Abstract
Objective:
The study evaluated the cost effectiveness of deferasirox (Exjade) compared to non-proprietary desferrioxamine (DFO) for the control of transfusional iron overload in lower risk myelodysplastic syndromes (MDS) patients. A UK National Health Service perspective was adopted.
Methods:
Recent clinical evidence has demonstrated the efficacy and safety of deferasirox in transfusion-dependent MDS patients with elevated serum ferritin levels. An economic model was used to extrapolate the clinical benefits of iron chelation therapy (ICT) in a cohort of lower risk MDS patients. Costs for drug acquisition, drug administration and monitoring, and quality of life (utility) outcomes associated with mode of drug administration were derived from a variety of sources. The incremental cost per QALY gained for deferasirox was estimated. Costs and outcomes were discounted at 3.5% in line with UK standards.
Results:
The base-case cost effectiveness of deferasirox versus DFO was estimated to be £20,822 per QALY gained, the key driver being the additional quality of life benefits associated with a simpler mode of administration for deferasirox. A mean survival benefit for both forms of ICT of 4.5 years was estimated. The results were sensitive to drug dose, days of DFO administration, and patient weight.
Conclusions:
In the UK, a cost per QALY below £20,000–30,000 is considered cost effective. Hence, the results from this economic analysis suggest deferasirox is cost effective in lower risk, transfusion-dependent, MDS patients. Limitations with the analysis include a lack of comparative randomised controlled trial evidence, in particular to differentiate survival and clinical outcomes for deferasirox and DFO.
Introduction
The myelodysplastic syndromes (MDS) cover a range of bone marrow disorders that mainly affect the elderly, with a median age at diagnosis of between 60 and 75 years, and an incidence of between 20 and 50 per 100,000 persons in patients aged over 60 years compared to 5 per 100,000 in the general population across the worldCitation1. A main clinical feature of MDS is anaemia, estimated to be present in 80% of patients at diagnosis and requiring frequent red blood cell transfusions in order to delay disease progression and improve patient outcomesCitation2,Citation3. A US study using a longitudinal, retrospective claims database demonstrated the healthcare cost burden associated with transfusion-dependent MDS. This study estimated the total annual cost of hospital inpatient, outpatient, emergency and physician office visits as $19,811 per patient for a cohort of patients who were transfusion independent (n = 2,864), but which rose to $51,066 in a transfusion-dependent MDS cohort (n = 336), a statistically significant difference of $31,255 per patient per yearCitation4. However, transfusion dependence is associated with a risk of chronic iron overload (typically defined as a serum ferritin >1,000 ng/ml)Citation5 which in MDS may cause or contribute to cardiac and other organ failure, onset of diabetes and progression to acute myeloid leukaemia (AML), and infection riskCitation2,Citation6. Therefore, there are mortality and quality of life implications associated with iron overload in MDS patientsCitation6. Evidence- and consensus-based guidelinesCitation2,Citation6,Citation7 recommend iron chelation therapy (ICT) for the management of iron burden in MDS patients with chronic iron overload. A number of recent observational studies have all indicated that there are survival benefits associated with ICT in transfusion-dependent MDS patients, especially in lower risk patientsCitation8–11, i.e. those with best survival prognosis, defined as low or intermediate-1 risk according to the International Prognostic Scoring System (IPSS), or defined by WHO criteria as (refractory anaemia (RA), refractory anaemia with ringed sideroblasts (RARS) and isolated 5q deletion syndrome)Citation5,Citation12.
The primary treatment to date in UK clinical practice for transfusional iron overload in lower-risk MDS has been desferrioxamine (DFO). DFO is self-administered parenterally as a subcutaneous infusion which due to a short plasma half-life is ideally infused for 24 hours per day for 7 days per week in order to ensure maintenance of iron chelation. In practice (and in trials), the usual maximum treatment duration tolerated by patients is 8–12 hours for between 5 and 7 nights per weekCitation13. Deferasirox (Exjade) received a licence in the UK for use in patients with β-thalassaemia and other anaemias, including MDS, in August 2006. Deferasirox has a longer half-life than DFO so can be administered as a once-daily oral tablet to provide 24 hours chelation. Both deferasirox and DFO are dosed according to patient weight on a mg per kg basis. Another, three times daily oral, ICT product, deferiproneCitation14, is available although it is not licensed in MDS and due to concerns over toxicity, in particular agranulocytosis, clinical opinion obtained from three UK haematologists involved in the treatment of MDS was that it is unlikely to be the primary treatment of choice for MDSCitation15.
There have been no previous published evaluations of the cost effectiveness of iron chelation therapy in MDS patients. Karnon et al., in a UK setting, evaluated the cost effectiveness of deferasirox versus DFO for a reference case consisting of a patient population with β-thalassaemia and chronic iron overloadCitation16. This analysis used a 1-year time horizon and found that deferasirox was associated with lower healthcare costs and greater health-related quality of life outcomes compared to DFO, driven by the simpler and more convenient oral mode of drug administration for deferasirox. Karnon et al. did not explicitly assess cost effectiveness in MDS patients due to a lack of clinical efficacy data at the time. However, there is now more evidence from trials of deferasirox with 1–2-year follow-up, albeit single-arm studies, demonstrating the clinical efficacy of deferasirox in controlling iron burden in transfusion-dependent MDSCitation17–19.
As the evidence base improves it therefore can be anticipated that there will be increasing clinical pressure in developed countries to treat transfusion-dependent low-risk MDS patients with ICT. Therefore, a question of interest to decision makers is whether deferasirox is a cost-effective ICT option as an alternative to DFO in transfusion-dependent MDS patients. This paper reports the results from the use of an economic model developed to address this question. As a secondary objective the model has also been used to estimate the long-run patient outcomes and healthcare cost consequences of ICT in the MDS patient population of interest.
Methods
Patient population
For this analysis the target MDS patient population have an IPSS prognostic rating of low risk or intermediate-1 (int-1) risk. These patients are transfusion-dependent but with a relatively good survival prognosis compared to intermediate-2 and high-risk patients, and with the capacity to significantly benefit from iron chelation therapyCitation20. These are the patients with MDS most likely to be receiving ICT in clinical practice.
Model structure and outcomes
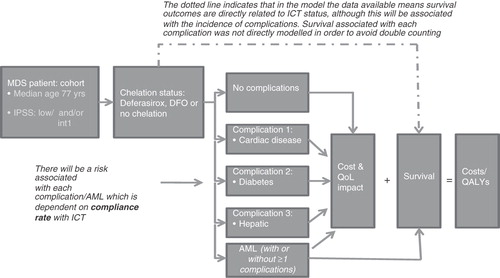
A Markov model with monthly cycles was developed in Microsoft Excel to evaluate the cost effectiveness of deferasirox compared to DFO for the treatment of iron overload in low and intermediate-1 risk patients with transfusion-dependent MDS (these are patients within ICD10 code D46 who are defined by IPSS risk status as low or intermediate-1 risk). In the model a cohort of 1,000 low and intermediate-1 risk transfusion-dependent MDS patients receiving each type of ICT are predicted through time to experience disease-related events and complications over a lifetime horizon (set at 20 years in the base case) according to a set of risks specified for each type of event. The cohort is assigned baseline characteristics – defined by age and IPSS risk category which, with chelation status, determines the risk of death. In addition, parameters for the risk of death and impact on patient health-related quality of life outcomes associated with risk of progression to AML are incorporated in the model.
The model has the capacity to investigate the impact of drug therapy compliance on the risk of AML progression and the risk of complications. The methodology for this is described later. Patient quality of life is directly affected by the mode of ICT administration: once daily deferasirox compared to slow (8–12-hour, 5–7 days per week) subcutaneous infusion with DFOCitation21,Citation22. presents an outline of the treatment pathway and health states within the model.
The model runs over a 20-year time horizon when all patients in the cohort are predicted to have died. The duration of ICT is assumed to be continuous until patient death, unless patients progress to AML in which case ICT ceases. Patients in the model, while alive, accumulate life-years and quality-adjusted life-years (QALYs), and healthcare costs (e.g., including hospital inpatient/outpatient and non-hospital healthcare costs) associated with drug therapy, blood transfusions and risk of complications or AML. Using the model the impact of ICT on patient survival, HRQoL and lifetime costs can be estimated, and results relating to these outcomes are reported in this paper. However, for the cost-effectiveness comparison of deferasirox and DFO the main driver of QALY outcomes are the differences in patient HRQoL associated with mode of drug administration for the two types of ICT.
The analysis was performed from the perspective of the UK National Health Service, which meant only healthcare costs to the public payer were considered.
The key clinical outcomes considered in the model are overall survival, the risk of progression to AML and the risk of complications (i.e., cardiac, hepatic and diabetes) associated with iron overload in transfusion-dependent low and int-1 risk MDS patients. While data were available for assessing the difference in these outcomes for ICT versus no ICT in MDS patients, similar data are not available to enable a direct comparison of deferasirox with DFO. However, it is assumed that the predicted survival and clinical outcomes associated with ICT apply equally to both DFO and deferasirox. This is based on evidence of deferasirox compared to DFO in β-thalassaemia patientsCitation13. Consistent efficacy results for deferasirox are also seen across a range of haemoglobinopathies, including sickle cell disease and other rare anaemiasCitation23. More definitive evidence is now emerging in low and int-1 risk MDS patientsCitation17–19,Citation23, supported by clinical opinion (three MDS-treating UK haematologists consultedCitation15). The physiological impact of cardiac iron overload has also been found to be similar across haemoglobinopathies, further supporting an assumption of equivalent clinical outcomes for the different types of ICTCitation24,Citation25. As the model is a lifetime model, data on survival and clinical outcomes enables estimation of life-years gained, QALYs and lifetime healthcare costs associated with ICT with deferasirox or DFO.
Survival outcomes associated with ICT
A number of observational studies have now demonstrated a relationship between ICT and survival outcomes in transfusion-dependent MDS patients, especially those classified as lower riskCitation8–11. A sufficient level of data for the economic model on survival outcomes for MDS patients receiving ICT compared to those not receiving ICT was available from one of these studies by Rose et al. in 165 regularly transfused MDS patients with a 2-year follow-up conducted in 18 treatment centres in FranceCitation26,Citation27. Median age in the study for all MDS patients was 77 years (as specific age data was not available for IPSS low and int‐1 risk patients it was assumed they had the same median age). The Kaplan–Meier plot from this study demonstrated a median overall survival of 115 months (9.58 years) versus 51 months (4.25 years) in the ICT and non-ICT groups, respectively (p < 0.0001)Citation26. Survival results from this study were also available specifically for IPSS low- and int-1 risk groups (n = 52 receiving chelation therapy, and n = 45 receiving no chelation therapy), and these data were incorporated into the economic model. Survival data were not available by age groups from the Rose et al. study, hence mortality hazards for MDS patients by IPSS score and age were obtained from a study examining factors predicting survival prognosis in MDS patientsCitation20. In this study mortality data were available by age ≤70 and >70 years, hence it had to be assumed that rates observed applied also to patients aged ≤77 and >77 years in order to fit to the Rose et al. survival data. The median survival was obtained from the Kaplan–Meier curves and used to estimate the resulting hazards/rates in patients with and without ICT by IPSS low and int‐1 risk groups stratified by age. It was assumed that within each strata the death hazard is constant. For the IPSS low risk chelation group, median survival was not reached at 250 months. Therefore, a parabolic function was fit to the data to permit estimation of the median time to survival of 285 months. The distribution of patients assumed in the model to have a baseline low or int‐1 IPSS score was 45.8% and 54.2%, respectively, derived from the Rose et al. study (2007)Citation26. The proportion of patients aged over 77 years or ≥77 years of 56.5% and 43.5%, respectively was estimated from an MDS database in a large UK treatment centreCitation28. The same proportions were applied to both low and int‐1 risk IPSS groups.
AML progression risk
MDS patients are clinically at risk of progression to AML with a consequent higher hazard of death and quality of life decrementCitation29. An annual risk of progression to AML of 19.5% for patients not receiving ICT compared to 5.3% for patients receiving ICT (based on assuming 100% compliance to ICT) was derived from a study using retrospective data for 178 MDS patients either receiving or not receiving DFO between 1981 and 2006 in a large Canadian hospitalCitation11. Once patients had progressed, an annual risk of mortality associated with AML in MDS patients of 42.5% was applied in the modelCitation5, which was assumed independent of ICT status. In the model, patients who have progressed to AML are assumed to no longer receive chelation therapy.
Complications of chronic iron overload
Chronic iron overload in haemoglobinopathies and transfusion-dependent MDS is associated with a risk of cardiac disease, diabetes and hepatic complicationsCitation6. Although the impact of complications on survival is already indirectly taken into account in the Rose et al. survival data, these data do not enable calculation of the risks of complications and impact on health-related quality of life. Direct data on the risks of complications in patients with MDS according to ICT status and type of ICT were not available. Hence, a number of sources were combined to estimate the risk of complications by ICT versus no-ICT to use in the model. Firstly, an annual incidence of 14.1%, 3.2% and 7.6% for cardiac disease, diabetes mellitus and hepatic complications, respectively was derived from a study in 46 transfusion-dependent MDS patientsCitation30. Secondly, the risk of complications for patients receiving iron chelation compared to those without chelation was derived from a large retrospective, case-controlled study using a US health insurance claims database with over 4,500 MDS patientsCitation31. In this study <1% of patients were receiving ICT, so those patients with ≥4 transfusion episodes were assumed to represent patients with risks of complications associated with non-chelation, and patients with <4 transfusion episodes were used as a proxy for the complication rate associated with ICT. Odds ratios for decrease of complications of 0.35 for cardiac disease, 0.07 for diabetes and 0.35 for hepatic complications for <4 (ICT proxy) and ≥4 transfusion (no-ICT proxy) groups were calculated from this study and used as inputs into the economic model.
Compliance
Although there is published evidence of better compliance to deferasirox (and other oral therapies) relative to DFO in β-thalassaemia patientsCitation32 similar published comparative data for differences in compliance in MDS patients are not available. The 1‐year EPIC study of the efficacy and safety of deferasirox involving patients with transfusion-dependent MDS previously treated with DFO estimated 85.7% (n = 36) at the end of study reported that they always adhered to treatment compared to 62.5% (n = 35) at baselineCitation33. Therefore, it is likely that in clinical practice compliance would be lower for DFO in MDS patients but due to limited data it was assumed in the economic model that both ICTs would have the same average compliance rate of 85.7% (75% and 100% compliance was explored in sensitivity analysis).
A linear association between compliance and annual risk of AML progression, and with complication rates is assumed. Hence, a 0.14% increase in risk of AML associated with each 1% decrease in compliance produced an estimated compliance adjusted risk of AML progression with ICT of 7.3% which was applied in the economic model. Adjusting the odds ratios for complications with ICT, compliance adjusted annual risks of 6.69%, 0.67% and 3.5% for cardiac disease, diabetes and hepatic complications, respectively were applied in the model.
Doses administered
In the base case the dose of deferasirox administered was estimated to be 20 mg/kg/day for 7 days per week, which was similar to the mean doses in the recent clinical studies of deferasirox in transfusion-dependent MDS patientsCitation17,Citation19, and was validated by UK clinical opinion consultedCitation15. There is limited information on the dose of DFO used in transfusion-dependent MDS patients in clinical practice. A dose of 40 mg/kg/day for 5 days per week was applied in the economic analysis, which was the standard dose for continuous subcutaneous infusion in the patients receiving DFO in the prospective study of Rose et al.Citation8. This was verified as reasonable by the three UK haematologists consultedCitation15.
Utility outcomes
The quality of life associated with various health states for transfusion-dependent patients with MDS receiving ICT was estimated on a 0–1 utility scale (zero representing a state equivalent to death, and one a state approximating to perfect health) in order to derive QALY outcomes. Patients receiving deferasirox were estimated to have a quality of life (utility) score of 0.84, whereas those receiving DFO had an estimated utility of 0.66, with a mean difference of 0.176. These values were derived from a published study using a time trade-off methodology in a UK general public sample designed to elicit utilities for ICT provided via a once-daily oral ICT formulation (representing deferasirox) and for a subcutaneous infusion administered for 8–12 hours per day for 5 days per week (representing DFO)Citation16.
If a complication associated with ICT was experienced this was associated with a decrement in quality of life for patients receiving deferasirox or DFO. Utility decrements of 12.5% and 11.1% were estimated for cardiac and diabetes complications, respectively, derived from the Beaver Dam Health Outcomes study, a longitudinal cohort study of health-related quality of life in a random sample of 1,356 US adultsCitation34. A utility decrement of 8% was estimated for hepatic complications using data derived from a study in patients with hepatitis BCitation35. For each complication event, the utility decrement was subtracted from the utility for MDS patients receiving either deferasirox or DFO.
Patients who progressed to AML were assumed to cease ICT and were assigned a quality of life (utility) value of 0.257. This was based on the weighted average utility for patients with and without bone marrow transplants derived from a published study using a generic utility measure, the Health Utilities Index 2 (HUI2)Citation36.
The adverse event profiles of deferasirox and DFO are similar, and are assumed not to have a differential impact on patient quality of life or costs of treatment, hence have not been further considered in the economic model.
Costs
Drug costs
The unit drug acquisition cost per mg/kg for deferasirox is £16.80 per 500 mg and is £4.26 per 500 mg of non-proprietary desferrioxamine. The costs for deferasirox and DFO were derived from the British National Formulary 59 (March 2010)Citation37. This cost of non-proprietary DFO was used rather than the cost for the brand Desferal as the majority of DFO prescriptions in the UK are for the former (the unit cost for 500 mg of Desferal is £4.66 per 500 mg)Citation38. The daily drug cost for deferasirox is £47.04 for a 70 kg patient receiving an average dose of 20 mg/kg/day, 7 days per week. For DFO drug acquisition, cost is £23.86 per day for 5 days per week (i.e., £17.04 per day averaged over 365 days) for a 70 kg patient receiving 40 mg/kg/day. Adjusted for 85.7% compliance in the base case, total daily costs for deferasirox were estimated to be £45.26 compared to £35.40 for DFO.
Drug administration
As deferasirox is an oral formulation dispersed in water, no additional cost of administration is assumed to be incurred. For DFO, infusion for 8–12 hours a day incurs an administration cost related to the infusion appliance and disposables. DFO is administered as a subcutaneous infusion using either a battery-operated pump or an elastomeric balloon infuser. An annual cost of £8,344 from the Karnon et al. economic evaluation of deferasirox versus DFO in β-thalassaemia patients (uprated to 2009 values)Citation16 was applied for DFO equipment and disposable costs in the current model. This equates to an average daily cost for DFO administration of £22.86 per day.
Monitoring costs
Monitoring requirements have been specified for deferasirox including monthly, yearly and as needed testsCitation21. The monitoring requirements as specified in the SPC for DFO are less explicitCitation22. There are a number of tests listed in the deferasirox SPC that would also be expected to be recommended for DFO (e.g., monthly tests for serum ferritin test, bilirubin, alkaline phosphate, proteinuria), but are not explicitly mentioned in the DFO SPC. DFO has been on the market for about four decades and generally SPCs for older products are less detailed and explicit than for newer products. However, to be conservative only the tests explicitly specified for deferasirox and DFO have been utilised in the base-case analysis (). In sensitivity analysis, based on clinical opinionCitation15, the only additional test assumed to be required for deferasirox is a monthly blood test for serum creatinine, as deferasirox has been found to result in raised serum creatinine in some patients that can be kept within normal limits once identified.
Table 1. Costs of monitoring tests for deferasirox and DFO.
Costs of AML, complications and blood transfusions
The annual costs associated with the treatment and care of MDS patients with AML, cardiac, diabetes and hepatic complications are estimated based on published data sources for these conditions. There are no published estimates for the healthcare costs of AML in the UK, hence an estimate of £20,485 derived from a US studyCitation39 and converted from a dollar to a UK cost estimate using Gross Domestic Product (GDP) purchasing power parities ($1 = £0.66, OECD 2009) has been applied. The costs estimated for AML include treatment, hospitalisation, blood transfusion and other related medical costs. The annual costs of cardiac disease, diabetes and hepatic complications use data from UK published sourcesCitation40–42 and are estimated to be £6,208, £4,187 and £2,144 per patient, respectively in 2008 values. Analysis was performed from a healthcare payer perspective, hence indirect costs such as loss of productivity were not considered.
In addition, patients who remain alive without AML are assumed to continue requiring blood transfusions and incurring specific costs associated with these until progression to AML occurs. The annual cost of blood transfusions per patient applied in the model was £11,052 assuming an average requirement of 3 units per month for transfusion-dependent MDS patients, and a unit cost of £307 per red blood cell transfused (estimate uprated to 2008 values). The cost was derived from a published study on the costs of blood transfusions in the UKCitation43.
Analyses
As no difference in clinical efficacy and compliance between deferasirox and DFO is assumed, the model base-case analysis provides a prediction of life-years, complications and AML incidence for patients receiving ICT with either deferasirox or DFO. The outcome measure used to assess cost effectiveness is the quality-adjusted life-year (QALY). Deferasirox generates additional QALYs due to the quality of life benefits associated with oral administration but at higher drug-related costs, hence cost effectiveness is presented as the incremental cost per QALY gained estimated for deferasirox versus DFO. Discounting of costs and QALYs has been performed at the rate of 3.5% per annum for both, in line with current UK standards for economic evaluationsCitation44.
To examine the impact of uncertainty in key variables on the cost-effectiveness results, sensitivity analysis was performed on variables affecting drug-related costs: patient weight, average daily dose of deferasirox and DFO, days/week of treatment with DFO, and DFO administration costs. There is considerable local and regional variation in the type of equipment used for the administration of DFO, which affects DFO administration costs. The base-case cost assumes 79% use of a balloon infuser and 21% use of a battery-operated pump for the subcutaneous infusion of DFO based on a retrospective audit in the UK covering MDS, β-thalassaemia, and SCD patientsCitation45. The balloon infuser has advantages in terms of noise, convenience and potentially compliance but is more expensive than a battery-operated pump, hence a scenario was examined in which only 50% of patients used a balloon infuser, consequently reducing the cost of DFO administration.
In the base case, significantly higher monitoring costs were assumed for deferasirox. As in clinical practice the difference in monitoring is not likely to be as extensive, a scenario was also explored in which the only additional monitoring cost assumed for deferasirox over DFO was monthly serum creatinine monitoring. Utility associated with mode of drug administration was varied using the 95% confidence intervals for mean utilities. Finally, the assumptions regarding compliance were also tested in sensitivity analysis.
presents an overview of the key input data in the model for the base case, and a summary of the specific sensitivity analyses performed on key variables.
Table 2. Base case model inputs and sensitivity analysis.
Results
Base case
The long-run clinical outcomes estimated from the model for deferasirox or DFO based on an assumption of equal compliance (of 85.7%) are presented in for a cohort of 1,000 transfusion-dependent MDS patients who are IPSS low or int-1 risk. Mean survival was estimated from the model as 8.41 life-years (not discounted, or 6.83 life-years discounted) with ICT, compared to 3.95 life-years without ICT (deferasirox or DFO treatment). From the model it is estimated that per 1,000 MDS patients, 566 patients will progress to AML with either ICT therapy, 350 will have cardiac disease, 214 hepatic complications and 48 will become diabetic ().
Table 3. Base-case results (per patient and for 1000 cohort, lifetime outcomes and costs).
In terms of QALY outcomes there is a total estimated discounted QALY gain of 1.03 per patient for deferasirox over DFO. To provide a benchmark, the outcomes predicted by the model for a no chelation scenario was a mean of 1.73 QALYs (discounted).
These estimated HRQoL benefits for deferasirox over DFO are obtained at an additional discounted lifetime drug, administration and monitoring cost of £21,500 per patient (£26,337 if not discounted). There were no differences in costs of blood transfusions, management complications or AML between deferasirox and DFO due to the assumption of equivalent iron burden and compliance assumptions ().
Therefore, combining the cost and QALY results, the base-case incremental cost effectiveness of deferasirox versus DFO is estimated to be £20,822 per QALY gained (discounted) ().
Sensitivity analyses
The results from sensitivity analysis are presented in . Cost effectiveness is sensitive to the mean dose of deferasirox assumed. A lower average daily dose of deferasirox of 15 mg/day would lead to deferasirox ‘dominating’ DFO (i.e., lower net costs and higher QALYs than DFO), whereas a higher average daily dose of 25 mg/day would result in an incremental cost per QALY gained of over £40,000 for deferasirox (). The results were reasonably sensitive to varying other cost parameters including patient weight, DFO dose, and the administration costs of DFO (). A scenario assuming the only additional monitoring cost for deferasirox is for serum creatinine monitoring improved the cost effectiveness of deferasirox over DFO to £13,861 per QALY gained ().
Table 4. Sensitivity and scenario analysis on key variables.
The analysis of the impact of varying compliance shows that if patients fully complied to both treatments the estimated cost effectiveness of deferasirox would be £22,813 per QALY gained, whereas a scenario whereby compliance was lower (assumed 75%), the incremental cost per QALY gained for deferasirox is £19,291 ().
Discussion
The cost effectiveness of ICT with deferasirox has been demonstrated in patients with β-thalassaemia in the UK and US healthcare contextsCitation16,Citation46. However, there have been no previous published analyses of the cost effectiveness of ICT in transfusion-dependent MDS patients. We have performed a cost-effectiveness analysis comparing deferasirox to DFO iron chelation therapy for the treatment of chronic iron overload (defined as a serum ferritin of over 1000 μg/L) in transfusion-dependent MDS patients with low or int-1 IPSS risk. This demonstrated an incremental cost per QALY gained for deferasirox compared to DFO of £20,822 for a cohort of low and int-1 risk MDS patients with an average weight of 70 kg simulated over a lifetime. This is at the lower end of the threshold of £20,000 and £30,000 per QALY gained used by UK HTA bodies (NICE, SMC and All Wales Medicines Strategy Group) to represent a cost-effective use of healthcare resourcesCitation44,Citation47.
Supporting this economic evaluation are recent clinical studies demonstrating the efficacy of deferasirox in reducing serum ferritin in transfusion-dependent MDS patients with iron overload over 1 year (the EPIC study)Citation17, and over a 2-year follow-up periodCitation19. In addition, an empirical relationship has been found between transfusional iron overload and poorer survival outcomes in MDSCitation5, with risk highest for low/int-1 risk MDS patients due to their better survival prognosis, making it more likely that they will require more red blood cell transfusions over an extended periodCitation29.
Four recent observational studies have all shown an association between use of ICT in transfusion-dependent low/int-1 risk MDS patients and improved survival outcomesCitation8–11. The current modelled analysis of survival outcomes for low and int-1 risk patients utilised data from the prospective Rose et al. studyCitation26, which has recently been publishedCitation8. Mean survival from ICT therapy was estimated through the economic model to be 8.41 years per patient, an undiscounted survival benefit of 4.46 years compared to no ICT. This is similar to the direct estimates of Rose et al. (estimated to be 5.9 years benefit)Citation8 and Raptis et al. (estimated to be 4 years benefit)Citation10, and higher than the benefit estimated by Fox et al. (2 years)Citation9. The current study Markov model has indicated that ICT with deferasirox or DFO (assuming the same compliance rate) produces survival benefits, although there are additional healthcare costs incurred in the additional survival time.
There is a lack of comparative data on the relative clinical efficacy, AML and complications risk or survival outcomes associated with deferasirox versus DFO in MDS. Therefore, in the economic model both drugs were assumed to result in equivalent clinical and survival outcomes in the base case. However, deferasirox was estimated to produce over a lifetime horizon additional QALYs per patient of 1.03 (discounted) over DFO. This outcome is driven by the additional quality of life (utility) benefits associated with the once-daily oral mode of administration for deferasirox compared to subcutaneous infusion over 5–7 days per week with DFO. Use of DFO has been found to have a detrimental impact on a number of areas of patients’ lives including their emotional well-being, physical functioning, self-esteem, work and sleepCitation48. In contrast, once-daily oral deferasirox has been shown to be associated with an increased treatment satisfaction and improved health-related quality of lifeCitation33,Citation49,Citation50. The utility estimate used in the model for this benefit was from a study of the UK public comparing once daily oral ICT with subcutaneous infusion ICT which found a 0.18 utility difference in favour of the former mode of administrationCitation16. This value is less than the 0.23 difference estimated between the two modes of administration in a similarly designed Australian studyCitation51. As the UK utility study was conducted in members of the public, the utilities generated represent a societal valuation of the quality of life benefits of once daily oral ICT. This is in line with the approach preferred by Health Technology bodies in the UK when assessing the value of treatmentsCitation44,Citation47.
The outcomes generated by the model are based on an equivalent compliance rate of 85.7% for both deferasirox and DFO. An equivalent rate was chosen in the base case due to a lack of evidence in MDS for differences in compliance rate, although there is indicative data suggesting a poorer compliance for DFOCitation33. This is also intuitive given the relatively burdensome mode of DFO administration, and is supported by a greater body of data in β-thalassaemiaCitation46,Citation52.
The economic analysis has demonstrated that deferasirox is associated with additional drug acquisition and monitoring costs compared to non-proprietary DFO, which are partly offset by the higher drug administration costs associated with DFO. There is still an estimated additional total discounted cost of £21,500 per patient for deferasirox drug, administration and monitoring cost over the remaining life expectancy of the patient cohort. The cost differential is lower if more moderate differences in monitoring requirements are assumed. The deferasirox SPCCitation21 specifies a range of monthly tests, including serum ferritin, serum creatinine, bilirubin, proteinuria, cardiac dysfunction and other tests. In contrast, the DFO SPCCitation22 is less detailed and only mentions auditory/ophthalmic testing but is highly likely in practice to also involve a number of the same tests as for deferasirox (e.g., serum ferritin, cardiac dysfunction monitoring). Additional costs and/or disutilities may be associated with possible infections and injection site reactions associated with DFO administration which have not been included in the economic analysis. A scenario was examined in sensitivity analysis in which the only additional costs of monitoring for deferasirox was serum creatinine monitoring, with this improving the cost effectiveness of deferasirox to £13,827 per QALY gained. In contrast, deferasirox cost effectiveness is reduced to over £30,000 per QALY gained if a greater proportion of patients than assumed in the base case receive the cheaper battery pump for administration of DFO.
In further sensitivity analyses the cost-effectiveness results were most sensitive to scenarios relating to deferasirox dose, days of DFO administration and patient weight. If deferasirox dose were as high as 25 mg/kg/day then the cost per QALY gained would be over £40,000. However, this is a particularly high dose for MDS patients and all deferasirox trials in MDS have shown a daily maintenance dose of about 20 mg/kg/day, which is also supported by UK clinical practice. It is also likely that in the limited number of patients who require a high dose of deferasirox, the equivalent dose required of DFO would also be higher hence improving the cost-effectiveness results. A low dose of 15 mg/kg/day of deferasirox would lead to lower net costs for deferasirox and is an option for MDS patients once serum ferritin has become stabilised at consistently low levels. Assuming the same outcomes with only 3 days of DFO administration increases the cost/QALY of deferasirox to £33,160 while an assumption of 7 days improves cost effectiveness to £8,485, although both extremes are relatively unlikely in clinical practice. The results were also dependent on mean patient weight – varying this from 60 kg to 80 kg produced cost per QALY gained results of between £13,000 and £28,000.
As with all economic models, there were several limitations with the economic analysis performed. In particular, there is a lack of comparative RCT data on clinical and survival outcomes associated with deferasirox and DFO in MDS patients. All the data on serum ferritin reduction, AML risk and survival are from observational studies. Another limitation is the lack of comparative data on compliance in MDS patients. Also the published utilities used for mode of administration are based on a reference case in β-thalassaemia rather than MDSCitation16. While the study was conducted in a general public sample rather than patients (hence reflecting the societal perspective recommended by NICE) and the health state descriptions for mode of administration of oral versus subcutaneous ICT infusion are broadly generalisable across patient populations (see Karnon et al. for the descriptions)Citation16, it would be useful in further research to verify these utility values using a low/int-1 risk MDS reference case. Finally, the comparator has been assumed to be non-proprietary DFO which has a lower unit cost to branded DFO (Desferal). Although non-proprietary DFO is the most used ICT in the UK, there is some use of Desferal in clinical practice which as shown in the sensitivity analysis would further improve the relative cost effectiveness of deferasirox. A further potential limitation of the analysis is that a probabilistic sensitivity analysis (PSA), in which the joint uncertainty of key parameters in the model are assessed, has not been performed. The economic analysis was developed originally for potential use with a UK health technology assessment body (the Scottish Medicines Consortium) that does not require PSA, but does require sufficiently extensive univariate sensitivity analysisCitation47. Hence, as PSA is not essential for all key UK decision makers the focus of the present sensitivity analysis was to use univariate sensitivity analysis in order to illustrate the key variables that the base-case incremental cost-effectiveness ratio is sensitive to.
Conclusions
While ICT has become standard in β-thalassaemia there has been lower utilisation in transfusion-dependent MDS patients – a US study found that only 41% of ICT-eligible patients with lower risk MDS received ICT in clinical practice, with treatment initiated later than recommendedCitation10. Similar utilisation evidence is not available for the UK, although clinical opinion consulted was that UK practice is variable across treatment centres, with DFO typically the standard ICT usedCitation15.
However, for the first time an assessment of the cost effectiveness of deferasirox versus non-proprietary DFO in lower risk transfusion-dependent MDS patients has been produced. It has demonstrated that deferasirox has the potential to be a cost-effective alternative to DFO for use in these patients, driven by the lifetime benefits in quality of life associated with the oral mode of administration for deferasirox. The value of the economic model for policy making could be enhanced further if comparative RCT evidence on clinical and survival outcomes for deferasirox versus DFO in lower risk transfusion-dependent MDS patients were to become available.
Declaration of interest
This study was funded by Novartis Pharmaceuticals, Inc.
Transparency
Declaration of financial/other relationships
K.T. has disclosed that he is an employee of Tolley Health Economics Ltd., a company that received funding from Novartis to conduct this study. Likewise, K.M.-W., D.B. and Q.L. have disclosed that they are employees of United BioSource, a company that also received financial support from Novartis to conduct this study. N.O. and E.M. have disclosed that they are employees of Novartis.
Notes
*Exjade is a registered trade name of Novartis AG, CH-4002, Basel, Switzerland.
*Exjade is a registered trade name of Novartis AG, CH-4002, Basel, Switzerland.
*Subsequent to economic model development the observational study of Rose et al. has been published. This reports a 2.5-year follow-up, with similar results as in the source used for the economic model (i.e., median overall survival of 124 months vs. 53 months in the ICT and no ICT patients, respectively, p < 0.0001)Citation8.
*Desferal is a registered trade name of Novartis AG, CH-4002, Basel, Switzerland.
References
- Gattermann N. Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol 2008;88:24-29
- Wells RA, Leber B, Buckstein R, et al. Iron overload in myelodysplastic syndromes: a Canadian consensus guideline. Leuk Res 2008;32:1338-1353
- Srinivasan S, Schiffer CA. Current treatment options and strategies for myelodysplastic syndromes. Expert Opin Pharmacother 2008;9:1667-1678
- Frytak JR, Henk HJ, De Castro CM, et al. Estimation of economic costs associated with transfusion dependence in adults with MDS. Curr Med Res Opin 2009;25:1941-1951
- Malcovati L, Porta MG, Pascutto C, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 2005;23:7594-7603
- Bennett JM. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol 2008;83:858-861
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology™: Myelodysplastic Syndromes. 1-31 2008. www.nccn.org
- Rose C, Brechignac S, Vassilief D, et al. Does iron chelation therapy improve survival in regularly transfused lower risk MDS patients? A multicenter study by the GFM (Groupe Francophone des Myelodysplasies). Leuk Res 2010;34:864-870
- Fox F, Kundgen A, Nachtkamp K, et al. Matched-pair analysis of 186 MDS patients receiving iron chelation therapy or transfusion therapy alone. Blood (ASH meeting abstracts) 114, 2009
- Raptis A, Duh MS, Wang ST, et al. Treatment of transfusional iron overload in patients with myelodysplastic syndrome or severe anemia: data from multicenter clinical practices. Transfusion 2009
- Leitch HA, Wong DHC, Leger CS, et al. Improved leukaemia-free and overall survival in patients with myelodysplastic syndrome receiving iron chelation therapy: a subgroup analysis. Abstract 1469. 49th American Society of Haematology meeting, Atlanta Georgia USA, 8-11th December, 2007
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 2002;100:2292-2302
- Cappellini MD, Cohen A, Piga A, et al. A phase 3 study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood 2006;107:3455-3462
- Swedish Orphan International Ltd. Ferriprox Summary of Product Characteristics, 2008
- Novartis Pharmaceuticals. Opinions of three UK clinicians treating MDS patients. Data on file, 2009
- Karnon J, Tolley K, Oyee J, et al. Cost-utility analysis of deferasirox compared to standard therapy with desferrioxamine for patients requiring iron chelation therapy in the United Kingdom. Curr Med Res Opin 2008;24:1609-1621
- Cappellini MD, Porter J, El-Beshlawy A, et al. Tailoring iron chelation by iron intake and serum ferritin: prospective EPIC study of deferasirox in 1744 patients with transfusion-dependent anemias. Haematologica 2009;95:557-566
- Schmid M, Guerci-Bresier A, Porta MD, et al. Deferasirox (Exjade®) is effective and well tolerated in chelation-naive and previously chelated patients with transfusion-dependent myelodysplastic syndromes (MDS). Abstract 3806. 51st American Society of Haematology meeting, New Orleans, LA, USA, 6--9th December, 2009
- List A, Baer M, Steensma D, et al. Two-year analysis of efficacy and safety of deferasirox (Exjade®) treatment in myelodysplastic syndrome patients enrolled in the US03 study. Abstract 3829. 51st American Society of Haematology meeting, New Orleans, LA, USA, 6-9th December, 2009
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997;89:2079-2088
- Novartis Pharmaceuticals UK Ltd. Deferasirox summary of product characteristics. 2009. http://emc.medicines.org.uk/
- Novartis Pharmaceuticals UK Ltd. Desferrioxamine summary of product characteristics. http://emc.medicines.org.uk/
- Vichinsky E, Onyekwere O, Porter J, et al. A randomised comparison of deferasirox versus deferoxamine for the treatment of transfusional iron overload in sickle cell disease. Br J Haematol 2007;136:501-508
- Porter JB. Concepts and goals in the management of transfusional iron overload. Am J Hematol 2007;82:1136-1139
- Glanville J, Eleftheriou P, Porter J. MRI evidence of cardiac iron accumulation in myelodysplasia and unusual anaemias. Blood (ASH annual meeting abstracts) 108, 2006
- Rose C. Iron chelation therapy improves survival in regularly transfused MDS Patients. A prospective analysis by the GFM. Slides presented at American Society of Haematology (ASH) meeting 2007 Data on file, 2007
- Rose C, Brechignac S, Vassilief D, et al. Positive impact of iron chelation therapy (CT) on survival in regularly transfused MDS patients. A prospective analysis by the GFM. Abstract 249, 49th American Society of Haematology meeting, Atlanta Georgia USA, 8-11th December, 2007
- Novartis Pharmaceuticals. Western General Hospital data on MDS. Personal correspondence data on file, 2008
- Sanz G, Nomdedeu B, Such E, et al. Independent impact of iron overload and transfusion dependency on survival and leukaemic evolution in patients with myelodysplastic syndrome. Blood (American Society of Haematology Annual Meeting Abstracts) 2008 112: Abstract 640, 2008
- Jaeger M, Aul C, Sohngen D, et al. Iron overload in polytransfused patients with MDS: Use of L1 for oral iron chelation. Drugs Today 1992;28(Suppl A):143-147
- Delea TE, Hagiwara M, Phatak PD. Retrospective study of the association between transfusion frequency and potential complications of iron overload in patients with myelodysplastic syndrome and other acquired hematopoietic disorders. Curr Med Res Opin 2009;25:139-147
- Cappellini MD, Bejaoui M, Agaoglu L, et al. Prospective evaluation of patient-reported outcomes during treatment with deferasirox or desferroxamine for iron overload in patients with beta-thalassemia. Clin Ther 2007;29:909-917
- Porter JB, Bowden D, Ganser A, et al. Satisfaction and adherence significantly improves in patients with β-thalassaemia and myelodysplastic syndromes treated with deferasirox (Exjade®). Abstract 1306. 50th American Society of Haematology meeting, San Francisco, California USA, 6-9th December, 2008
- Fryback DG, Dasbach EJ, Klein R, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making 1993;13:89-102
- Wong JB, Koff RS, Tine F, et al. Cost effectiveness of interferon-alpha 2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med 1995;122:664-675
- Barr R, Furlong W, Henwood J, et al. Economic evaluation of allogeneic bone marrow transplantation: a rudimentary model to generate estimates for the timely formulation of clinical policy. J Clin Oncol 1996;14:1413-1420
- British National Formulary 59. March 2010. Deferasirox. http://bnf.org/bnf/bnf/59/129929.htm?q=exjade&t=search&ss=text&p=1#_129929
- Sourced from Chemist and Druggist website 26/02/2010. http://www.chemistanddruggist.co.uk/
- Menzin J, Lang K, Earle CC, et al. The outcomes and costs of acute myeloid leukemia among the elderly. Arch Intern Med 2002;162:1597-1603
- Luengo-Fernandez R, Leal J, Gray A, et al. Cost of cardiovascular diseases in the United Kingdom. Heart 2006;92:1384-1389
- Diabetes UK. Diabetes. Beware the silent assassin. A report from Diabetes UK, October 2008. http://www.diabetes.org.uk/Documents/Reports/Silent_assassin_press_report.pdf
- Shepherd J, Jones J, Hartwell D, et al. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007;11:1-205
- Varney SJ, Guest JF. The annual cost of blood transfusions in the UK. Transfus Med 2003;13:205-218
- National Institute of Health and Clinical Excellence (NICE). Guide to the methods of technology appraisal. June 2008
- Desrosiers MP, Payne K, Proskorovsky I, et al. Estimated total annual costs of infused iron chelation therapy in the United Kingdom. Poster 0810. 11th Congress of the European Haematology Association, Amsterdam, The Netherlands, 15-18th June 2006
- Delea TE, Sofrygin O, Thomas SK, et al. Cost effectiveness of once-daily oral chelation therapy with deferasirox versus infusional deferoxamine in transfusion-dependent thalassaemia patients: US healthcare system perspective. Pharmacoeconomics 2007;25:329-342
- The Scottish Medicines Consortium (SMC). Guidance to Manufacturers for completion of New Product Assessment Form (NPAF) (Clinical and Economic Sections combined). 2010. Available from: URL: http://www.scottishmedicines.org.uk/
- Abetz L, Baladi JF, Jones P, et al. The impact of iron overload and its treatment on quality of life: results from a literature review. Health Qual Life Outcomes 2006;4:73
- Taher A, Cappellini MD. Update on the use of deferasirox in the management of iron overload. Ther Clin Risk Manag 2009;5:857-868
- Porter JB, Bowden D, Ganser A, et al. Improved health-related quality of life in patients with haematological disorders receiving deferasirox (Exjade®). Blood (American Society of Haematology Annual Meeting Abstracts) 2008 112. Abstract 1307
- Osborne RH, De Abreu LR, Dalton A, et al. Quality of life related to oral versus subcutaneous iron chelation: a time trade-off study. Value Health 2007;10:451-456
- Arboretti R, Tognoni G, Alberti D. Pharmacosurveillance and quality of care of thalassaemic patients. A large scale epidemiological survey. Eur J Clin Pharmacol 2001;56:915-922