Abstract
Objective:
Pre-eclampsia (PE), a leading cause of maternal and perinatal morbidity and mortality, is only detected after symptomatic onset. Early diagnosis may be possible with a new serum test, with resulting clinical and economic benefits versus standard practice. The authors evaluated the financial impact to the UK National Health Service (NHS).
Methods:
A decision-analytic model was developed in which a cohort of 1,000 pregnant women receiving UK obstetric care was simulated. The economic impact of improved sensitivity and specificity of the novel PE test [Roche Diagnostics, Rotkreuz, Switzerland] over current diagnostic practice was modeled. While there is no specific approved diagnostic test to detect PE, physicians are using a combination of tests including blood pressure, proteinuria, Doppler, serum uric acid, etc. The novel PE test constitutes two novel biomarkers Placenta Growth Factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) which can be quantitatively analyzed using an automated system widely available in hospitals or laboratories (Elecsys/Cobas, Roche Diagnostics) and measures the levels of PlGF and sFlt-1 growth factors in pregnant women. The analysis assumed administration of the £31.13 test (the equivalent of 52 Swiss Francs [CHF]) after 20 weeks of gestation as an addition to current practice. True-positive and false-negative patients were assumed to develop mild or severe PE, eclampsia, or death. A hybrid research approach was adopted; when available, data for model inputs were obtained from published literature and public databases. Interviews with obstetricians, laboratory managers, and healthcare payers were used to validate model inputs and fill utilization-related data gaps.
Results:
The model estimates that the costs associated with managing a typical pregnancy are £1,781 per patient when the new test is used versus £2,726 with standard practice. This represents savings of £945 per pregnant woman, if the test is used as a supplementary diagnostic tool. The savings are attributed to the new test's improved performance and its ability to better classify the pregnant patients.
Conclusions:
The novel test has the potential to provide substantial cost savings for NHS. Even when the novel test's cost is added to the current cost of care, the benefits exceed the additional cost, driven by the test's ability to reduce the rates of false-positive and false-negative diagnoses compared to current standard of care. Potential study limitations include the use of a pooled average of the individual sensitivities and specificities of currently used tests since no data were available on combination testing, the reliance on clinical trial data versus actual practice, and the use of clinical expert opinion when published data were unavailable.
Key words::
Introduction
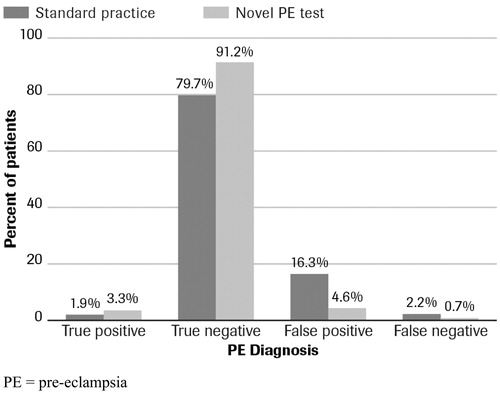
Pre-eclampsia (PE) complicates approximately 4–8% of all pregnancies and remains a leading cause of maternal and perinatal morbidity and mortalityCitation1,Citation2. Resource-intensive interventions are the current standard of care, requiring frequent monitoring of maternal hypertension and proteinuria; similarly, fetal growth is routinely monitoredCitation3. Time-sensitive treatment, including seizure prophylaxis, the use of corticosteroids, and early delivery, is crucial for prevention of serious complicationsCitation3. However, no reliable diagnostic tests for PE exist to allow detection prior to the development of clinical symptoms, and diagnosis depends on the frequent assessment of pregnant women with known risk factors, although many may never develop PE. Furthermore, analysis of the published performance data of current PE tests reveals that about 2.2% of PE cases remain undetected (see ), resulting in sub-clinical PE that often requires aggressive late-term intervention and managementCitation3,Citation4.
Angiogenic growth factors such as placenta growth factor (PlGF) and soluble fms-like tyrosine kinase-1 (sFlt-1) have been found to play a major role in the development of pre-eclampsiaCitation5–12. Elevated sFlt-1Citation5–10 while decreased PlGF has been demonstrated in women diagnosed while pre-eclampsiaCitation5,Citation8,Citation10,Citation11, making them potential biomarkers for use in confirming diagnosis of pre-eclampsia. Elevation of sFlt-1 has also been shown to be able to differentiate between women with pre-eclampsia and those with gestational hypertension, and could play a role in differential diagnosisCitation7. Noori et al. 2010Citation1Citation2 demonstrate the development of pre-eclampsia by providing evidence of temporal sequence of changes in maternal vascular function and circulating levels of PlGF and sFlt-1 in women who later develop pre-eclampsia and the relationship between these measures.
The novel PE test constitutes the two novel biomarkers PlGF and sFlt-1 which can be quantitatively analyzed using an automated system widely available in hospitals or laboratories (Elecsys/Cobas, Roche Diagnostics) and measures the levels of PlGF and sFlt-1 growth factors in pregnant women. A multicenter case-control studyCitation13 evaluated the novel PE test for sFlt-1 and PIGF and tested the value of the sFlt-1/PIGF ratio in the assessment of PE. The study included 351 patients in two arms – 71 patients with PE and 280 gestational age-matched control subjects from five European study centers. A total of 595 serum samples were measured for sFlt-1 and PIGF using an automated platform. Results showed that maternal serum concentrations of sFlt-1 and PIGF significantly separated healthy women and women with PE. An optimal cut-off for the sFlt-1/PlGF ratio of 85:1 was determined, resulting in a calculated sensitivity of 82% and a specificity of 95% for testing after 20 weeks of gestationCitation13.
An improved diagnostic test may have the potential to decrease the number of false-positive diagnoses and appropriately identify the false-negative patients (sub-clinical presentations). The former will improve patient classification and has the potential to reduce the costs of monitoring these patients and possibly avert unnecessary hospital admissions. The latter can potentially decrease the likelihood of complications among these patients as they benefit from earlier detection and appropriate management.
Economic analyses of new clinical tools are becoming increasingly important for healthcare policy decisions to support institutional and public planning efforts, especially in an environment characterized by limited resources and rising costs. The goal of this study was to provide an economic evaluation of the new PE diagnostic test from the UK and German healthcare payer perspectives. A budget impact model, a decision-analytic software tool, was developed to quantify the potential financial impact of adding the novel PE test to the standard PE testing paradigm. The current paper reports the results of the model developed for the UK. The work conducted for the German market will appear in Hypertension in Pregnancy journal. To the best knowledge of the authors, this is the first UK budget impact model to evaluate in vitro PE testing.
Methods
Model objectives and overview
A budget impact model was developed to assess the economic impact resulting from improved sensitivity and specificity of the novel PE test over current, standard PE diagnostic practice. Using a series of linked worksheets, the model compared two PE testing paradigm scenarios: (1) standard practice, as currently followed by UK physicians in diagnosing PE. Physicians currently use a combination of interventions to diagnose PE, including blood tests such as serum uric acid, urine tests (to screen for proteinuria), blood pressure measurements, and uterine artery Doppler ultrasounds; (2) novel PE test, which simulates patient classification and management after the novel PE test is added to the existing testing armamentarium to aid in the diagnosis of PE. For the model, a conservative approach was taken, assuming that while physicians familiarize themselves with the test, they will continue to use standard approaches. Hence, in the model, the novel PE test was used as an additive versus a replacement test. For the purposes of discussion, the two scenarios will be referred as (a) ‘standard practice’ and (b) ‘novel PE test’.
As shown in , the patient population encompassed by the model is a hypothetical cohort of 1,000 pregnant women in the UK. Per the antenatal guidelines from the National Institute for Clinical Excellence (NICE), physicians are required to (1) measure blood pressure and analyze urine for protein at each antenatal visit to screen for PE, and (2) stratify the patients as high risk for PE at the booking period. The ‘booking period’ is defined as the period of time when a doctor confirms that a patient is pregnant and conducts baseline exams to assess the patient's condition; this usually takes place during weeks 8–12 of gestation. Furthermore, per the NICE guidelines, high risk PE patients are required to visit more frequently between weeks 12 and 20 of gestation (until PE can be clinically diagnosed) compared to the normal patients. The model, therefore, assumes that between weeks 12 and 20, blood pressure measurements and urinalysis will be conducted at each antenatal visit and high risk patients will visit more frequently than the normal patients.
Figure 1. Clinical overview of the budget impact model. *Per pre-eclampsia community guidelines, PE cannot be diagnosed before week 203. PE, pre-eclampsia.
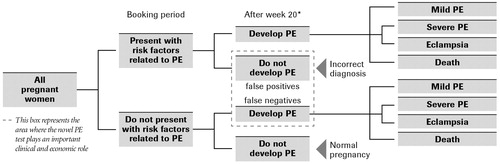
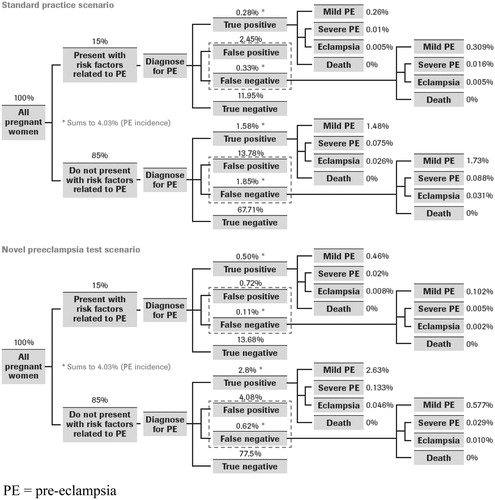
The model simulates pregnant patients from booking period (week 12) to term (week 40 of gestation). In the standard practice scenario, the model assumes that standard practice testing (blood pressure, urine analysis, blood tests such as serum uric acid, and ultrasound) occurs during all antenatal visits. In the novel test scenario, the model assumes the novel pre-eclampsia test is added to the standard practice testing during week 20 of gestation, since PE cannot be clinically diagnosed before 20 weeks of gestationCitation3. The model then tracks the proportions of patients with and without risk factors who do or do not develop PE. Patients who are (1) improperly classified as high risk during the booking period (and never declassified using current tests) and (2) improperly classified as low risk during the booking period and go undetected until they present with symptoms in the hospital (i.e., sub-clinical patients) are the target of the modeling analyses as they represent the patient cohorts where the novel PE test plays an important clinical and economic role. In the model, the term false positive is used to describe patients that are incorrectly classified as being at a high risk for preeclampsia, and hence unnecessarily receive high-intensity care and follow up services. According to probabilities derived from underlying clinical data (), the patients who develop PE are tracked in the model as having mild or severe PE, as progressing to eclampsia, or suffering from maternal death due to PE complications.
Figure 2. Distribution of the patient population in the model: standard practice vs. novel pre-eclampsia test. PE, pre-eclampsia.
Acute treatment and follow-up costs associated with PE diagnosis (or misdiagnosis) and PE management are assigned to the proportion of patients in each of the health states described above, and then all costs are summed, resulting in total cost estimates for patients in the two scenarios – ‘novel PE test’ and ‘standard practice.’ The difference between the sums for the two scenarios represents the ‘budget impact’ of the novel PE test.
Data sources
Data pertaining to treatment practices, healthcare resource utilization, probability of clinical events and outcomes, and costs for detection and management of PE in the UK were obtained through the data from published literature and public databases, and supplemented with interviews conducted with OB/GYNs, laboratory managers, and healthcare payers in the UK. All clinical and economic input parameters for the model, their values, and sources, are summarized in and . While in the current practice risk factors are used to stratify patients, shows that they are an imperfect method and not all patients with risk factors develop PE.
Table 1. Summary of clinical model inputs.
Table 2. Summary of economic inputs.
Currently, there is no published literature that holistically defines the resource utilization involved in diagnosing, treating and managing PE. Thus, to supplement public databases, interviews were conducted during April–May 2009 with ten UK OB/GYNs, five laboratory managers, and five payers. The goal was to collect reliable, UK-specific data on pregnant patients, from patient volume seen, frequency of visits, and current tests used to assess PE, to how patients were managed, and what resources were utilized during the course of the pregnancy. Data were collected for specific pregnancy sub-groups defined as normal, mild pre-eclamptic, severe pre-eclamptic and eclamptic. The in-depth interviews also explored the respondent's reaction to the novel PE test, and its perceived value proposition. The OB/GYNs interviewed were required to (1) have been practicing between 2 and 30 years, (2) treat at least 50 pregnant patients per month, and (3) have at least 3% of their patients with PE. Laboratory managers were required to (1) have between 2 and 30 years experience, (2) participate in decisions regarding selection and acquisition of diagnostic tests, and (3) be affiliated with a hospital that regularly treats and manages PE patients. Payers were required to (1) have between 2 and 30 years of experience, (2) including experience with organizational or governmental policy and legislative decisions regarding coverage and reimbursement related to diagnostic practice (see and ).
Using PE incidence data in the UKCitation4, most of the model's inputs () pertain to distributional probabilities of patients within the model structure (), based on sensitivity and specificity statistics associated with PE testing. Sensitivity and specificity data for standard practice were estimated as a pooled average of various sensitivity and specificity estimates of current PE tests, as presented by Meads et al. 2008Citation1Citation4, while sensitivity and specificity for the novel PE test were taken from Verlohren et al. 2010Citation1Citation3.
Utilization and incidence data obtained through the published literature and expert interviews were used as the basic inputs to the economic model. Inputs were subsequently converted into resource utilization by applying unit costs as cited by the UK drug and healthcare cost databaseCitation15, National Health Service (NHS) Payment by Results DatabaseCitation16, and other published literatureCitation3,Citation4,Citation17–19. Drug costs listed in are from various therapies prescribed by physicians to patients who test positive for PE, including aspirin, α-methyldopa, nifedipine, etc. Drug dosage and duration of therapy depend upon PE severity. PE management costs include physician office visits, physical exams, regular blood pressure checks, blood and urine tests, and cardiotocography, as well as hospital stays for day assessments, intensive care, inpatient monitoring, and delivery or termination of pregnancy. Cost of the novel PE test (which is assumed to be additive to the standard practice costs of PE testing) was provided by the test manufacturer. The cost was set at £31.13 per test (converted from 52 Swiss Francs [CHF]), with the initial testing being administered on all pregnant patients at 20 weeks of gestation. Because pre-eclampsia is highly variable, Lapaire et al. 2010Citation19 and Levine et al. 2004Citation20 suggest repeating the sFlt-1/PlGF test at 4-week intervals. In order to be conservative in the model, patients whose initial PE test result was negative were assumed to undergo two additional rounds of testing, every 6 weeks, during the remainder of pregnancy, with an associated testing cost of £93.39.
Results
In a hypothetical cohort of 1,000 pregnant women, including the novel PE test as an additive diagnostic test to detect PE yielded a positive economic effect. The use of the novel PE test caused a shift in the patient population from false diagnoses to true diagnoses due to better sensitivity and specificity of the novel test compared to tests used in standard practice (). The novel PE test was estimated to reduce false-negative diagnoses of PE by 67% (15/1,000 pregnant patients) and false positives by 71% () (115/1,000 pregnant patients). In turn, this shift produced cost increases arising from true diagnoses and the additional expense of treating patients with those diagnoses (true positive, £159 per patient; true negative, £185 per patient) (). However, these costs were offset by cost savings from reduced numbers of false-negative patients not receiving timely management (false negative, −£85 per patient), and by an even greater amount by the reduction in the number of false-positive patients who ordinarily would be unnecessarily tested, treated, and managed for PE in the standard practice scenario (false positive, −£1,204 per patient).
Table 3. Comparison of total costs in the two scenarios – standard practice and novel pre-eclampsia test.
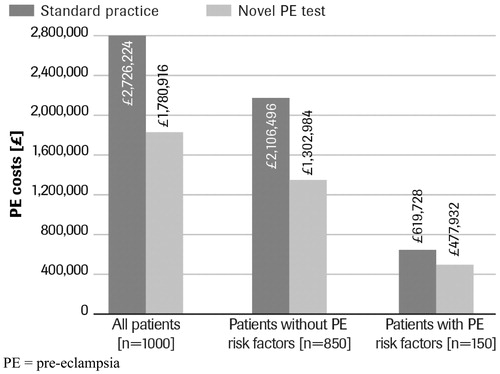
In the current practice, the key cost drivers to payers are the treatment and management costs associated with PE patients, including the costs associated with the false-positive patients who are currently tested, treated, and managed for PE. In the ‘novel PE test’ scenario, the proportion of patients incurring these costs is reduced, given the reduction in false-positive and false-negative patients. Based on the hypothetical cohort of 1,000 pregnant women, a total of 115 fewer patients are treated and monitored in the novel PE test scenario compared to standard practice. While 67% of previously undetected (sub-clinical) PE patients (15/1,000 pregnant patients) would be detected using the novel PE test and would require management for the condition, that increase in cost is offset by the cost savings associated with ‘de-classifying’ 71% patients (115/1,000 pregnant patients) who would have been wrongly diagnosed as having PE using currently-available tests, with associated resource intensive treatment and management. For the cohort of 1,000 pregnant women, total costs were estimated to be £1,780,916 (£1,781 per patient) with the novel PE test and £2,726,224 (£2,726 per patient) with standard practice, representing a cost savings of nearly 35%, or £945,309 (£945 per patient) ( and ).
Figure 4. Budget impact of novel pre-eclampsia test in three screening scenarios: a comparison. PE, pre-eclampsia.
Several sensitivity analyses were performed to test the model findings and assess the value of the novel PE test under a variety of scenarios. Specifically, the following model parameters were altered: (1) PE incidence rate, (2) sensitivity of current tests, (3) specificity of current tests, (4) the proportion of patients stratified as high risk for PE, and (5) the cost of the novel PE test. The findings of each analysis are discussed below:
PE incidence rate: To test the scenario where the PE incidence is lower than that quoted in Bhattacharya et al. 2005, the incidence rate was reduced by 20%; from 4.03% to 3.22%. In this case, total costs per patient were estimated to be £1,711with the novel PE test and £2,680 with standard practice scenario, representing a cost savings of £969 per patient (compared to $945 in the base case). The impact was modest but favorable for the novel PE test, driven by the proportional increase in the number of patients with true-negative and false-positive diagnoses.
Sensitivity of current tests: If the average sensitivity of the current tests were to be improved by 10%, from 46% to 51% while other parameter values were held constant, to base-case values, the total per patient costs would increase for standard practice by £10, to £2,736, while per patient costs under the novel PE test scenario would remain the same. This would represent an overall increase in cost savings by £10, or £955 per patient. This result is derived from the fact that more patients will be identified as PE patients with improved sensitivity.
Specificity of current tests: If the average specificity of the current tests were to be improved by 10%, from 83% to 91% while the other parameter values were held constant, to base-case values, the total per patient costs for standard practice will be reduced significantly to £1,989, while the costs under the novel PE test scenario would remain constant (£1,781). Hence, the novel PE test scenario would represent only modest cost savings to NHS (about £208 per patient).
Proportion of high risk patients: In the primary research, physician experts reported that an estimated 15% of patients were likely to be stratified as high risk for PE on average. While this finding was relatively consistent across interviews, since the 15% ‘incidence’ is not available as a cited data input, the authors provide a sensitivity analysis by altering the proportion of high-risk patients. When this incidence is reduced by 5% points to 10%, the total per patient costs would fall by £82 across both the standard practice and novel PE test scenarios resulting in no change in net cost savings between the two scenarios, i.e. £945 per patient. Similarly, if the proportion of high risk patients is increased by 5% points, to 20%, the total costs would increase by £82 in both the scenarios resulting in no change in the net cost savings.
Cost of the novel PE test: If the cost of the novel PE test was increased by 20% to £37.36 per test, the total per patient costs for the novel PE test scenario would increase to £1,798 thereby decreasing the cost savings by 2% or £928 per patient.
Discussion
Budget impact analyses are useful analytical tools for helping physicians, healthcare payers, and reimbursement authorities to evaluate the economic efficiency of healthcare technologies. Although current PE diagnostic tools are generally inexpensive and convenient to implement, they lead to significant misclassification of patients. Therefore, once downstream clinical effects and resulting patient management costs are taken into account, they have a substantial financial impact on healthcare budgets. Nonetheless, standard biochemical and hematological parameters continue to be the suboptimal mainstay for detecting PECitation21.
In this study, the budget impact of adding a novel PE test with higher sensitivity and specificity than current tests was modeled as a supplementary diagnostic tool added in the standard practice testing, quantifying the economic impact that the test would have for a given patient population. Few studies have been published that address the economics of diagnostic PE testing, even though such knowledge would lead to better decision-making, promote more efficient use of health resources, and decrease healthcare spend. To the authors’ knowledge, this is the first model of the economics of serum diagnostic tests for PE. As part of a systematic review of methods for PE prediction and prevention, Meads and colleaguesCitation14 discuss the accuracy and cost effectiveness of tests for PE in their health technology assessment report. The authors found that while the tests were relatively inexpensive, the quality of the tests was generally poor, and while some tests appeared to have high specificity, they achieve that result at the expense of compromised sensitivity, and hence question their clinical utility. The authors concluded that there is a need for rigorous evaluation of tests with modest cost that have high levels of both sensitivity and specificity. This study pursues that goal by demonstrating that a novel PE test with improved sensitivity and specificity has the potential to provide substantial cost savings for the NHS.
In Europe, the automated measurement of the sFlt-1/PlGF ratio has been recently approved and is increasingly available as a supplementary aid in the diagnosis of pre-eclampsiaCitation19. The model simulations and the budget impact analyses provide substantial data that the NHS could save nearly £945 per pregnant patient by using the novel PE test at or after week 20 of gestation. With approximately 772,000 viable pregnancies in the UK each yearCitation22, total national savings could theoretically amount to £730 million annually.
There are several limitations of this study. First, the model assumes sensitivity and specificity of the novel PE test as provided by clinical trial data (Verlohren et al., 2010Citation1Citation3) which may vary in actual clinical practice. In addition, given that there was no sensitivity and specificity data available with respect to combination testing of current standard practice tests, the model used a pooled average of the individual sensitivities and specificities. Third, some of the resource utilization inputs such as frequency of visits, etc. where published data were unavailable, were obtained from expert interviews. On the one hand, while primary data collected from physicians and financial decision-makers who have experience with PE patients, provided a more clinically- and economically-relevant perspective to the model, these data input were not validated in a peer-reviewed process. Sensitivity analyses were hence needed to test the impact of data inputs on model results.
Furthermore, the model in this study focused on maternal health. To the extent that the clinical and economic impacts of PE are suffered by the fetus, resulting in economic impact to a payer for neonatal care, the cost savings represented by the novel PE test are underestimated. Future research could analyze the economic argument for the use of this test from a neonatal perspective in order to fully assess the value of a PE diagnostic test to society at large.
In addition, the model did not account for the substantial health problems that can be caused by PE later in life in both women and their childrenCitation23–25. The direct and indirect costs of such complications are believed to be highCitation26. Since the novel PE test may help in timely identification and appropriate management of PE patients, it also has the potential to prevent long-term complications and associated costs. However, such benefits of the novel PE test were not captured by the short-term scope of the model. Finally, the test performance could be enhanced either by improved assays and/or by sequential testing. These scenarios with likely benefits to the payer are not discussed or estimated in this paper.
Conclusion
The results of this budget impact model suggest that the novel diagnostic test has the potential to create significant savings to healthcare payers. Savings are due to the novel PE test's ability to reduce false-negative and false-positive diagnoses, by correctly identifying the subclinical PE patients and preventing unnecessary spend on patients that are unlikely to develop PE. Costs for the management of pregnancy after 20 weeks of gestation total an estimated £1,781 with the novel PE test and £2,726 with standard practice diagnostic testing; thus, the novel PE test would save nearly £945 per tested patient. The results of the study are particularly important in the UK where substandard care contributes to 72% of maternal deaths that are related to hypertensive disease in pregnancyCitation27, and PE complicates over 4%Citation4, or about 31,000 pregnancies each yearCitation22. Results from this study provide, quantitative evidence about the favorable impact of adding a novel PE test to a well-established but underperforming PE diagnostic armamentarium.
Transparency
Contribution to authorship
All authors of this paper are qualified for authorship based on their substantial contributions to conception and design of the study, data acquisition and analytical work, and for drafting, editing, and approving all of the versions of this paper, including the final version submitted for publication. In particular, N.H. was responsible for overall project direction and oversight; S.G. contributed substantially to reviewing literature, acquisition and management of data, statistical analyses and reporting, and model programming and analytics; C.C. had responsibility for day-to-day oversight of all technical lines of research, as well as reviewing and editing drafts of the paper; J.D.M. maintained technical oversight of model development, model programming, and with T.F. wrote drafts of the paper; W.v.d.H. initiated the design of the study with J.C. and was involved with the development and conceptualization of the study, and reviewed and edited drafts of the paper.
Disclosure of interest
N.H., consultancy to Roche Diagnostics Ltd; S.G., consultancy to Roche Diagnostics Ltd; C.C. was an employee of Abt Bio-Pharma Solutions, Inc. at the time of this study and provided consultancy to Roche Diagnostics Ltd; J.D.M. was an employee of Abt Bio-Pharma Solutions, Inc. at the time of this study and provided consultancy to Roche Diagnostics Ltd; W.v.d.H. employment with Roche Diagnostics Ltd; T.F. consultancy to Roche Diagnostics Ltd; J.C. employment with Roche Diagnostics Ltd; N.H., T.F. and S.G. are currently employees of United BioSource Corporation which acquired Abt Bio-Pharma Solutions, Inc.
Declaration of funding
This study was sponsored by Roche Diagnostics Ltd (Rotkreuz, Switzerland).
Acknowledgment
The authors express their appreciation to Juliane Gartemann (Economic Strategy Manager, Roche Diagnostics Ltd) for reviewing and editing drafts of this paper.
References
- Stella CL, Sibai BM. Preeclampsia: Diagnosis and management of the atypical presentation. J Matern Fetal Neonatal Med 2006;19:381-6
- Wagner LK. Diagnosis and management of preeclampsia. Am Fam Physician 2004;70:2317-24
- Milne F, Redman C, Walker J, et al. The pre-eclampsia community guideline (PRECOG): how to screen for and detect onset of pre-eclampsia in the community. BMJ 2005;330:576-80
- Bhattacharya S, Campbell DM. The incidence of severe complications of preeclampsia. Hypertens Pregnancy 2005;24:181-90
- Buhimschi CS, Norwitz ER, Funai E, et al. Urinary angiogenic factors cluster hypertensive disorders and identify women with severe preeclampsia. Am J Obstet Gynecol 2005;192:734-41
- Muy-Rivera M, Vadachkoria S, Woelk GB, et al. Maternal plasma VEGF, sVEGF-R1, and PlGF concentrations in preeclamptic and normotensive pregnant Zimbabwean women. Physiol Res 2005;54:611-22
- Woolcock J, Hennessy A, Xu B, et al. Soluble Flt-1 as a diagnostic marker of pre-eclampsia. Aust N Z J Obstet Gynaecol 2008;48:64-70
- Robinson CJ, Johnson DD, Chang EY, et al. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol 2006;195:255-9
- Lee ES, Oh MJ, Jung JW, et al. The levels of circulating vascular endothelial growth factor and soluble Flt-1 in pregnancies complicated by preeclampsia. J Korean Med Sci 2007;22:94-8
- Kim YJ, Park H, Lee HY, et al. Paraoxonase gene polymorphism, serum lipid, and oxidized low-density lipoprotein in preeclampsia. Eur J Obstet Gynecol Reprod Biol 2007;133:47-52
- Teixeira PG, Cabral AC, Andrade SP, et al. Placental growth factor (PlGF) is a surrogate marker in preeclamptic hypertension. Hypertens Pregnancy 2008;27:65-73
- Noori M, Donald AE, Angelakopoulou A, et al. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation 2010;122:478-87
- Verlohren S, Galindo A, Schlembach D, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010;202:161.e1-161.e11 (Epub 2009 Oct 21)
- Meads CA, Cnossen JS, Meher S, et al. Methods of prediction and prevention of pre-eclampsia: systematic reviews of accuracy and effectiveness literature with economic modelling. Health Technol Assess 2008;12:1-270
- British National Formulary (BNF). (Accessed July 2009). Available online at https://www.medicinescomplete.com/mc/bnf/current/login.htm
- National Health Service (NHS). (Accessed July 2009). Payment by Results: Guidance and Tariff for 2008-09. Available online at http://www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_081096
- Mulhaven Medical Laboratory. (Accessed July 2009). Laboratory Testing Prices. Available at http://www.mullhaven.co.uk/index1.htm
- Murphy DJ, Stirrat GM. Mortality and morbidity associated with early-onset preeclampsia. Hypertens Pregnancy 2000;19:221-31
- Lapaire O, Shennan A, Stepan H. The preeclampsia biomarkers soluble fms-like tyrosine kinase-1 and placental growth factor: current knowledge, clinical implications and future application. Eur J Obstet Gynecol 2010;151:122-9
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004;350:672-83
- Cnossen JS, ter Riet G, Mol BW, et al. Are tests for predicting pre-eclampsia good enough to make screening viable? A review of reviews and critical appraisal. Acta Obstet Gynecol Scand 2009;88:758-65
- UK Office for National Statistics. (Accessed August 2009). Key Population and Vital Statistics, Series VS No 34, PPI No 30, 2007 data, corrected version issued on 27 April 2009. Available online at http://www.statistics.gov.uk/downloads/theme_population/KPVS34-2007/KPVS2007.pdf
- Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. Epub 2007 Nov 1
- McDonald SD, Malinowski A, Zhou Q, et al. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 2008;156:918-30
- Funai EF, Friedlander Y, Paltiel O, et al. Long-term mortality after preeclampsia. Epidemiology 2005;16:206-15
- Sibai BM. Caring for women with hypertension in pregnancy. JAMA 2007;298:1566-8
- National Institute for Clinical Excellence, Scottish Executive Health Department, Department of Health, Social Services and Public Safety, Northern Ireland. Saving mothers's lives;2003–2005. The seventh report of the confidential enquiries into maternal deaths in the United Kingdom. London: CEMACH, 2007