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc 2007;55:1499-1507
- Schmader K, Oxman MN, Levin M. Persistence of zoster vaccine efficacy. Poster presented at the 48th annual ICAAC/IDSA 46th annual meeting, Washington DC, USA, 25-28 October. Poster number G-409 (Abstract)
- Szende A, Williams A. Measuring self-reported population health: an international perspective based on EQ-5D. Budapest, Hungary: SpringMed Publishing Limited, 2004
- Cleemput I, Kind P, Kesteloot K. Rescaling social preference data: implications for modelling. In: Kind P, Macran S, eds. 19th Plenary Meeting of the EuroQol Group Discussion Papers York: Centre for Health Economics, University of York, 2004:13-123
- McDermott AM, Toelle TR, Rowbotham DJ. The burden of neuropathic pain: results from a cross-sectional survey. Eur J Pain 2006;10:127-135
- Statistics Belgium (2008). Employment rates 2007/2004. 2008
- SECUREX Social Management. (2004). Het Absenteisme in Belgie 2004. Kosten, benchmarks, medische redenen en personeelstevredenheid, 2004
- Rodriguez MJ, Diaz S, Vera-Llonch M. Cost-effectiveness analysis of pregabalin versus gabapentin in the management of neuropathic pain due to diabetic polyneuropathy or post-herpetic neuralgia. Curr Med Res Opin 2007;23:2585-2596
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001;19:3076-3090
- Pellissier JM, Brisson M, Levin MJ. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007;25:8326-8337
- Bala MV, Wood LL, Zarkin GA. Valuing outcomes in health care: a comparison of willingness to pay and quality-adjusted life-years. J Clin Epidemiol 1998;51:667-676
- Coplan PM, Schmader K, Nikas A, et al. Development of a measure of the burden of pain due to herpes zoster and postherpetic neuralgia for prevention trials: adaptation of the brief pain inventory. J Pain 2004;5:344-356
- van Hoek AJ, Gay N, Melegaro A, et al. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009;27:1454-1467
- Dworkin RH, Gnann JW Jr, Oaklander AL, et al. Diagnosis and assessment of pain associated with herpes zoster and postherpetic neuralgia. J Pain 2008;9:S37-44