Abstract
Background:
Pseudomonas aeruginosa (PA) is the most common airway pathogen in cystic fibrosis (CF) patients. The objective of this analysis was to determine the costs of managing PA infection in CF patients with a chronic regimen of tobramycin inhalation solution (TIS).
Methods:
A budget impact model of CF patients was developed to evaluate the costs of TIS from a US managed-care organization (MCO) perspective. The Microsoft Excel model compared TIS treatment plus standard care with standard care alone over a 4-year time horizon and included the cost of drugs, medical care, and annual probabilities of hospitalization and IV anti-pseudomonal (anti-PA) antibiotics administration.
Results:
For an MCO with 5,000,000 members, 389 members 6 years of age or older were estimated to have CF, and 218 (56%) had PA infection. Assuming that use of TIS increased from 20% to 25%, the 1-year budget increased $231,251 or from $0.049 to $0.053 per member per month (PMPM). The net drug budget increase was $243,919, while medical costs associated with exacerbation management decreased $12,669 over the first year. Increasing utilization of TIS, from 20% to 40% over 4 years resulted in an incremental overall budget increase of $925,002, a 3% decrease in hospitalizations, and a 4% decrease in administrations of IV anti-PA antibiotics. These reductions translated to a medical care cost saving of $50,676 over 4 years. Limitations of this study include that the clinical data for the model are from clinical trials conducted in 1996 and the estimation of TIS use for CF patients with chronic PA infections can be impacted by TIS adherence.
Conclusion:
Model results suggest that increasing the use of TIS decreases medical care costs due to decreased hospital admissions and the use of IV anti-PA antibiotics at the expense of higher drug costs.
Introduction
Cystic fibrosis (CF), one of the most prevalent lethal hereditary diseases among Caucasians, affects approximately 30,000 individuals in the United States (US) and 70,000 individuals worldwideCitation1. CF is a complex disease, involving multiple organs, but the leading cause of mortality in CF patients is chronic lung disease. CF lung disease presents as progressive, recurrent episodes of respiratory symptoms (e.g., coughing, sputum overproduction, permanent airway obstruction, and bronchiectasis) and eventually respiratory failureCitation2. Together, these signs and symptoms are collectively termed pulmonary exacerbations and account for more than 90% of the morbidity in CF patientsCitation3–5.
Pulmonary exacerbations in CF patients are primarily caused by chronic infection with Pseudomonas aeruginosa (PA) and the resulting inflammatory response. While this association has not been explicitly demonstrated, this hypothesis is generally accepted based on the prevalence of PA in this patient populationCitation6 and the decrease of PA concentration in sputum samples following antibiotic treatment for pulmonary exacerbationsCitation7,Citation8. The 2008 Cystic Fibrosis Foundation Patient Registry estimates that approximately 30% of CF patients aged 6–10 years had respiratory infections due to PA; PA prevalence increased to 50% for aged 11–17 years, 68% for 18–24 years, and 80% for 25 and olderCitation6. Similarly, the prevalence of pulmonary exacerbations resembles the trend observed in PA infectionsCitation6 – increasing with increasing age – 23% for patients ages <6 years to 63% for patients ages ≥18 yearsCitation9. In addition, newly acquired PA infection was associated with a pulmonary exacerbation odds ratio of 3.7 (95% CI: 1.4–4.7) for patients aged 6–12 years and 3.2 (95% CI: 1.0–10.1) for patients aged ≥18 yearsCitation9.
Only a limited number of studies have been conducted to estimate the economic impact of CF. A 1996 study of inpatient and outpatient clinic and medication costs estimated $13,300 (1996, US dollars [US$]) per patient costs in the USCitation10. A more recent US study examined CF healthcare expenditures among privately insured individuals and found that annual direct costs for an actively treated CF patient were $48,098, which is 22 times higher than an individual without CFCitation11. These findings highlight the economic burden of CF.
Current Cystic Fibrosis Foundation pulmonary guidelines (2007) strongly recommend the chronic use of inhaled tobramycin to improve lung function and reduce exacerbations for CF patients aged 6 years and older who have moderate-to-severe lung disease with PA persistently present in cultures of the airwaysCitation12.
Amidst increasing healthcare costs, assessing the budget impact of changes in therapies is an important consideration for health plan decision makers. The budget impact model (BIM) provides quantitative methods to estimate the financial consequences on the health plan budget of adoption and increased uptake of therapiesCitation13. Although tobramycin inhalation solution (TIS) has been on health plan formularies for more than a decade, no studies have examined the budget impact of its use for treating chronic PA infection in CF patients. A BIM is presented to compare the costs and resource utilization associated with increasing TIS use for the treatment of chronic PA infection in CF patients ≥6 years of age, over a 4-year time horizon.
Methods
Model overview
The TIS BIM is a simple, population-based Microsoft Excel model designed to simulate the annual budgetary impact of adding or increasing the use of TIS for chronic PA infection. The model uses Ramsey et al. 1999 trial dataCitation14 to estimate the rate of hospital admissions and antibiotic infusions required to manage pulmonary exacerbations and assumptions developed with key opinion leaders (KOL). Two practicing CF clinicians provided clinical expert opinion and guidance on the CF treatment patterns and model assumptions. Model outcomes included incremental per member per month (PMPM), per treated patient per month (PTMPM), and annual costs of TIS plus standard therapy compared to standard therapy alone over a 1–4-year period from a US managed-care perspective (direct costs only, no indirect costs included).
The population was estimated from 2006 US census population estimatesCitation15 and prevalence estimates of CF and PA infection among CF patients aged ≥6 years were obtained from the Cystic Fibrosis Foundation Patient Registry Annual Data Report and Razvi et al. 2009, respectively ()Citation1,Citation16.
TIS trial data
The nebulized tobramycin trials were two identically designed, randomized, parallel-group, double-blind, placebo-controlled, phase III 24-week clinical studies conducted in 69 cystic fibrosis centers in the USCitation14. A total of 520 patients (mean age 21) was randomized to receive either 300 mg of TIS and standard of care or placebo and standard of care, twice daily in three 28-day cycles (study drug was administered for 28 days and then suspended for 28 days). For standard of care, patients were allowed to use their routine medication for the management of CF, with the exception of other inhaled antibiotics. In addition, use of dornase alfa or a pneumatic vest was permitted if used 4 weeks prior to the start of the study and maintained throughout the study period. TIS was administered using the Pari LC Plus reusable nebulizer and DeVilbiss Pulmo-Aide air compressor. All patients had confirmed PA infections and baseline FEV1 between 25% and 75%. The primary outcome of both trials was lung function (FEV1) and density of PA in sputum at week 20. Secondary outcomes included hospitalization and treatment with IV anti-PA antibiotics. Safety was monitored during the trials by clinical observations, auditory testing, and laboratory testing of blood and urine samples.
Baseline characteristics and results of the pivotal nebulized tobramycin trials are presented in . Baseline characteristics of the two treatment groups were similar. Approximately 49.6% of the pooled study population was assigned to TIS, and 50.4% were assigned placebo. At week 20, an average FEV1 increase of 10% was observed in the TIS group, and a decrease of 2% was observed in the placebo group (p < 0.001). Of patients receiving TIS, 39% (100 of 258) received one or more courses of IV anti-PA antibiotics, and 37% (95 of 258) were hospitalized at least once. Of placebo patients, 52% (135 of 262) received one or more courses of IV anti-PA antibiotics, and 45% (117 of 262) were hospitalized at least once. Patients in the TIS group were 26% (95% CI: 2–43%) less likely to be hospitalized and 36% (95% CI: 17–51%) less likely to be treated with IV anti-PA antibiotics treatments than patients receiving placebo. These data are used to calculate the annual rates of hospital admissions and use of IV antibiotics.
Table 1. Baseline characteristics, secondary outcomes, and derived annual probabilities (From Ramsey et al. 1999Citation14).
Model assumptions
A number of assumptions were made in constructing the model. First, the model is prevalence-based and assumes the proportion of CF patients seeking treatment remains constant for the duration of the model. The model assumes that 14 days of IV anti-PA antibiotics (a combination of two antibiotics) are required in the event of an acute pulmonary exacerbation per published guidelinesCitation17 and they are administered concurrently (as opposed to serially). The current utilization of TIS is assumed to be approximately 20% based upon an estimate from the manufacturer of the number of patients treated for the recommended cycle duration over a year’s time. Lastly, no patient cost sharing is assumed.
Resource use estimates
The resource use for pulmonary exacerbations was obtained from the literature (). The model included: (1) chronic suppressive treatment drug costs, (2) acute drug costs, and (3) annual medical costs. Chronic suppressive treatment drug costs included drug acquisition costs and durable medical equipment (DME) costs. Drug acquisition costs are calculated using wholesale acquisition costs (WAC) obtained from Red Book ()Citation18. Based on the TIS package insert and agreement with the clinical trial dosing, a treatment cycle is a 300-mg dose of TIS twice a day for 28 days followed by 28 days of no treatment with an estimated 6.5 cycles per yearCitation19.
Table 2. Model inputs and sources for use of tobramycin inhaled solution in cystic fibrosis.
The costs of each type of DME were determined by the standard Medicare reimbursement for the Healthcare Common Procedure Coding System (HCPCS) codeCitation20. The amount of equipment required for 1 year of TIS therapy () was based on manufacturer recommendationsCitation21,Citation22.
The combination regimen of IV anti-PA antibiotics included ceftazidime and tobramycinCitation17. The dose assumed is 2 grams (g) of IV ceftazidime given every 8 hours for 14 days and 3 mg/kg of IV tobramycin given every 8 hours for 14 daysCitation23,Citation24.
Costs associated with home health visitsCitation25 and lab monitoringCitation26 were included as other related costs. Annual hospitalization costs were based on the average hospitalization cost per CF patient as reported by Lieu et al. 1999, inflated to 2008 US$ using the US Consumer Price IndexCitation10.
Sensitivity analysis
One-way sensitivity analyses (SA) were conducted using a relative change of ±30% from base-case values with the exception of probability of hospitalization and use of IV anti-PA antibiotics where the 95% confidence intervals were used.
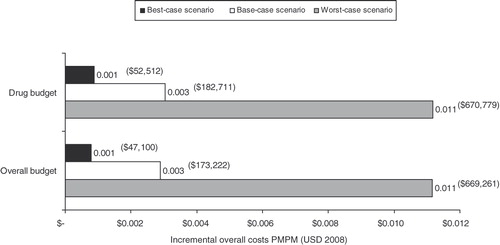
Two scenario analyses were conducted to evaluate the worst-case (where the incremental BIM and annul budget are highest) and best-case (where the incremental BIM and annual budget are lowest). In these analyses, the model parameters were simultaneously varied from base-case values presented in . For the worst-case scenario analysis, the cost of TIS, prevalence of CF and prevalence of PA infection, costs of hospitalization and home infusions per patient were increased 40%, the probability of hospitalization with TIS increased 14% and probability of home IV infusion increased 20%. For the best-case scenario analysis, the above parameter values were decreased by the listed amounts.
Results
Base-case analysis
For a health plan population of 5 million enrollees, the model estimated that approximately 389 patients would have CF, and of these, 218 CF patients would be chronically infected with PA (). The estimated incremental annual per-patient drug and related DME costs for TIS treatment were $22,481. The estimated medical and drug costs to treat each acute pulmonary exacerbation were $2,261 (including antibiotic costs, duration of treatment, laboratory diagnostics, and per diem rate for home infusion).
Based on a current TIS utilization of 20% within the CF eligible population, approximately 44 patients were treated with TIS therapy; an MCO with 5 million members would pay $2,923,103 ($0.049 PMPM) of which approximately 37% were drug costs and 63% were medical care costs relating to pulmonary exacerbations (). Increasing the proportion of the treated CF population from 20% to 25% over 1 year resulted in an approximate overall budget increase of $231,251, of which the drug budget increased 22% ($243,919) and medical costs decreased $12,669. The incremental overall impact on the members and treated members per month and per year are shown. Note that as drug expenditures increased, the number of hospital admissions and IV therapies for exacerbations decreased thus avoiding $12,669 in medical care costs ().
Table 3. Budget impact results of increasing use of tobramycin inhaled solution in cystic fibrosis patients.
After 4 years, the population of TIS-treated CF patients was assumed to increase to 87 patients and total TIS therapy costs increased accordingly – by 90% over 4 years, while the medical care budget decreased about 3%.
Sensitivity analysis
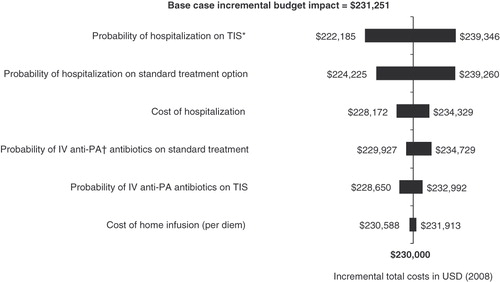
The one-way sensitivity analysis explored the impact of model assumption on the results. The parameters with the greatest impact on the overall costs are illustrated in the tornado diagram (). The most sensitive variables excluding the prevalence of CF were the probability of hospitalization with either therapy. The costs of managing acute pulmonary exacerbations had relatively small budget impacts. For example, varying the cost of hospitalization by ±30% resulted in a 1.3% change in the incremental budget impact.
Figure 1. One-way sensitivity analysis of incremental budget impact of tobramycin inhaled solution comparing current formulary to year 1 for health plan of 5,000,000 members. *TIS, tobramycin inhaled solution; †IV anti-PA, IV anti-pseudomonal.
The results of the worst-case and best-case scenario analyses are illustrated in . The worst-case scenario resulted in incremental overall costs of $669,261 ($0.011 PMPM). In the best-case scenario, the incremental overall costs decreased $47,100 ($0.001 PMPM) as compared to the base-case results, due to decreasing the prevalent population and treatment costs.
Discussion
This study evaluated the budgetary impact of increasing the use of TIS as suppressive treatment of chronic PA infection for CF patients from a US MCO perspective. Budget impact analyses are used to estimate the financial consequences of alternative treatment strategies from the perspective of a health plan or payer. Approaches to budget impact analyses vary; they can evaluate solely drug costs (a formulary budget impact analysis) or include other direct costs, such as administration costs. In the present study, a more expansive approach to evaluate important treatment-related costs (i.e., hospitalization and home infusion costs of anti-PA antibiotics) was adopted.
The results of the study indicate that for a hypothetical health plan of 5 million members, the increased use of TIS suppressive treatment for chronic PA infection over 4 years can decrease hospital admissions and use of IV anti-PA antibiotics by approximately 2.6–3.6%. These improvements in patient outcomes are achieved at the expense of increased drug costs. The study analysis has shown that a 5% increase in TIS use has a nominal impact on the incremental drug budget ($0.004 increase in PMPM) – an average annual increase of $1,120 for CF patients.
The extreme scenario analyses showed notable differences between the incremental overall budget under the best-case scenario ($0.001 PMPM) and worst-case scenario ($0.011 PMPM) (). The likelihood of either scenario is uncertain and depends upon the number of CF patients managed within the MCO. The recent advent of newer inhaled antibiotics for chronic treatment of PA infection would suggest that the cost of TIS may decrease in the future as a more competitive market would result in lower drug costs for payers.
This is the first study to assess the budget impact of TIS suppressive treatment for chronic PA infection in CF patients. It provides a tool for evaluating the financial impact of adopting a new therapy; it answers the key question of affordability and should be used widely by decision makers. Secondly, this study was based on randomized clinical trials directly comparing standard of care with and without TIS for suppressive treatment for chronic PA infection in CF patients. Including trial-derived probabilities of hospitalization and IV anti-PA antibiotics use allowed for a more comprehensive analysis of the costs associated with PA infection in CF patients.
The objective of this model was to quantify the budget impact of increased utilization of TIS for a US MCO. Relative to other therapies, such as tegaserod for irritable bowel syndrome (PMPM of $0.01)Citation27 and sildenafil for erectile dysfunction ($0.18 PMPM)Citation28, the budget impact of TIS is nominal. The low PMPM cost for TIS is due in part to the relatively low prevalence of CF in the US. MCOs should consider the population characteristics of their membership when generalizing these budget impact results to their situation.
Limitations
This study has several limitations. The BIM was based on a 12-year old study (Ramsey et al. 1999)Citation14, since there have not been any other large US multicenter, double-blind, placebo-controlled trials reported, but this trial was the basis for guideline development by the Cystic Fibrosis Foundation Pulmonary Therapies CommitteeCitation12 and is the best data available. There were no direct comparators for TIS until the recent FDA approval of aerosolized form of aztreonam. This compound is only beginning to be used in MCOs, thus this analysis did not include it. The model can be adapted to incorporate information for new therapies as the information becomes available.
The resources and costs associated with the pulmonary exacerbations requiring hospital admission and home IV antibiotic therapy were based upon KOL opinion. To evaluate the role of variation in treatment-related costs, a one-way sensitivity analysis was conducted on the cost of hospitalization and the cost of home infusion. These parameters were found to have only a small impact on the model results.
The model assumed that all patients were fully compliant with prescribed medications and this assumption may not be valid for all CF patient age groups. Decreased compliance may increase the number of hospitalizations and use of IV anti-PA antibiotics while simultaneously decreasing drug costs and vice versa. Several studies of adherence to inhaled antibiotics among CF patients found that adherence was highly variable and subject to patient reporting biasCitation29,Citation30. Objective measurements of adherence (via prescription refill history, daily diaries, and/or medication monitoring devices) resulted in lower estimates than self-reportCitation30. For US CF patient populations, Modi et al. 2006 reported an adherence rate of 36% for inhaled antibiotics based on daily phone diary entries; in contrast, patient self-reports of adherence were as high as 85%Citation31.
In the context of this BIM, it seems reasonable to estimate adherence based on pharmacy refill date from insurance claim database. Briesacher et al. 2009 reported that among CF patients who used TIS over 1 year in a large administrative database from private insurers, 72% of them received ≤2 cycles, 22% received 3 cycles, and 6% received ≥4 cyclesCitation32. O’Sullivan et al. 2008 reported that among CF patients who used TIS over 1 year, 48% had one to two prescriptions, 33% had three to five prescriptions, and 19% had six or more prescriptionsCitation33. The targeted prescriptions (cycles) should be 6.5 per year for TISCitation19. Therefore, the average utilization of TIS is about two to three cycles (31–49% adherence rate) from claims data analysis. Based on these data, a 20% TIS utilization was chosen as a conservative estimate, which is a limitation of this approach. However, the BIM model itself allows health plans to input their specific assumptions of TIS utilization to assess the budget impact. Additionally, compounded tobramycin is used for inhalation as an alternative to TIS, which results in lower utilization of the TIS. Compounded tobramycin varies in strength and it cannot be considered to have the same outcomes as TIS; furthermore, there is no published evidence on the efficacy for compounded tobramycin as inhalation therapy for PA.
Conclusion
The results of this budget impact analysis suggest that increasing the use of suppressive treatment with TIS by 5% for CF patients with chronic PA infection in a 5 million MCO population will result in decreased hospital admissions and reduced use of IV anti-PA antibiotics, plus an overall MCO drug and medical budget increase of $231,251 (PMPM $0.004 and PTMPM $88 increases). Major drivers of costs were the number of patients treated. Given variability in health plan populations, MCOs should execute individual budget impact analyses using their own pharmacy and claims data and costs.
Transparency
Declaration of funding
This research was funded by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
T.C.W. and R.B. have disclosed that they are employed by United BioSource Corporation, a company that received funding from Novartis to conduct this study. P.S. and J.Z. have disclosed that they are employees of Novartis Pharmaceuticals Corporation.
References
- Cystic Fibrosis Foundation. About Cystic Fibrosis – What You Need to Know, 2008. http://www.cff.org/AboutCF/
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 2007;62:360-367
- Britto MT, Kotagal UR, Hornung RW, et al. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64-72
- Liou TG, Adler FR, Fitzsimmons SC, et al. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001;153:345-352
- Ramsey BW. Management of pulmonary disease in patients with cystic fibrosis. N Engl J Med 1996;335:179-188
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation: Patient Registry Annual Data Report 2006. Bethesda, MD
- Ordonez CL, Henig NR, Mayer-Hamblett N, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 2003;168:1471-1475
- Regelmann WE, Elliott GR, Warwick WJ, et al. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am Rev Respir Dis 1990;141:914-921
- Rabin HR, Butler SM, Wohl ME, et al. Pulmonary exacerbations in cystic fibrosis. Pediatr Pulmonol 2004;37:400-406
- Lieu TA, Ray GT, Farmer G, et al. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics 1999;103:e72
- Ouyang L, Grosse SD, Amendah DD, et al. Healthcare expenditures for privately insured people with cystic fibrosis. Pediatr Pulmonol 2009;44:989-996
- Flume PA, O'Sullivan BP, Robinson KA, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Am J Respir Crit Care Med 2007;176:957-969
- Mauskopf J, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices – budget impact analysis. Value Health 2007;10:336-347
- Ramsey BW, Pepe MS, Quan JM, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. N Engl J Med 1999;340:23-30
- Population Division US Census Bureau. Monthly estimates of the population by sex, race, and Hispanic origin for the United States: July 1, 2006. Available at: http://www.census.gov/popest/national/asrh/2007-nat-res.html
- Razvi S, Quittell L, Sewall A, et al. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 2009;136:1554-1560
- Driscoll JA, Brody SL, Kollef MH. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007;67:351-368
- Thomson PDR. Red Book for Windows. Greenwood Village, CO: Thomson, 2008
- Novartis. TOBI: Tobramycin Inhalation Solution, USP. Prescribing information, 2007
- Center for Medicare & Medicaid Services. HCPCS Alpha-Numeric Index, 2008. Available at: http://www.cms.hhs.gov/HCPCSReleaseCodeSets/Downloads/INDEX2008.pdf
- DeVilbiss Healthcare. Products: pulmonary drug delivery, 2008. Available at: http://www.devilbisshealthcare.com
- PARI Respiratory Equipment Inc. Products: the lower airway, the upper airway, 2008. Available at: http://www.pari.com/home/pari-products.htm
- American Pharmaceutical Partners Inc. Tobramycin. Prescribing information
- American Society of Health-System Pharmacists. Ceftazidime (Systemic), 2008. Available at: http://www.ashp.org/s_ashp/docs/files/practice_and_policy/ceftazidime.pdf
- Tice AD, Hoaglund PA, Nolet B, et al. Cost perspectives for outpatient intravenous antimicrobial therapy. Pharmacotherapy 2002;22:63-70S
- MAG Mutual. Physician's Fee and Coding Guide 2008, 19th edn. Atlanta, GA: MAG Mutual Healthcare Solutions, Inc., 2007
- Bloom MA, Barghout V, Kahler KH, et al. Budget impact of tegaserod on a managed care organization formulary. Am J Manag Care 2005;11:S27-34
- Cooke CE, Wong W, Lee H. Utilization and cost of sildenafil in a large managed care organization with a quantity limit on sildenafil. J Manag Care Pharm 2005;11:674-680
- Velasco MV ZI, Gonzalez A, et al. Inhaled antibiotics treatment adherence in cystic fibrosis. Paper presented at: American Thoracic Society International Conference, 2002
- Weiner JR, Toy EL, Sacco P, et al. Costs, quality of life and treatment compliance associated with antibiotic therapies in patients with cystic fibrosis: a review of the literature. Expert Opin Pharmacother 2008;9:751-766
- Modi AC, Lim CS, Yu N, et al. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros 2006;5:177-185
- Briesacher BA, Saiman L, Fouayzi H, et al. Adherence to tobramycin inhaled solution and health care utilization. Am J Respir Crit Care Med 2009;179:A1183
- O’Sullivan A, Sullivan JK, Higuchi K. Medication compliance and impact on health-care resource utilization and mortality among cystic fibrosis patients with P. aeruginosa. Pediatr Pulmonol 2008;S31:473-484
- Centers for Medicare & Medicaid Services. HCPCS Alpha-Numeric Index, 2008. Available at: http://www.cms.hhs.gov/HCPCSReleaseCodeSets/Downloads/INDEX2008.pdf