Abstract
Objective:
To compare healthcare resource utilization and costs of postherpetic neuralgia (PHN) patients initiating lidocaine patch 5% (lidocaine patch) or oral gabapentin/pregabalin.
Methods:
Patients with PHN diagnosis, or herpes zoster diagnosis and ≥30 days PHN-recommended treatment were selected from de-identified Medicaid claims data from Florida, Iowa, Missouri, and New Jersey, 1999–2007. Patients initiated monotherapy with lidocaine patch or gabapentin/pregabalin after PHN diagnosis, had continuous eligibility 6 months before (baseline) and 6 months after (study period) medication index date, and were ≥18 years old. Lidocaine patch patients were matched to gabapentin/pregabalin patients based on their propensity to initiate treatment. Study period resource utilization and costs from a Medicaid perspective were compared between treatment groups using univariate analysis.
Results:
Matched patients were on average 61.3 years old, approximately 73% were women, and 55% had other painful conditions during the baseline period. 6-month per patient PHN-related prescription drug costs were similar for matched lidocaine patch (n = 312) and gabapentin/pregabalin (n = 312) patients ($854 vs. 820, p = 0.75), while PHN-related medical costs appeared lower in the lidocaine patch group ($145 vs. 353, p = 0.12). Furthermore, there were no statistically significant differences between treatment groups during the observation period in overall resource utilization, total prescription drug costs, and total medical costs per patient.
Conclusions:
In spite of higher list prices, PHN patients treated with lidocaine patch cost no more than patients treated with gabapentin or pregabalin in terms of overall healthcare costs over the 6-month study period. The study suggests that PHN-related medical costs may be lower among lidocaine patch patients.
Limitations:
Findings are based on a Medicaid sample and may not be generalizable to all PHN patients.
Introduction
This is the second of two papers examining Medicaid patients with postherpetic neuralgia (PHN). The first paper provided a descriptive analysis of Medicaid patients with PHN, to better understand patient characteristics, and, more specifically, the characteristics of patients treated with lidocaine patch 5%, a leading topical treatment for PHN. This paper takes the analysis a step further, conducting an economic analysis of patients treated with lidocaine patch 5% compared with similar patients treated with oral gabapentin or pregabalin
Background
Postherpetic neuralgia (PHN) is a painful condition which affects approximately 10–20% of herpes zoster (HZ) patientsCitation1,Citation2. Age is a leading risk factor, with PHN 15 times more likely in patients over age 50Citation2,Citation3. Effective management of PHN can be complicated and costly, with total annualized costs associated with PHN patients estimated at $10,054 for commercially insured patients, and $17,972 for Medicaid patients (2001–2003)Citation4.
Lidocaine patch 5% (lidocaine patch), gabapentin and pregabalin are three of the leading therapies indicated for treating PHNCitation1. The authors’ observations of published drug price lists and the recommended doses as outlined in the FDA-approved labelsCitation5–8 suggest that daily treatment of PHN with the lidocaine patch may cost more than its systemic comparators (branded or generic). However, it is unclear whether PHN patients treated with the lidocaine patch incur higher healthcare costs from a payer perspective over the course of their treatment, compared with gabapentin and pregabalin. In terms of cost-effectiveness, previous studies have demonstrated that the lidocaine patch compares favorably with gabapentin and pregabalin in treating PHN patients in EuropeCitation9,Citation10.
No study, however, has compared the economic costs associated with the lidocaine patch vs. gabapentin and pregabalin among US patients with PHN, and more specifically among the Medicaid population. The age profile of PHN patients suggests that analysis of an older patient population is of clinical and policy relevance. Analysis of Medicaid data offers two main advantages. While the main source of insurance for the elderly population in the US is Medicare, close to 9 million Americans participated in both Medicare and Medicaid in 2005 (‘dual eligibles’)Citation11, indicating that Medicaid data provide substantial coverage of the target population. Moreover, Medicaid coverage includes prescription drug benefits, which were not covered by Medicare prior to 2006. Therefore the data offer a more comprehensive perspective on costs, as prescription drug treatment is observable in Medicaid claims.
The objective of this study was to compare health-resource utilization and direct (medical and prescription) costs of two comparable, mutually exclusive, cohorts of PHN Medicaid patients: those treated with the lidocaine patch, and those treated with gabapentin and/or pregabalin. As the analysis was based on observed utilization and actual Medicaid payment data, it can inform Medicaid policy makers regarding the costs associated with treating PHN patients.
Methods
Data source
The study sample was selected from Medicaid claims databases in four states (Florida, Iowa, Missouri and New Jersey), covering approximately 10 million lives (1999–2007). The data contain de-identified information on patients’ demographics (e.g., age, gender), monthly enrollment history, and medical and pharmacy claims. Medical services use was recorded with dates of service, associated diagnoses (up to nine codes, using the International Statistical Classification of Diseases and Related Health Problems, ICD-9-CM), performed procedures (Current Procedural Terminology, CPT), and actual amounts paid to providers. The database also includes pharmacy claims with prescribed medications identified by National Drug Code (NDC), date of prescription fill, days of supply, quantity, and actual Medicaid payments.
Sample selection
Identification of PHN patients
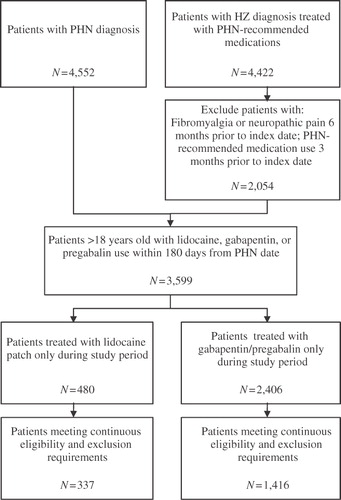
Patients were identified as having PHN if they met one of the following criteria: (1) had ≥1 medical claim with a PHN diagnosis (ICD-9-CM: 053.12, 053.13 or 053.19, n = 4,552); or (2) had ≥1 medical claim with a HZ diagnosis (ICD-9-CM: 053.0 x, 053.10, 053.11, 053.2 x, 053.7 x, 053.8 x, or 053.9 x) and ≥30 days of treatment with a PHN-recommended medication beginning within 30 days of HZ diagnosis (‘PHN-likely patients’, n = 4,422). Gabapentin, pregabalin, lidocaine patch, capsaicin, duloxetine and tricyclic antidepressants were considered PHN-recommended medicationsCitation12. Given the established clinical relationship between HZ and PHNCitation13, we followed a similar approach as Dworkin et al. and assumed that patients diagnosed with HZ and soon-thereafter treated with PHN-recommended medication were, with reasonable probability, PHN patientsCitation4,Citation14. Unlike previous studies, we include only those patients who initiated treatment with PHN-recommended medication, rather than any form of analgesic medication. Given the nature of retrospective claims data, and the clinical association of HZ and PHN, this approach balances careful sample selection and real world representativeness. Note, however, that excluding these patients does not qualitatively alter the reported results, though the decline in sample size reduces the statistical precision of the estimates.
As PHN treatment may be initiated soon following HZ diagnosis, the PHN date was defined as the date of the first claim with a HZ diagnosis, except for: (1) patients with a PHN diagnosis, but no diagnosis for HZ; (2) patients with a HZ diagnosis which precedes their PHN diagnosis by more than 6 months; (3) patients with a PHN diagnosis which precedes their HZ diagnosis. For these three exceptions the PHN date was defined as the PHN diagnosis date. The study index date was defined as the date of first prescription for lidocaine patch, gabapentin or pregabalin on or after the PHN date, provided that treatment was initiated ≤180 days of the PHN date. A 6-month baseline period prior to the index date and study period of 6 months following the index date were examined. As less than 25% of PHN patients still experience pain at 6 months after the HZ eruptionCitation2, a 6-month study period following initiation of treatment (typically 1–3 months after eruption) was deemed appropriate to capture PHN-related utilization and costs.
Treatment groups and exclusion criteria
To increase the likelihood that PHN-likely patients were indeed initiating treatment for PHN (rather than other conditions for which the same treatment may be used), they were required to have no claims for PHN-recommended medications during the 90 days prior to the PHN date, and no claims with diagnoses for neuropathic pain or fibromyalgia 6 months prior to the PHN date. This exclusion means that treatment was initiated only after diagnosis with PHN or HZ, and was not a direct continuation of pre-existing treatment. The remaining PHN-likely cohort consisted of 2,054 patients.
Two mutually exclusive treatment groups were defined, based on index medication (i.e., initiation of pharmaceutical therapy):
Lidocaine patch – patients who initiated lidocaine patch as their first PHN-indicated treatment within 180 days of the PHN date, provided that they had no claims for gabapentin or pregabalin during the study period;
Gabapentin/pregabalin – patients who initiated gabapentin or pregabalin as their first PHN-indicated treatment within 180 days of the PHN date, provided that they had no claims for lidocaine patch during the study period.
The resulting sample consisted of 337 lidocaine patch patients and 1,416 gabapentin/pregabalin patients. Sample selection is summarized in .
Patient characteristics
Patient characteristics were compared between groups over the 6-month baseline period, including demographics (age, gender, geographic location), baseline rates of selected comorbidities and baseline severity measures (Charlson Comorbidity Index (CCI)Citation15,Citation16, medication use, healthcare resource utilization and medical and drug costs). Baseline comorbidities and their appropriate ICD-9-CM codes were identified based on related, previously published studiesCitation4,Citation17.
Treatment and medical resource utilization
For each patient, days of supply (DOS) of the index medication (lidocaine patch or gabapentin/pregabalin) was calculated as the sum of DOS across all observed prescriptions during the 6-month study period. The mean and median DOS were calculated for each treatment group.
Medical services were categorized as inpatient (i.e., hospitalizations), Emergency Department (ED), long-term care (LTC) or outpatient/other using a combination of provider type, provider specialty, service type, category of service, claim type and procedure code variables. The precise definitions were adjusted to match state-level differences in Medicaid coding practices.
The definition of LTC included the following categories: long-term care, skilled nursing facility, intermediate care facility (ICF) and swing beds. Rehabilitation centers, mental health LTC, nursing facilities for mental illness and ICF for mental retardation were excluded. All services not categorized under LTC, inpatient, or ED were labeled as outpatient/other.
Resource utilization was compared in terms of rates (share of patients with at least one visit) and counts (number of visits).
Cost calculation
Healthcare costs, including medical and prescription drug costs, were calculated during the 6-month follow-up period. Cost analyses were conducted from the payer’s perspective (i.e., costs defined as Medicaid payments to providers). Since actual Medicaid payments to providers were directly reported in the claims database, medical and pharmaceutical costs were calculated independently of observed resource utilization. Medical costs were calculated for inpatient, ED, LTC and outpatient/other medical services (e.g., outpatient surgery, physician services, laboratory, and other ancillary services, etc.). PHN-related medical costs were estimated using claims associated with ICD-9-CM codes for PHN or HZ.
All costs were inflated to 2007 US dollars, the most recent year of available medical and pharmacy claims, using the Consumer Price Index for Medical Care.
Statistical analyses
Matching lidocaine patch 5% and gabapentin/pregabalin patients
Propensity score matchingCitation18 was used to control for baseline differences (e.g., demographics, comorbidities) between lidocaine patch and gabapentin/pregabalin patients that may confound with resource use and cost outcomes. A logistic regression model was used to estimate the likelihood of lidocaine patch use as a function of baseline characteristics (including demographics, other painful conditions, CCI, baseline medication, resource use and costs). The predicted probability of lidocaine patch use was defined as the propensity score. Optimal matchingCitation19,Citation20 was used to match lidocaine patch patients to patients in the gabapentin/pregabalin group on propensity score and age. Including age both in the propensity score model and directly in the matching algorithm contributed to a more balanced match at baseline. Note that indicator variables for the index year were included in the logistic regression to account for time-varying unobservables possibly correlated with assignment to treatment groups. This would include, for instance, the impact of Medicare Part D introduction in 2006. The matched sample included 312 patients in each treatment group.
Lidocaine patch patients were compared to matched gabapentin/pregabalin patients using univariate analyses. Categorical variables (e.g., rates of comorbidities) were compared using McNemar tests for matched pairs; continuous variables (e.g., costs, CCI) were compared using bias-corrected bootstrapping to account for their skewed distributionCitation21–23.
Sensitivity analysis
As a sensitivity analysis, multivariate analyses were used to compare healthcare costs between lidocaine patch and gabapentin/pregabalin patients eligible for the study prior to propensity score matching. Multivariate generalized linear models with a log link and gamma distribution were used to compare costs controlling for baseline characteristics (demographics, PHN diagnosis type, comorbidity profile, CCI, medication use, healthcare resource utilization, baseline medical and drug costs, and index date medication and healthcare resource use). Risk-adjusted costs were then estimated for PHN patients in both treatment groups.
All analyses were conducted using SAS version 9.1 (SAS Institute Inc., Cary, NC). P-values ≤0.05 were considered to indicate statistically significant differences.
Results
Baseline characteristics and comorbidities
Prior to matching, the study sample consisted of 337 lidocaine patch patients and 1,416 gabapentin/pregabalin patients (). Compared with gabapentin/pregabalin patients, unmatched lidocaine patch patients were, on average, 2 years younger (p = 0.05), had a higher proportion of patients with a PHN-likely diagnosis type (60.5 vs. 50.7%, p = 0.001), higher proportion of patients with low back pain (20.5 vs. 15.9%, p = 0.04) and other spinal and neck pain (19.0 vs. 13.9%, p = 0.02), and a higher proportion with promethazine use (12.2 vs. 8.3%, p = 0.02). Note that baseline period use of lidocaine patch, gabapentin or pregabalin was not excluded, therefore baseline utilization rates greater than zero were observed.
Table 1. Baseline characteristics and comorbidities.
In each group, 312 patients were successfully matched. Of the matched gabapentin/pregabalin patients, 163 patients (52.2%) initiated treatment with branded gabapentin, 125 (40.1%) with generic gabapentin, and 24 (7.7%) with pregabalin. Matched PHN patients were on average 61.3 years old, and approximately 73% were female. There were no significant differences in baseline comorbidities, medication or resource use between treatment groups after matching.
Study period treatment
During the 6-month study period, lidocaine patch patients received an average (median) of 48.1 (30) DOS of their index medication, compared with 77.3 (60) DOS among gabapentin/pregabalin patients (). These numbers may understate lidocaine patch use because patches may be cut into smaller sizesCitation6.
Table 2. Study period (6-month) healthcare resource utilization.
Apart from the use of their index medications, lidocaine patch patients and gabapentin/pregabalin patients had similar utilization rates of tricyclic antidepressants (16.3 vs. 15.7%, p = 0.83), duloxetine (1.9 vs. 1.0%, p = 0.32), analgesic medication (85.9 vs. 84.3%, p = 0.57), antiviral therapy (37.2 vs. 34.6%, p = 0.50) and corticosteroids (20.5 vs. 25.0%, p = 0.18) during the 6-month study period.
Study period healthcare resource use
Overall resource utilization during the 6-month study period was not significantly different between lidocaine patch and gabapentin/pregabalin patients (). For example, in both groups, approximately 40% of patients were treated in the ED, 3% of patients were treated in a LTC setting, and patients averaged about one outpatient/other visit per week. PHN-related resource use was also similar, though gabapentin/pregabalin patients had more PHN-related outpatient/other visits during the study period (1.3 vs. 1.0, p = 0.04).
Costs
reports descriptive comparisons of study period (6-month) costs between lidocaine patch patients and propensity score-matched gabapentin/pregabalin patients. On average, total costs were $9,446 among lidocaine patch patients, compared with $9,448 for gabapentin/pregabalin patients (p = 0.98). There were no statistically significant differences in total medical or in total prescription drug costs.
Table 3. Mean study period (6-month) healthcare costs.
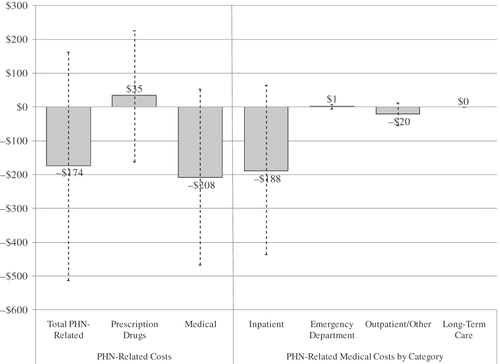
In both patient groups, PHN-related costs accounted for less than 12.5% of total costs. In addition to , illustrates mean differences in PHN-related costs during the study period. The point estimate of PHN-related costs, while not statistically significant, was $174 lower (p = 0.31) among lidocaine patch patients, with PHN-related medical costs $208 lower (p = 0.12).
Figure 2. Study period (6-month) differences in mean PHN-related costs – lidocaine patch vs. gabapentin/pregabalin. Dotted lines represent 95% confidence intervals; costs were compared using bias-corrected bootstrapping. PHN-related costs were calculated over the 6-month study period and inflated to 2007 US dollars using the CPI for medical care. Differences in mean costs were defined as mean per-patient costs in the lidocaine patch group minus mean per-patient costs in the gabapentin/pregabalin group.
The multivariate sensitivity analysis found that risk-adjusted total costs for lidocaine patch patients were $9,000, compared with $9,317 among gabapentin/pregabalin patients (difference: −$317, p = 0.56). Total medical costs were $617 lower in the lidocaine patch group (p = 0.20), while total drug costs were $236 higher (p = 0.25). PHN/HZ-related costs display a similar cost offset pattern.
Discussion
Multiple studies have established the substantial costs associated with HZ and PHN, including direct costs due to prescription medication and medical resource utilization, indirect costs associated with work-loss and disability, and reduced quality of life due to debilitating painCitation4,Citation14,Citation24–28. While much of the recent literature has focused on the cost effectiveness of the varicella zoster vaccine and prevention of PHN/HZCitation25–29, improvement in disease management could lead to significant cost savings, given the high costs associated with treating PHN. Studies examining costs associated with established PHN treatments can improve practitioners’ and payers’ ability to manage PHN patients more effectively.
While retrospective analyses of claims data have estimated the costs associated with PHN patients, including the Medicaid populationCitation4,Citation14,Citation30, no study, to the authors’ knowledge, has examined the economic costs of lidocaine patch, the leading topical treatment for PHN, versus its systemic comparators, gabapentin and pregabalin, among Medicaid patients.
Measured from a wholesale acquisition cost perspective, daily treatment of PHN with lidocaine patch appears more expensive than treatment with gabapentin (branded or generic) or pregabalin. However, comparison of propensity score-matched PHN patients who initiated monotherapy with lidocaine patch with those treated with gabapentin/pregabalin reveals that patients treated with lidocaine patch had similar rates of resource utilization as gabapentin/pregabalin patients, lower prescription drug costs and cost no more, in terms of total healthcare costs, over a 6-month study period.
A more detailed breakdown of direct cost components () reveals that PHN-related drug costs among lidocaine patch patients were only nominally higher ($35 over 6 months, p = 0.75). Higher drug costs would be consistent with the higher published prices associated with lidocaine patch, though it is worth noting that the results suggest possible cost savings among lidocaine patch patients due to lower costs associated with concomitant systemic therapies (e.g., analgesic medication). At the same time, while not significant at the 5% level, the results suggest PHN-related medical costs may be lower among patients treated with the lidocaine patch.
These cost results should be interpreted as hypothesis-generating findings, as no differences were statistically significant at the 5% level, likely due to limited sample size. Further research with additional data sources is necessary to confirm the main findings presented above.
At approximately $9,000 over a 6-month period, total healthcare costs estimated for PHN Medicaid patients in this study are consistent with results previously reported among PHN patients in the Medicaid populationCitation4,Citation14. Previous studies, however, did not compare resource utilization and costs between PHN patients treated with lidocaine patch and patients treated with gabapentin/pregabalin.
The study findings should be interpreted in the context of the sample selection criteria. The study sample was drawn from a pool of Medicaid beneficiaries in four states, and the findings for this sample may not be generalizable to the overall population of patients with PHN. In addition, utilization and costs captured in the study only reflect Medicaid claims, and do not capture Medicare claims for dual eligible patients. With approximately 40% of the study sample aged 65 and older, costs may be underestimated. However, there is no reason to suspect systematic differences in cost estimates across treatment groups, as assignment to treatment groups is likely uncorrelated with Medicare dual eligibility.
The study intentionally compared patients initiating treatment with lidocaine patch during the study period, but not treated with gabapentin or pregabalin, with patients initiating treatment with gabapentin/pregabalin during the study period, but not treated with lidocaine patch. Given the objective of this study, patients who received both kinds of medication during the study period (approximately 20% of the original sample) were excluded. While the proportion of excluded patients is non-trivial, the exclusion was necessary to facilitate appropriate comparison between treatments. Clearly, this exclusion is artificial, as physicians can prescribe additional medications to patients if they do not respond to their index treatment. Therefore, the design may result in selection of a study sample who respond well to their index medication. This too may suggest that the costs reported above are an underestimate of the true costs associated with treating PHN Medicaid patients, though the approach is unlikely to bias the comparative nature of the results.
Conclusion
PHN is an extremely painful complication which affects many HZ patients. Despite higher observed list prices for the lidocaine patch compared with gabapentin and pregabalin, no differences are found in total healthcare costs between PHN patients initiating treatment with lidocaine patch and those initiating with pregabalin/gabapentin among the Medicaid population. The study suggests that PHN-related medical costs may be lower among lidocaine patch patients.
Transparency
Declaration of funding
This study was funded by Endo Pharmaceuticals, Inc.
Declaration of financial/other relationships
N.K., H.G.B., R.W., E.K., and J.I.I. have disclosed that they are employed by Analysis Group, Inc., a company that received financial support from Endo Pharmaceuticals to conduct this study. R.A.P., R.H.B-J., and K.H.S. have disclosed that they are employees of Endo Pharmaceuticals, Inc.
Acknowledgments
The authors wish to thank Dr Stephen Camper of Endo Pharmaceuticals, Inc. for his assistance in manuscript preparation and review.
Material from this study was presented as a poster at AMCP’s 22nd Annual Meeting & Showcase, San Diego Convention Center, San Diego, CA, April 7–10, 2010, under the title: ‘Economic value of lidocaine patch 5% vs. gabapentin or pregabalin in Medicaid patients with postherpetic neuralgia’.
Notes
* Kirson et al. Descriptive analysis of Medicaid patients with postherpetic neuralgia treated with lidocaine patch 5%, pp. 472-481 of this issue.
References
- Dubinsky RM, Kabbani H, El-Chami Z, et al. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2004;63:959-65
- Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (shingles) and postherpetic neuralgia. Am Fam Physician 2000;61:2437-2444, 2447-2448
- Kost RG, Straus SE. Postherpetic neuralgia—pathogenesis, treatment, and prevention. N Engl J Med 1996;335:32-42
- Dworkin RH, White R, O’Connor AB, et al. Health care expenditure burden of persisting herpes zoster pain. Pain Med 2008;9:348-353
- Red Book Drug Topics. Montvale, NJ: Thomson Healthcare, 2009
- Lidoderm (lidocaine patch 5%) label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020612s008lbl.pdf. Accessed November 30, 2009
- Neurontin (gabapentin) label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020235s041,020882s028,021129s027lbl.pdf. Accessed November 30, 2009
- Lyrica (pregabalin) label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021446s013s014lbl.pdf. Accesses November 30, 2009
- Liedgens H, Hertel N, Gabriel A, et al. Cost-effectiveness analysis of a lidocaine 5% medicated plaster compared with gabapentin and pregabalin for treating postherpetic neuralgia: a German perspective. Clin Drug Invest 2008;28:583-601
- Dakin H, Nuijten M, Liedgens H, et al. Cost-effectiveness of a lidocaine 5% medicated plaster relative to gabapentin for postherpetic neuralgia in the United Kingdom. Clin Ther 2007;29:1491-1507
- Holahan J, Miller DM, Rousseau D. Dual eligibles: Medicaid enrollment and spending for Medicare beneficiaries in 2005. Kaiser Commission on Medicaid and the Uninsured Issue Brief, February 2009
- Tyring S. Management of herpes zoster and postherpetic neuralgia. J Am Acad Dermatol 2007;57:s136-142
- Gnann JW, Whitley RJ. Clinical practice. Herpes zoster. N Engl J Med 2002;347:340-346
- Dworkin RH, White R, O’Connor AB, et al. Healthcare costs of acute and chronic pain associated with a diagnosis of herpes zoster. J Am Geriatr Soc 2007;55:1168-1175
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-383
- Romano PS, Roos LL, Jollis J. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data. J Clin Epidemiol 1993;46:1075-1079
- Oster G, Berger A, Dukes E, et al. Use of potentially inappropriate pain-related medications in older adults with painful neuropathic disorders. Am J Geriatr Pharmacother 2004;2:163-170
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-55
- Rosenbaum PR. Optimal matching for observational studies. J Am Stat Assoc 1989;84:1024-1032
- Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat 1985;39:33-38
- Rascati KL, Smith MJ, Neilands T. Dealing with skewed data: an example using asthma-related costs of Medicaid clients. Clin Ther 2001;23:481-498
- Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993
- SAS Institute. Jackknife and Bootstrap Analyses. http://support.sas.com/kb/24/982.html
- Gauthier A, Breuer J, Carrington D, et al. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2009;137:38-47 Epub 2008 May 9
- Rothberg MB, Virapongse A, Smith KJ. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Clin Infect Dis 2007;44:1280-1288
- Harpaz R, Ortega-Sanchez IR, Seward JF. Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2008;57:1-30
- Johnson RW, Wasner G, Saddier P, et al. Herpes zoster and postherpetic neuralgia: optimizing management in the elderly patient. Drugs Aging 2008;25:991-1006. doi: 10.2165/0002512-200825120-00002
- Johnson RW. Herpes Zoster and postherpetic neuralgia: a review of the effects of vaccination. Aging Clin Exp Res 2009;21:236-243
- Hornberger J, Robertus K. Cost-effectiveness of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Ann Intern Med 2006;145:317-325
- Dworkin RH, Malone DC, Panarites CJ, et al. Impact of postherpetic neuralgia and painful diabetic peripheral neuropathy on health care costs. J Pain 2010;11:360-368. Epub 2009 Oct 22