Abstract
Objectives:
Escitalopram is the S-enantiomer of citalopram and is the most discriminating of the selective serotonin reuptake inhibitors (SSRI). The aim of the current analysis was to assess the cost effectiveness of escitalopram versus the serotonin norepinephrine reuptake inhibitors (SNRI) duloxetine and generic venlafaxine as second-step treatment of major depressive disorder.
Methods:
The analysis was based on a decision analytic model. Effectiveness outcomes were quality-adjusted life-years (QALYs) and remission rates; cost outcomes were direct medical costs, including impact of treating adverse events, and indirect costs associated with lost productivity. The analysis was performed from the societal perspective in Sweden over a 6-month timeframe.
Results:
Estimated remission rates showed an incremental effectiveness in favour of escitalopram of 16.4 percentage points compared with both SNRI comparators. The escitalopram strategy was associated with a 0.025 increase in QALYs. Sensitivity analyses demonstrated that the model is robust and that escitalopram remains a cost-effective option when considering future predicted price reductions of generic venlafaxine.
Limitations:
The main limitation in this study was the lack of data available for second-step treatment. The remission rates, which are a key input to the model, were obtained from a relatively small sample of patients on second-step treatment and there are no published relapse rates for second-step treatment. The model also assumed that there was no difference in the adverse event (AE) rates between treatments after the first 8 weeks.
Conclusions:
This cost-effectiveness analysis indicates that, at a willingness-to-pay threshold of £30,000, escitalopram is the most cost-effective second-step treatment option for MDD in Sweden in over 85% cases compared with both venlafaxine and with duloxetine. Benefits for escitalopram included both increased effectiveness and reduced overall costs. The major contributing costs were those associated with productivity loss.
The model was shown to have internal validity and robustness through the use of stochastic simulations and sensitivity analyses, which were conducted around the key efficacy parameters.
Introduction
Major depressive disorder (MDD) is a public health problem that results in a substantial burden of illness for healthcare providers, payers, and patients worldwide. In Sweden, the lifetime risk of MDD is 20% for men and 30–35% for womenCitation1,Citation2. The economic burden of MDD in Sweden rose from €1.7 billion in 1997 to €3.5 billion in 2005, driven primarily by the indirect costs associated with depression-related productivity lossCitation3.
The selective serotonin reuptake inhibitors (SSRI), such as citalopram, are the most widely prescribed first-line antidepressants (AD)Citation4. However, the effectiveness of SSRIs is limited; for instance, the STAR*D effectiveness study reported that after 12 weeks of treatment with citalopram 20–60 mg per day, less than half of patients had a clinical response and 28–33% of patients were in remissionCitation5. For patients that fail to respond to initial treatment for MDD, a common strategy for second-step treatment is to switch to an AD of a different class with a different mechanism of action, such as the serotonin norepinephrine reuptake inhibitors (SNRIs)Citation6,Citation7. However, there is no consistent evidence of improved treatment response or remission associated with such a switch compared with switching within the same classCitation8–11.
Escitalopram, the S-enantiomer of citalopram, is the most selective SSRI availableCitation12. In the second-step treatment of MDD, it was shown to be more efficacious and better tolerated than the SNRIs venlafaxine and duloxetineCitation13.
While evidence of efficacy and tolerability is important in the evaluation and comparison of available therapeutic options, other factors such as cost effectiveness and treatment impact on quality of life are becoming increasingly important. Sweden has a single-payer healthcare system, a strong health technology assessment outlook and a focus on the societal perspective, and therefore adequate up-to-date evidence of cost effectiveness is essential for decision making. The objective of the present analysis was to assess the cost effectiveness of escitalopram versus duloxetine and generic venlafaxine as second-step treatment of MDD over a 6-month timeframe. The study took into account the full clinical profile (efficacy and tolerability) of the three ADs of interest, adopted the societal perspective in Sweden and identified the major cost drivers associated with the second-step management of depression to provide a robust evaluation of the cost effectiveness of escitalopram in this indication.
Patients and methods
Model description
The model reported is based on a decision analytic model using Monte Carlo simulation that has been previously used to compare the cost utility of escitalopram and sertralineCitation14. This model has been adapted to reflect second-step MDD treatment patterns in Sweden, as detailed below. In this model, escitalopram has been compared with:
Generic venlafaxine extended-release (XR), an antidepressant of the SNRI class commonly used in the second-step treatment of MDD;
Duloxetine, another commonly used SNRI with a different adverse event (AE) profile, which makes a substantial contribution to the direct cost of this class of medication in Sweden.
Model structure
The decision tree is presented in . The initial 2-month acute second-step treatment was assumed to start on either 10 mg escitalopram, 75 mg venlafaxine XR or 60 mg duloxetine, with a possible dose adjustment during the second month to escitalopram 20 mg/day or venlafaxine 150 mg/day for patients receiving those treatments. During the acute treatment period, patients could achieve symptom remission (MADRS ≤ 12). Patients who achieved remission during this period were assumed to continue medication for another 4 months. During this maintenance treatment, patients could either relapse or remain in sustained remission. Patients who did not achieve remission were assumed to have a decreased quality of life/utility that impacted the QALY outcome.
Figure 1. Model framework for second-line treatment. (1) Remission by 24 weeks: Patients who achieved remission by 8 weeks following initiation of second-step therapy and remained in remission by 24 weeks. Patients were to continue taking the same medication for another 4 months. (2) Relapse at 24 weeks: Patients who achieved remission by 8 weeks following initiation of second- step therapy but experienced relapse during the following 4-month period. (3) No remission at 8 weeks: Patients who did not achieve remission during the first 8 weeks of second-step therapy.
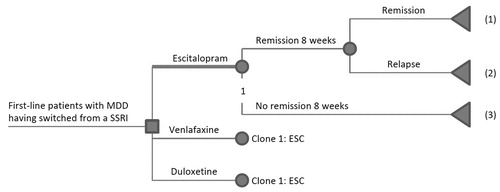
The results of the analysis were estimated based on utilities and costs associated with different health states (as detailed below). The model was run as a Monte Carlo simulation comprising 10,000 iterations, resulting in 95% credibility intervals of point estimates of incremental costs and effectiveness (QALYs and sustained remission rate) for escitalopram compared with venlafaxine.
The model was developed using Data 4.0 software from TreeAge Software Inc., Williamstown, MA, USA.
Data sources – clinical parameters
Remission
Remission rates following second-step treatment with escitalopram, venlafaxine or duloxetine were determined from a pooled analysis of individual patient data on the subgroup of patients with second-step treatment from three randomized controlled trials (RCTs) involving patients with a diagnosis of MDD according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteriaCitation13. Four relevant clinical trials were identified which had a head-to-head comparison of escitalopram and one of the comparator treatments. This pooled analysis included the two head-to-head studies that compared escitalopram (10–20 mg/day) to duloxetine (60 mg/day) over 8 and 24 weeks, respectivelyCitation15,Citation16 and one study that compared escitalopram (20 mg/day) to venlafaxine (225 mg/day) over 8 weeksCitation17. Only those patients who had received AD therapy in the 6 months prior to randomization were included in the subgroup analysis of the data from these trials. No patients were recorded as having used any AD before baseline in the second venlafaxine study and it was therefore not included in the pooled analysisCitation18. Data for the three trials were combined to increase the statistical power.
For the purposes of the model, the 8-week second-step remission rates were estimated to be 62.1% for escitalopram and 40.9% for the SNRIs ().
Table 1. Clinical probabilities – remission, relapse, adverse events, titration rates.
Relapse
In the absence of direct comparative data on relapse following remission from head-to-head studies of escitalopram versus venlafaxine or duloxetine as second-step treatment, relapse rates were obtained from the General Practitioners Research Database (GPRD) database (The General Practice Research Database (GPRD) www.gprd.com) [accessed 5th June 2010]. The GPRD is one of the world’s largest databases of anonymous longitudinal medical records compiled from primary care throughout the United Kingdom (UK). In a study by Wade comparing escitalopram and venlafaxine as second-step treatmentCitation19,Citation20, the relapse rates were defined as a subsequent AD prescription within and 1–6 months after the AD stop date (i.e., an unsuccessful AD stop). Using this definition, 19.2% of the escitalopram patients and 18.1% of the venlafaxine patients were estimated to have relapsed ().
Duloxetine-specific data were not reported in this study. However, venlafaxine and duloxetine relapse rates were assumed to be equal based on data from two head-to-head clinical trialsCitation21. Combined relapse rates of 16.7% for venlafaxine and 13.0% for duloxetine were reported and a post-hoc analysis (chi-squared test) showed that there was no significant difference between the treatments. Therefore, the relapse rate for duloxetine in the model was assumed to be the same as for venlafaxine.
Adverse events
The incidence of adverse events during the initial 8 weeks of treatment was estimated using data from two RCTs of escitalopram versus venlafaxineCitation17,Citation18 and two RCTs of escitalopram versus duloxetineCitation15,Citation16. All AEs (not just those considered to be related to study medication) occurring in more than 7% (to enable the selection of most relevant AEs) of patients in at least one treatment arm during the first 8-week period following initiation of treatment with escitalopram, venlafaxine or duloxetine were considered. The odds ratios (ORs) were calculated from pooled data for each AE in the escitalopram versus venlafaxine and escitalopram versus duloxetine comparisons. The AE rates used in the model were estimated from the pooled AE rate for escitalopram across all four RCTs combined with the OR of escitalopram versus venlafaxine, and escitalopram versus duloxetine.
summarizes AE rates and the associated resource use and costs. All AEs were assumed to occur in the first 8 weeks of therapy. This assumption is supported by trial data showing that the AEs being considered generally occur early in therapy. It is also assumed that there is no difference in AE rates across the treatments beyond the first 8 weeks of therapy.
Table 2. Unit costs (€2009), including additional medications for adverse events.
Doses and titration
Patients receiving escitalopram or venlafaxine could have been titrated to a higher dose of the original medication (from 10 mg to 20 mg for escitalopram and from 75 mg to 150 mg for venlafaxine). These are the approved doses in the respective summary of product characteristics (SPC) and in the source RCTsCitation15–17. Titration rates over the first 8 weeks of the model were estimated to be 15.5% for escitalopram and 29.2% for venlafaxine from the analysis of second-step patients in the GPRD databaseCitation19 (). In this model, titration was not applied to duloxetine as the evidence shows close to 0% titration ratesCitation19.
Healthcare resource use, sick leave and unit costs
Twenty-four week data on resource consumption, including inpatient care, outpatient care and productivity losses due to sick leave days, were obtained from the HEADIS cohort study for patients in remission and for those who did not achieve remissionCitation22. The HEADIS study is a naturalistic longitudinal survey, capturing data on socio-demographics, treatment patterns, healthcare resource use, working ability (sick leave) and quality of life on adult MDD patients from 56 primary care centres in Sweden, over the 6-month follow-up period. From this study, the mean estimates for each type of resource use category were calculated () and associated unit costs assigned (). Patients who were not in remission at 6 months were assumed to have the same resource use as the ‘no remission’ category.
Table 3. Resource utilization per patient for inpatient care, outpatient care, production loss (24-week data), according to remission status and titration.
The number of additional general practitioner (GP) contacts was assumed to be the mean number of up-titrations amongst patients who had been titrated, which was ascertained for patients receiving second-step therapy in the GPRD study ().
Pharmaceutical use included consumption of AD medication, which was estimated from the daily dose of usage in the relevant clinical trials, taking account of the dose increments and based on assumptions regarding duration of treatment (). The cost of venlafaxine in the analyses was the current cost of generic venlafaxine (base-case scenario).
AD medication was assumed to continue for 180 days. Titration, if applicable, was assumed to occur after day 30 and the patient was switched to an alternative AD treatment after day 60 if there was no remission. The estimated costs for treating AEs were based on relevant medication costs. No assumptions were made regarding possible additional physician visits that may have been required to manage the AEs. Drug use for the treatment of AEs was assumed to be one prescription for the treatment of each AE (). Medications used to treat AEs were based on assumptions. The cost of treatment for an AE was assumed to be the price of the lowest preparation and was taken from The Dental and Pharmaceutical Benefits Agency (Tandvårds Och Läkemedelsförmånsverket, TLV) database. If the two experts reported different medications for a particular AE, the average cost for the two different drugs was considered.
Productivity loss in this study was defined as absenteeism, which was measured as sick leave based on the HEADIS survey, for MDD patients in remission and for those who did not achieve remission at 6 months. Expected per patient cost associated with productivity loss was estimated using the human capital approach, as a product of sick leave and daily wage rate.
Unit costs associated with resource use and daily wage rate (for the productivity loss assessment) were obtained from published national sources including the Dental and Pharmaceutical Benefits Agency database (TLV), April 2009; the Regional Joint Committee of the Southern Sweden Healthcare Region, price list for 2007; the Confederation of Swedish Enterprise, 2007 (www.svensktnaringsliv.se) [accessed December 18, 2009]. An inflation correction was applied as follows: unit costs for 2009 were calculated using the published inflation rates of 2.2% in 2007 and 3.5% in 2008 (www.ekonomifakta.se/en) [accessed June 26, 2009].
The model inputs and outputs were defined in SEK, but the corresponding values in euros are reported in the present manuscript to facilitate interpretation beyond the Swedish healthcare system. The exchange rate used was that for December 1, 2009 (1 SEK = €0.0960); costs were calculated to two decimal places (http://www.ecb.europa.eu/stats/eurofxref/eurofxref-hist-90 d.xml).
Utility values
QALYs for remission, relapse after remission and no relapse were calculated based on utilities published from the HEADIS studyCitation22. The mean baseline utility in this study was 0.47 [standard error 0.0013], with corresponding mean utilities for remission (0.81 [0.015]) and no remission (0.57 [0.020]). QALY computation is shown in . Additionally, a utility decrement was applied for patients with the most common AEs (). The decrements were obtained from a published depression economic modelCitation23 which had derived age-adjusted utility decrements based on data from the 2000 Medical Expenditure Panel Survey (MEPS)Citation24. A utility decrement for each of the AEs included in the model was obtained from this analysis.
Table 4. Utilities for QALY calculation.
Discounting
No discounting was applied due to the length of the model follow-up.
Model analysis
Probabilistic cost-effectiveness modelling was applied to determine the average joint effects of costs and effectiveness, using Monte Carlo simulation allowing for uncertainty in all parameters.
Cost-effectiveness acceptability curves were generated for each pair of treatments. The curves showed the probability that escitalopram was cost effective versus the comparator at each level of willingness-to-pay (€ per QALY gained ranging from €0 to €50,000).
Sensitivity analyses
One-way sensitivity analyses were conducted on the following key parameters of the model:
Remission rate for escitalopram (key clinical parameter) was varied according to the confidence limit values calculated from the pooled RCT analysis;
The number of sick leave days (key cost driver) and the numbers of GP visits (the key component of outpatient costs) were varied to the confidence limit values, for each remission status.
The rates of nausea (AE with the biggest impact on utility) were computed using 95% confidence interval values for escitalopram.
Relapse rates for escitalopram were varied within the confidence limit values.
Conservative case analysis for venlafaxine
A sensitivity analysis was conducted in which the cost of venlafaxine was assumed to be 5% of the current branded price of venlafaxine XR to anticipate the future price decrease of venlafaxine generics (the 5% threshold was chosen to reflect a hypothetical venlafaxine price drop in order to create a conservative scenario).
Results
Model effectiveness and costs outcomes
Results showed an estimated rate of sustained remission at 24 weeks following the introduction of second-step therapy of 50.1% for escitalopram and 33.6% for venlafaxine or duloxetine, resulting in an incremental effectiveness in favour of escitalopram of 16.4 percentage points (95% CI [2.7%, 30.2%]) compared with both comparators (). The escitalopram strategy was associated with a 0.025 increase in QALYs (95% CI [0.009, 0.042]) compared with the SNRIs, driven primarily by QALY differences for remission and smaller decrements due to AEs ().
Table 5. Model effectiveness results: escitalopram versus venlafaxine and duloxetine.
The direct and indirect costs associated with escitalopram, venlafaxine and duloxetine are summarized in . There were estimated savings on the overall per patient cost of treating with escitalopram of €506.59 (95% CI [−€5,249, €3,427]) compared with venlafaxine and savings of €482.69 (95% CI [−€5,230, €3,444]) compared with duloxetine, with savings in both direct and indirect costs. Indirect costs amounted to approximately 86% of total costs for each second-step AD. Disaggregated direct costs showed that escitalopram was associated with higher acquisition costs (€149.57) compared with venlafaxine (€104.35) and lower acquisition costs compared with duloxetine (€162.05). Drug costs accounted for 15.4%, 10.3% and 16.4% of the total direct costs in the escitalopram, venlafaxine and duloxetine treatment groups, respectively.
Cost effectiveness
The cost-effectiveness plane for the comparison of escitalopram and venlafaxine ( and ) shows that the probability of escitalopram being dominant is 65.4%. In the comparison of escitalopram and duloxetine the probability of escitalopram being dominant is 64.6% ( and ). The cost-effectiveness acceptability curve () shows that at a willingness-to-pay threshold of €22,080 and €33,600 per QALY gained, the probability of escitalopram being cost effective is 81.5% and 85.8%, respectively, compared with venlafaxine, and 80.9% and 85.5%, respectively, compared with duloxetine from the societal perspective. These values correspond approximately to £20,000 and £30,000 per QALY gained, which are the upper limits of the incremental cost-effectiveness range within which a technology is considered to be an effective use of health service resources within a nationally based healthcare system as in the UKCitation25.
Figure 2. (a) The cost effectiveness of escitalopram compared with venlafaxine (base-case scenario): incremental effectiveness (on QALYs) versus incremental total costs. 1 SEK = €0.0960. (b)The cost effectiveness of escitalopram compared with duloxetine: incremental effectiveness (on QALYs) versus incremental total costs. 1 SEK = €0.0960.
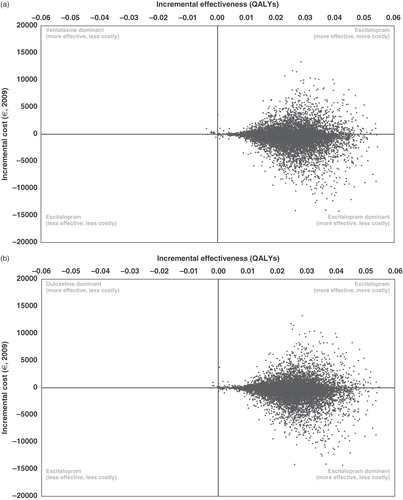
Figure 3. Cost-effectiveness acceptability curve – escitalopram compared with venlafaxine and duloxetine. 1 SEK = €0.0960.
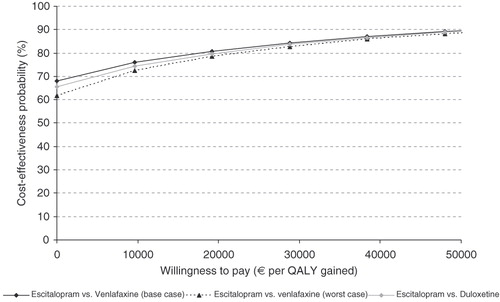
Table 6. Cost effectiveness of escitalopram.
Since the indirect costs were such a large proportion of the overall costs, a separate analysis was carried out on the differences in direct medical costs using the same thresholds. In this analysis, the probability of escitalopram being cost effective were 98.5% and 99%, respectively, compared with venlafaxine, and 98.1% and 98.9%, respectively, compared with duloxetine.
Sensitivity analyses
The conclusion on the cost effectiveness of the model did not change following the sensitivity analyses on remission rate, relapse rate, absenteeism, number of GP visits or incidence of nausea (). Escitalopram remained a cost-effective strategy compared with either venlafaxine or duloxetine.
Table 7. Sensitivity analyses – effect of variation in key effectiveness parameters on costs (mean, €2009) and QALYs for escitalopram versus venlafaxine and duloxetine.
Scenario analyses assuming lower venlafaxine costs
When the venlafaxine cost was assumed to be 5% of that of branded venlafaxine XR, the overall per patient cost for venlafaxine decreased slightly to €7,145.57 giving a saving of €431.14 for escitalopram. Direct costs for venlafaxine reduced to €938.98 with estimated savings in favour of venlafaxine of €34.27. Disaggregated direct costs showed that escitalopram was associated with higher acquisition costs (€149.57 vs. 28.90). However, this was offset by the reduction in outpatient costs with escitalopram versus venlafaxine (€661.92 vs. 751.58).
Although this is slightly lower than in the base-case analysis due to the lower AD medication costs for venlafaxine, the probabilities of escitalopram being cost effective at willingness-to-pay thresholds of €22,080 and €33,600 per QALY gained were 79.7% and 84.6%, respectively. As in the previous analyses, sensitivity analyses on the key model parameters did not change the cost-effectiveness outcomes.
Discussion
This is the first model to consider the effect of both efficacy and tolerability on QALY and cost outcomes for second-step treatment of MDD. The results demonstrate that for patients with a diagnosis of MDD receiving second-step therapy in Sweden, escitalopram is the most cost effective second-step treatment option for MDD in Sweden in over 85% cases compared with both venlafaxine and with duloxetine at a willingness-to-pay threshold of £30,000. This results from superior effectiveness outcomes and numerically lower costs whether or not costs due to loss of productivity were considered. A better tolerability profile of escitalopram compared with SNRIs contributed to higher expected QALY and lower healthcare resource utilization in terms of pharmacological treatment of AEs (although only a minor component of treatment costs).
The results of the sensitivity analyses demonstrate that the model is robust to the key parameters tested: remission rate (impacts QALYs and costs), number of days of sick leave (a key cost driver), the number of GP visits (key driver of outpatient costs) and nausea incidence (impacts QALYs). The scenario analysis clearly shows that, even when considering a possible reduction in the generic venlafaxine price in the coming years, escitalopram remains a cost-effective option.
The results are consistent with previously published findings of superior cost effectiveness and cost utility of escitalopram compared with other SSRIs and with venlafaxine XR when given as first-line therapy. Five modelled pharmacoeconomic analyses from Western Europe used a two-path decision analytic model with a 6-month time horizon and reported that escitalopram has a cost effectiveness and cost-utility advantage over other SSRIs and over venlafaxine XRCitation26,Citation27. A pharmacoeconomic comparison of escitalopram and venlafaxine carried out prospectively alongside a RCT suggested that escitalopram has similar effectiveness to venlafaxine in the treatment of MDD, and may be associated with lower healthcare costsCitation28.
Data on second-step treatment are scarce. The total cost per patient of achieving remission with second-step treatment was calculated to be slightly less for venlafaxine XR than for escitalopram ($14,275 vs. 16,100 [approximately €9,470 vs. 10,681]) when the incidence of AEs was assumed to be equal for all treatmentsCitation29. However, the importance of considering the impact of the incidence and economic consequences of treatment emergent AEs when comparing the effectiveness and costs of treatment has been noted in studies of first-line therapy, and the favourable tolerability profile of escitalopram is a key factorCitation14,Citation23. In the current study, a utility decrement for AEs and the cost incurred for treatment of AEs were both taken into account.
The cost effectiveness of escitalopram versus SNRIs is driven largely by improved productivity. For all treatments, the indirect costs associated with loss of productivity due to absenteeism accounted for about 86% of the total costs. Compared with duloxetine, escitalopram has been shown to be associated with significantly reduced absenteeismCitation30. Indirect costs due to sick leave account for the most substantial portion of the total cost and are therefore an important consideration when pharmacoeconomic comparisons between treatments are made from the societal perspectiveCitation30.
Limitations when using a decision analytic model arise from the model assumptions and from the data sources used. The remission rates in second-line treatment, which are key inputs for this model, are based on post hoc subgroup analysis from head-to-head clinical trials comparing escitalopram with SNRIs. The current analysis was based on a relatively small sample of patients on second-line treatment, mainly due to limited information on previous treatment. Also, the model assumed that there was no difference in the AE rates between treatments after the first 8 weeks. This was necessary because AE data are treatment specific and the best available data source is RCTs, in which only data for acute (8 weeks’) treatment were available for the comparison of escitalopram with venlafaxine. However, it is not unreasonable to expect that the majority of treatment emergent AEs would be reported during the acute treatment stage.
Another limitation that applies to all modelling studies is that the inputs for the model are primarily based upon the results of published literature from a range of different sources (RCTs, GPRD, MEPS survey) with different patient groups and different study designs. However, this approach does have the advantage of using patient level data rather than expert opinion, wherever possible. A key problem for this particular model was that data sources for second-step data are very limited. Remission rates were based on an analysis of the second-step subgroup in three RCTs and the two SNRI groups had to be combined to provide a reasonable sample size. The GPRD also provided second-step data, although the database is specific to the UK. The model also included data which were not specific to second-step treatment (HEADIS, clinical AE data).
There are no published relapse data specific to second-step therapy. The use of the GPRD allowed real-life data to be included, even though it was necessary to use a proxy measure for relapse. These data lack the stringent evaluation obtained from using scales such as MADRS or physician opinion. However, these disadvantages are offset by the benefits of using the GPRD data, which reflect real-life treatment patterns since they are based on a prescription database.
One of the RCTs of escitalopram and venlafaxine providing effectiveness and AE data for the model required rapid up-titration to maximum doses of 20 mg escitalopram and 225 mg venlafaxine, respectivelyCitation17. This could have resulted in an over estimation of efficacy and the incidence of AEs associated with the higher doses.
Conclusion
This cost-effectiveness analysis indicates that escitalopram is the dominant treatment option versus venlafaxine in 65.4% cases and versus duloxetine in 64.6% cases. Additionally, willingness-to-pay threshold of £20,000 and higher, escitalopram is the most cost-effective second-step treatment option for MDD in Sweden in over 80% cases compared with both venlafaxine and with duloxetine. Benefits for escitalopram included both increased effectiveness and reduced overall costs. The major contributing costs were those associated with productivity loss. Switching to escitalopram after first-line treatment failure results in a significantly higher rate of remission and consequently in higher QALYs and numerically lower costs compared with switching to either venlafaxine or duloxetine. Furthermore, switching to escitalopram is associated with fewer AEs and therefore lower AE treatment costs and a lower utility decrement.
Benefits for escitalopram included both increased effectiveness and reduced overall costs. The major contributing costs were those associated with productivity loss.
The model was shown to have internal validity and robustness through the use of stochastic simulations and sensitivity analyses, which were conducted around the key efficacy parameters.
Transparency
Declaration of funding
This study was supported by Lundbeck SAS.
Declaration of financial/other relationships
G.N. has disclosed that he has received consulting fees from Lundbeck SAS. N.D., F.M. and N.D. have disclosed that they are employed by Lundbeck SAS. K.M. has disclosed that he has received consulting fees from Lundbeck SAS.
Acknowledgements
The authors would like to acknowledge Carolyn McDonald and Jan McKendrick of Rx Communications Ltd, UK, a company that received funding from Lundbeck SAS, for medical writing assistance, project management and editorial support for the preparation of this article.
References
- Mattisson C, Bogren M, Nettelbladt P, et al. First incidence depression in the Lundby Study: a comparison of the two time periods 1947–1972 and 1972–1997. J Affect Disord 2005;87:151-160
- Nordström A, Bodlund O. Every third patient in primary care suffers from depression, anxiety or alcohol problems. Nord J Psychiatry 2008;62:250
- Sobocki P, Lekander I, Borgstrom F, et al. The economic burden of depression in Sweden from 1997–2005. Eur Psychiatry 2007;22:146-152
- Gelenberg AJ, Hopkins HS. Assessing and treating depression in primary care medicine. Am J Med 2007;120:105-108
- Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354:1243-1252
- American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder (revision). Am J Psychiatry 2000;157:1-45
- Anderson IM, Ferrier IN, Baldwin RC, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2000 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2008;22:343–396. Epub 2008 Apr 15
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Bupropion-SR, sertraline, or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006;354:1231-1242
- Ruhé HG, Huyser J, Swinkels JA, et al. Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry 2006;67:1836-1855. Review
- Papakostas GI, Fava M, Thase ME. Treatment of SSRI-resistant depression: a meta-analysis comparing within- versus across-class switches. Biol Psychiatry 2008;63:699-704
- Rush AJ. STAR*D: what have we learned?. Am J Psychiatry 2007;164:201-204
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry 2001;50:345-350
- Lam RW, Larsson Lönn S, Despiegel N. Escitalopram versus SNRIs as second-line treatment. Int J Psychiatry Clin Pract 2009;13(Suppl 1):35-36
- Armstrong EP, Skrepnek GH, Erder MH. Cost–utility comparison of escitalopram and sertraline in the treatment of major depressive disorder. Curr Med Res Opin 2007;23:251-258
- Khan A, Bose A, Alexopoulos CG, et al. Double-blind comparison of escitalopram and duloxetine in the acute treatment of major depressive disorder. Clin Drug Invest 2007;27:481-492
- Wade A, Gembert K, Florea I. A comparative study of the efficacy of acute and continuation treatment with escitalopram versus duloxetine in patients with major depressive disorder. Curr Med Res Opin 2007;23:1605-1614
- Bielski RJ, Ventura D, Chang C-C. A double-blind comparison of escitalopram and venlafaxine extended release in the treatment of major depressive disorder. J Clin Psychiatry 2004;65:1190-1196
- Montgomery SA, Huusom AKT, Bothmer J. A randomised study comparing escitalopram with venlafaxine XR in primary care patients with major depressive disorder. Neuropsychobiology 2004;50:57-64
- Wade AG, Milea D, Despiegel N, et al. A GPRD-based comparison of second-line antidepressant therapy with escitalopram and venlafaxine. Presented at the ISPOR 11th Annual European Congress, Athens, Greece, 8–11 November, 2008
- Wade AG, Milea D, Despiegel N, et al. A comparison of second-line antidepressant therapy with escitalopram and venlafaxine in the UK based on the General Practitioners Research Database. Clin Ther In press
- Benedict A, Arellano J, De Cock E, et al. Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. J Affect Disord 2009;120:94-104
- Sobocki P, Ekman M, Agren H, et al. The mission is remission: health economic consequences of achieving full remission with antidepressant treatment for depression. Int J Clin Pract 2006;60:791-798
- Sullivan PW, Valuck R, Saseen J, et al. A comparison of the direct costs and cost effectiveness of serotonin reuptake inhibitors and associated adverse drug reactions. CNS Drugs 2004;18:911-932
- MEPS, 2003: Agency for Healthcare Research and Quality, Center for Cost and Financing Studies. MEPS HC-052. 2000 medical conditions, June 2003 [online]. Available from URL: http://www.meps.ahrq.gov/PUFFiles/H52/H52doc.htm [Accessed (2003) Nov 10]
- National Institute for Clinical Excellence: Guide to the Methods of Technology Appraisal, April 2004. Available from URL: http://www.nice.org.uk/niceMedia/pdf/TAP_Methods.pdf [Accessed Aug 2009]
- Croom KF, Plosker GL. A pharmacoeconomic review of its use in depression. Pharmaeconomics 2003;21:1185-1209
- Llorca PM, Fernandez JL. Escitalopram in the treatment of major depressive disorder: clinical efficacy, tolerability and cost effectiveness vs. venlafaxine extended-release formulation. Int J Clin Pract 2007;61:702-710
- Fernandez J-L, Montgomery S, François C. Evaluation of the cost effectiveness of escitalopram versus venlafaxine XR in major depressive disorder. Pharmacoeconomics 2005;23:155-167
- Malone DC. A budget-impact and cost-effectiveness model for second-line treatment of major depression. J Manag Care Pharm 2007;13(6SupplA):S8-18
- Wade AG, Fernandez JL, Francois C, et al. Escitalopram and duloxetine in major depressive disorder: a pharmacoeconomic comparison using UK cost data. Pharmaeconomics 2008;26:969-981