Abstract
Background:
Thrombocytopenia is a significant risk for patients with chronic HCV infection and a common side-effect of treatment with pegylated (PEG) interferon (IFN). Thrombocytopenia predisposes patients to bleeding and requirements for platelet transfusions, and may thus place an increased burden on patients and on medical resource utilisation.
Scope:
In a retrospective analysis of an integrated, longitudinal database of medical and pharmacy claims and laboratory results in a US commercial health (insurance) plan, patients with chronic hepatitis C viral (HCV) infection were identified by reviewing ICD-9-CM HCV-, chronic liver disease-, and cirrhosis-related diagnoses. Medical resource utilisation and laboratory results were evaluated during the year following the HCV diagnosis index date as well as during the baseline year prior to that index date. Medical resource utilisation was determined by comparing outpatient visits, emergency department (ER) visits, and inpatient hospital stays for HCV patients with or without thrombocytopenia.
Findings:
HCV patients diagnosed with thrombocytopenia had a greater incidence of bleeding events (27.3 vs. 9.9%), platelet transfusions (8.5 vs. <1%), liver disease-related ambulatory visits (10.4 vs. 4.4; odds ratio [OR] = 2.3; p < 0.001), ER visits (OR = 8.6; p < 0.01), and inpatient hospital stays (OR = 17.7; p < 0.01) during the study period compared with HCV patients without a thrombocytopenia diagnosis. HCV patients with thrombocytopenia had significantly higher overall healthcare costs ($37,924 vs. $12,174; p < 0.001) and liver disease-related costs ($14,569 vs. $4107; p < 0.001) than patients without thrombocytopenia.
Limitations:
Administrative claims data are subject to coding errors; additionally, the patient population may not be completely representative of the general chronic HCV population.
Conclusions:
Diagnosis of thrombocytopenia in patients with HCV is associated with increased incidence of certain comorbidities, complications, and medical interventions, and significantly increased medical resource utilisation.
Introduction
The incidence of hepatitis C viral (HCV) infection in the United States has declined from an estimated peak of 232,000 new infections annually in the late 1980s to an estimated 19,000 new infections in 2006Citation1. HCV incidence has been relatively level since 2003, with a slight increase in new cases reported in 2006Citation2. Seroprevalence surveys estimate that 3.9 million persons were HCV positive during 1988 through 1994Citation3, and 4.1 million persons were HCV positive during 1999 through 2002Citation4. Despite the overall decrease in new cases, HCV remains the most common chronic blood-borne infection in the USCitation4.
Although new cases have declined during the past decade, a high percentage of individuals who become infected with HCV develop chronic infection. The clinical manifestations of chronic liver disease (CLD), such as liver fibrosis or cirrhosis, are typically not apparent until 10–20 years after the initial infectionCitation5. The Centers for Disease Control and Prevention (CDC) estimate that 3.2 million Americans have chronic HCV infection, i.e. approximately 75–85% of the total HCV-infected populationCitation6.
Case and public health management of HCV infection is complicated by its frequently asymptomatic or nonspecific clinical presentation, with jaundice or other overt signs occurring in only a third of acute casesCitation7. Asymptomatic patients serve as a reservoir capable of transmitting virus. There are currently no vaccines to prevent HCV infection and a large segment of the population remains susceptible.
CLD occurs in approximately 60–70% of persons chronically infected with HCVCitation6, and is the 12th leading cause of adult mortality in the US, with an annual death rate of over 29,000 individualsCitation8. The CDC estimates that 40–60% of all CLD is caused by HCVCitation5. Over a 20- to 30-year period, cirrhosis of the liver develops in 10–30% of persons with chronic HCV infectionCitation5,Citation7. The risk for hepatocellular carcinoma (HCC) risk is 1–4% annually once cirrhosis developsCitation5. Cirrhosis due to chronic HCV infection is the leading indication for liver transplantation in the USCitation9.
HCV infection is associated with an increased risk of thrombocytopenia, particularly in people with advanced-stage liver diseaseCitation10–12. In one study, a 10.2% incidence of thrombocytopenia (defined as platelet counts <100,000/µL) was reported in a population of HCV-seropositive patients (n = 294) versus a 1.3% incidence in seronegative patients (n = 1183). The odds ratio (OR) of thrombocytopenia occurring in HCV-seropositive versus seronegative patients was 6.0 (95% confidence interval [CI] = 3.2–11.2)Citation12.
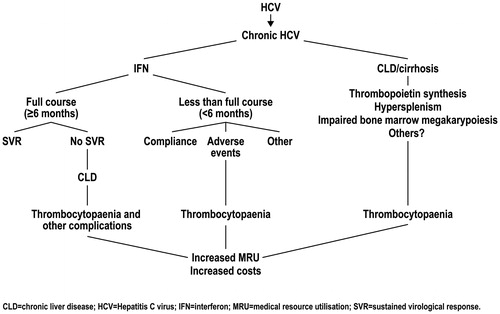
A variety of factors potentially contribute to the pathogenesis of thrombocytopenia, including decreased thrombopoietin (TPO) synthesisCitation13, splenomegalyCitation11, immunoglobulin-associated autoimmunity to plateletsCitation14, and idiopathic exhaustion of bone marrow progenitor cells (in some cases involving concurrent HCV infection of megakaryocytes), leading to impaired bone marrow megakaryopoiesisCitation11. HCV can be a primary cause of thrombocytopenia as a result of progressive hepatic fibrosis and impaired thrombopoietin productionCitation12. Thrombocytopenia is of clinical significance because it is associated with an increased risk of bleeding across a range of haemostatic challenges, especially in cases of severe thrombocytopenia (platelet counts <50,000–60,000/µL)Citation15. The increased likelihood of bleeding may necessitate platelet transfusions and delays or cancellations of invasive medical or dental procedures, including important interventions in patients with CLDCitation15–18.
The standard of care for chronic HCV infection is pegylated (PEG) interferon (IFN) given in combination with ribavirin (PEG-IFN/RBV); no new treatments have become available in the past 5 yearsCitation19–22. Evolving direct acting antiviral therapies will still use PEG as the backbone of the regimen. The benefits of this treatment have been well-established. PEG-IFN/RBV is given for 24 weeks or 48 weeks depending on HCV genotype. Achievement of sustained virological response (SVR) is assessed 6 months after the completion of treatment. Treatment with PEG-IFN/RBV produces an SVR in roughly half of treated patientsCitation19,Citation20. However, haematological abnormalities including thrombocytopenia are common side-effects of IFN treatment. IFN dose reduction or discontinuance is required in up to 25% of patients and 3% of patients, respectively, upon development of significant thrombocytopeniaCitation21. IFN-induced thrombocytopenia is especially problematic in patients with platelet counts that are already low prior to IFN initiation (PEG-IFN/RBV product labels do not recommend starting PEG-IFN/RBV in patients with platelet counts below 75,000–100,000/µL)Citation23 further jeopardising the ability to maintain dose or schedule of antivirals and reducing the likelihood of SVR.
Methods
The objective of our study was to analyse in a representative sample the clinical and economic burden of thrombocytopenia for patients with HCV. The prevalence of diagnosed thrombocytopenia in adult patients with HCV was analysed in a large medical claims database, and HCV patients were characterised in terms of their platelet counts, major bleeding events, and the relationship between platelet counts and bleeding events. Current antiviral treatment patterns and clinical practices in the management of thrombocytopenia were analysed by estimating the percentage of HCV patients receiving platelet transfusions, the occurrence of IFN treatment delays, and dose reduction or discontinuation of IFN due to thrombocytopenia, thrombocytopenia and anaemia, or thrombocytopenia and neutropenia. Medical resources and costs associated with liver disease progression such as liver transplant or liver cancer in patients with HCV were determined, as were costs associated with or without a full course of antiviral therapy (defined in this study as ≥6 months of continuous therapy). Incremental differences in medical resource utilisation and care costs between HCV patients with low platelet counts and HCV patients without substantively low platelet counts also were determined.
This retrospective analysis was performed on a longitudinal administrative claims database from a large, integrated commercial health insurance plan spanning the period January 1, 2000 through December 31, 2004. The database includes insurance eligibility, pharmacy and medical claims, and laboratory results data. Data were available from claims submitted by healthcare providers on behalf of 12.7 million commercially enrolled patients representing geographically diverse regions of the US. Comprehensive laboratory data were available for approximately 40% of patients in the claims database.
Claims or encounter data were collected from all available healthcare sites (inpatient hospital, outpatient hospital, emergency department (ER), physician’s office, surgery centre, etc.) for virtually all types of provided services, including specialty, preventive, and office-based treatments. Medical and pharmacy claims and coding conformed to insurance industry standards. Claims for ambulatory services submitted by individual providers, e.g. physicians, used the CMMS equivalent of the HCFA-1500 format. Inpatient or same day surgery claims for hospital facility services used the UB-82 or UB-92 format.
In this claims database, each facility service record can contain nine diagnoses, recorded with the International Classification of Diseases, Ninth Revision Clinical Modification (ICD-9-CM) diagnosis codes, and as many as six procedures recorded with ICD-9-CM procedure codes. Outpatient or clinic services and provider records are coded with fewer ICD-9-CM diagnoses, as well as current procedural terminology (CPT-4) or Health Care [Financing Administration] Common Procedure Coding System (HCPCS) codes. All transactions by category of service are submitted for payment using the respective claims forms. The cost of medical services used in this study corresponds to the transaction amount paid for the service to the provider by the health plan and patient (enrolees’ copays and coinsurance).
Claims for pharmacy services are typically submitted electronically by the pharmacy at the time prescriptions are filled. Pharmacy claims data include drug name, dosage form, drug strength, fill date, days of supply, prescription average wholesale price, and de-identified patient and prescriber data, allowing for longitudinal tracking of medication fills and refills. Laboratory test results are available for subpopulations of the research database. Standard Logical Observation Identifiers Names and Codes (LOINC) coding was used in identifying specific tests and results. Test results are linked to all other medical resource utilisation and pharmacy claims data.
Study population
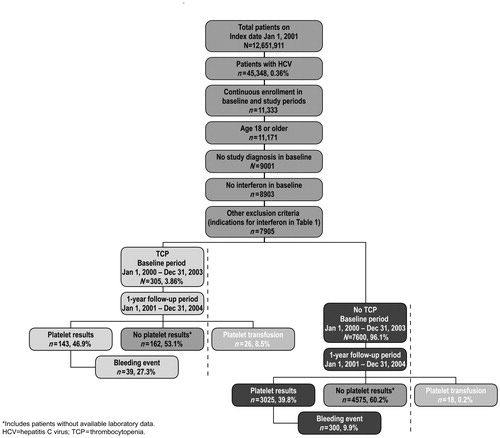
The study included commercial health plan members identified with a new diagnosis for HCV from January 1, 2001 through December 31, 2003. The date of the first claim with an HCV diagnosis for each patient was defined as the patient’s index date. Patients 18 years and older who were continuously enrolled with the insurer for at least 1 year before their index date (baseline period) and for at least 1 year after their index date (study period) were included in the analysis. Patients with HCV were identified from the ICD-9-CM HCV-related diagnosis codes 070.41, 070.44, 070.51, 070.54, 070.70, 070.71, and V02.62 in either the primary or secondary positions on individual medical claims. Patients with thrombocytopenia (287.3–287.5), anaemia (280.0–285.9, 790.1), and neutropenia (288.0) were identified (). For purposes of this analysis, liver disease was defined by diagnostic codes that included HCV hepatitis, CLD and cirrhosis, liver abscess and sequelae of CLD, liver transplant, liver cancer, and other liver disorders.
Table 1. ICD-9-CM diagnosis codes used in this study.
Patients were excluded from the study if they had any diagnosis for HCV during the baseline period (January 1, 2000 to January 1, 2002); any medical or pharmacy claims for IFN during the baseline period (identified by codes J9212, J9213, J9214, S0145, S0146, and NDC codes for Infergen [interferon alfacon-1], Intron A [interferon alfa-2b], Pegasys [peginterferon alfa-2a], Roferon-A [interferon alfa-2a], Rebetron [interferon alfa-2b/ribavirin], and PegIntron Redipen [peginterferon alfa-2b/ribavirin]); or a claim in either the baseline or study periods for any condition other than HCV in which IFN is indicated (i.e., hepatitis B, hairy cell leukaemia, Kaposi sarcoma, chronic myelogenous leukaemia, follicular lymphoma, condylomata acuminate, or malignant melanoma; ). shows the study population and the number of patients in each group.
Analyses
The prevalence of thrombocytopenia among patients with HCV during the 4-year study period was estimated by dividing the number of HCV patients with a thrombocytopenia diagnosis on a medical claim by the total number of HCV patients identified. These patients were identified from diagnoses in primary or secondary positions. Demographic data for HCV patients with and without a thrombocytopenia diagnosis included patients’ age and gender as of the index date, and select health characteristics or interventions during the baseline and the 1-year study period (anaemia, neutropenia, liver transplants [996.8, V42.7], liver cancer [155.x, 197.7, V10.07], liver disease [CLD and cirrhosis, 571.xx; liver abscess and sequelae of CLD, 572.xx; other disorders of the liver, 573.xx], platelet transfusions [99.05], or IFN treatment).
T-tests of number of days to treatment with IFN (treatment delay) were performed on patients receiving a full course or less than a full course of IFN therapy, with or without thrombocytopenia, including those with or without thrombocytopenia and anaemia, and with or without thrombocytopenia and neutropenia. Patients were considered to have a full course of IFN treatment if they were on IFN continuously for at least 6 months during the study period since no information was available to distinguish between those who were to receive 24 vs. 48 weeks of antiviral therapy. A chi-square test of difference in numbers with IFN dose reduction or discontinuation was performed for HCV patients with or without thrombocytopenia, with or without thrombocytopenia and anaemia, and with or without thrombocytopenia and neutropenia. Dose reduction was defined as at least a 50% reduction in recorded dose in at least two consecutive medical claims during the study period. Discontinuation was defined as no medical claim for IFN within 30 days of the last claim (date of service) for IFN (‘J‘ and ‘S‘ codes). For pharmacy claims, discontinuation was defined as no pharmacy claim for IFN within 1.5 times the day’s supply of the last prescription from the run-out date of the last prescription (i.e., no claim within the 45 days following the end date on a 30-day prescription). The number of bleeding events and the number of platelet transfusions during the study period were compared in patients with or without a diagnosis of thrombocytopenia. An alternative analysis was performed for a subset of HCV patients with complete laboratory results data who had thrombocytopenia as determined by low platelets (<100,000/µL). The correlation between thrombocytopenia and bleeding events was evaluated in these patients.
Medical resource utilisation was evaluated by examining the mean number of ambulatory visits, ER visits, and inpatient hospital stays during the study period. Negative binomial regression models were used in estimating or predicting incremental differences in medical resource utilisation between HCV patients with or without diagnosed thrombocytopenia. Medical resource utilisation also was compared in HCV patients with thrombocytopenia who received a full and uninterrupted full course (≥6 months) of IFN treatment and in those who discontinued therapy (received <6 months of therapy). Overall healthcare costs and liver disease-related costs during the study period were compared using multivariate modelling of HCV patients with and without a diagnosis of thrombocytopenia. Overall and liver disease-related healthcare costs were determined using claims information describing the nature of the service and corresponding diagnosis code. Costs discussed are allowable or transaction amounts paid by the patient and the commercial health insurer or plan to the provider of the service in 2003 US dollars.
To confirm results of the analysis of HCV patients diagnosed as thrombocytopaenic, medical resource utilisation and medical care costs were compared between the overall HCV population of patients diagnosed with thrombocytopenia and the subset of those patients who were defined as thrombocytopenic or having low platelets based on laboratory data. The latter defines clinically meaningful thrombocytopenia by platelet count (<100,000) rather than by diagnosis code.
To ensure the integrity of statistical comparisons, analysis cohorts matched on age and gender were selected from both the overall HCV population (complete medical and pharmacy claims histories) and the subset of that HCV population for whom complete laboratory claims and results histories were available. Patients with and without thrombocytopenia were selected in a 1 : 4 ratio. Creation of such comparison cohorts increases the likelihood of tractable regression models and ensures robust estimates of effects.
Binomial regression analyses were performed; age, gender, physician specialty, geographic region, comorbid conditions, medication history, and medical care use in the baseline or preindex diagnosis period were controlled in models to evaluate difference in medical resource use (liver disease-related ambulatory care visits, ER visits, and inpatient stays) between HCV patients with or without thrombocytopenia. Generalised linear estimating equations were used in estimating incremental cost differences (liver disease-related and overall healthcare costs) between HCV patients with or without thrombocytopenia, both in those receiving a full course of IFN and those receiving limited or no IFN. Liver-disease related costs during the follow-up period were defined as total costs incurred by the health plan and patients due to medical and pharmacy utilisation for patients with a primary diagnosis claim for liver disease identified by the ICD-9-CM diagnosis codes for HCV and CLD () and for procedures identified by CPT-4 or HCPCS codes for liver cancer, liver transplant, and procedures reflecting liver disease. Descriptive statistics also are provided.
Results
Demographic and health characteristics
The overall prevalence of HCV within the study database was 0.36% (45,348/12,651,911). After inclusion and exclusion criteria were applied, a final study population of 7905 adult patients with an HCV diagnosis code remained (). Using ICD-9-CM diagnosis codes, the prevalence of thrombocytopenia among patients with HCV was 3.86% (305/7905). Annual prevalence of thrombocytopenia among patients with HCV ranged from 4.25 to 5.74% during the 3-year identification period.
Demographic and selected health characteristics of the final study population are shown in and . The mean age of HCV patients with a thrombocytopenia diagnosis was 50 ± 7.8 years, and that of HCV patients without thrombocytopenia was 47 ± 9.2 years. The percentage of men in the HCV patients with and without thrombocytopenia diagnoses was 72.5 and 59.1%, respectively. HCV patients with thrombocytopenia diagnoses had higher rates of anaemia (50.2 vs. 13.1%), neutropenia (16.7 vs. 2.2%), liver transplantation (7.5 vs. 1.0%), liver cancer (3.6 vs. 0.6%), platelet transfusions (8.5 vs. 0.2%), and IFN treatment (30.5 vs. 17.0%) compared with HCV patients without thrombocytopenia diagnoses.
Table 2. Demographic and selected health characteristics of patients with hepatitis C virus at baseline.
Table 3. Summary of primary study outcomes in hepatitis C patients during the 12-month follow-up period.
Platelet counts, bleeding events, and transfusions
The number of platelet transfusions during the study period was compared among HCV patients with or without a diagnosis code of thrombocytopenia and in a subset of patients with laboratory results data. Complete laboratory data were available for 40.1% (3168/7905) of the overall HCV study population. This included 143 (46.9%) HCV patients with thrombocytopenia diagnoses and 3025 (39.8%) HCV patients without thrombocytopenia diagnoses. Mean platelet counts were nearly two-fold higher in HCV patients without thrombocytopenia diagnoses (223,723/µL) compared with HCV patients diagnosed with thrombocytopenia (112,898/µL). Among the subset of patients with complete laboratory data, an average of 4.4 platelet tests per HCV patient with thrombocytopenia diagnoses and 3.1 tests per HCV patient without thrombocytopenia diagnoses were recorded.
In HCV patients with laboratory results and diagnosed thrombocytopenia, 27.3% (39/143) had bleeding events compared with 9.9% (300/3025) of HCV patients with laboratory results but without diagnosed thrombocytopenia (). The correlation between mean platelet count and bleeding was <1, negative, and not significant (p = 0.55).
Table 4. Number of HCV patients with a bleeding event or receiving a platelet transfusion during the 12-month study periods among the analysis cohort.
As shown in , the percentage of HCV patients with thrombocytopenia who received a platelet transfusion was 8.5% (26/305), 36-fold greater than that observed in HCV patients without thrombocytopenia who received platelet transfusions (0.2%; 18/7600).
IFN treatment
More HCV patients with thrombocytopenia diagnoses (30.5%; 93/305) received IFN during the study period compared with HCV patients without thrombocytopenia diagnoses (17.0%, 1294/7600). The mean interval from HCV diagnosis to IFN treatment (139 days) was the same in HCV patients with or without thrombocytopenia diagnoses. Discontinuation of IFN treatment, defined as less than 6 months of continuous use, occurred in 49.5% (46/93) of HCV patients with thrombocytopenia diagnoses and in 40.4% (523/1294) of HCV patients without thrombocytopenia, a nonsignificant difference. A significantly higher percentage (p = 0.002) of HCV patients with thrombocytopenia and anaemia (62.5%; 30/48) discontinued IFN treatment compared with HCV patients without thrombocytopenia and without anaemia (39.7%; 401/1009). The percentage of HCV patients with thrombocytopenia and neutropenia who discontinued IFN treatment (48.1%; 37/77) was higher than that for HCV patients without thrombocytopenia or neutropenia (40.0%; 401/1003), but the difference was not statistically significant.
IFN dose reduction rates analysed in 25% increments varied somewhat between groups. There was no significant difference in the percentage of HCV patients with and without thrombocytopenia diagnoses who had an IFN dose reduction of >50% (2.2% for HCV patients with thrombocytopenia vs. 5.2% for HCV patients without thrombocytopenia). There was no significant difference in the percentage of HCV patients with thrombocytopenia and anaemia or HCV patients without thrombocytopenia or anaemia who had an IFN dose reduction. Differences in dose reduction for HCV patients with thrombocytopenia and neutropenia versus patients without thrombocytopenia or neutropenia were not significant except among those receiving a 26–50% dose reduction (p = 0.005). In that quartile, the IFN dose was reduced in 13.0% of HCV patients with thrombocytopenia and neutropenia compared with 4.3% of HCV patients without thrombocytopenia or neutropenia (p = 0.005).
Medical resource utilisation
Types of medical service included liver disease-related ambulatory visits, ER visits, and inpatient hospital stays. presents the healthcare utilisation for HCV patients by full course of treatment with IFN, defined as greater than 6 months. HCV patients who had a full course of IFN treatment averaged 3.8 times as many liver disease-related ambulatory visits per year compared with HCV patients who had had partial or no IFN treatment (14.16 vs. 3.68; p < 0.001). The mean number of liver disease-related ER visits was virtually the same in both groups (0.016 vs. 0.02; p = 0.459). The mean number of liver disease-related inpatient hospital stays was significantly lower (p = 0.016) in patients with HCV receiving a full course of IFN treatment compared with those who did not (0.015 vs. 0.03). Patients that did not receive full course of IFN treatment or did not receive treatment with IFN had significantly higher (p = 0.0041) baseline costs ($5650) as compared to patients in the group with no gaps in IFN therapy ($4161). Patients in the full IFN group had significantly lower number of liver-related hospital visits but higher number of ambulatory visits in the follow-up period (). The full IFN group had significantly higher liver-related costs ($22,592 vs. $2621; p < 0.0001) and significantly higher total costs ($28,856 vs. $11,527; p < 0.0001).
Table 5. Liver disease-related ambulatory visits, ER visits, and inpatient stays during the 12-month study period among patients with hepatitis C virus by thrombocytopenia status and interferon treatment status*.
A negative binomial regression analysis showed that HCV patients with thrombocytopenia diagnoses had 2.3 times more liver disease-related ambulatory visits compared with HCV patients without thrombocytopenia diagnoses (95% CI = 2.0–2.6; p < 0.01). Similarly, logistic regression estimates suggest that HCV patients with thrombocytopenia diagnoses were more than eight times more likely to have liver disease-related ER visits (OR = 8.6; 95% CI = 5.4–13.7; p < 0.01) and almost 18 times more likely to have liver disease-related inpatient hospital stays (OR = 17.7; 95% CI = 11.9–26.3; p < 0.01) than patients without thrombocytopenia (see Appendix).
Overall healthcare costs and liver disease-related costs during the study period
Overall healthcare costs and liver disease-related costs during the study follow-up period were evaluated in HCV patients with or without coding of thrombocytopenia diagnoses. Estimated or predicted overall healthcare costs were significantly higher in HCV patients with thrombocytopenia diagnoses compared with HCV patients without thrombocytopenia diagnoses ($37,924 vs. $12,174; p < 0.001; ). Liver disease-related costs were also significantly higher in HCV patients with thrombocytopenia diagnoses ($14,569 vs. $4107; p < 0.001). Driving this cost difference are the significantly greater numbers of liver-related hospitalisations, ER visits, and ambulatory care or outpatient visits in the study period among HCV patients with thrombocytopenia.
Figure 2. Regression-based estimates of annual liver disease-related and overall medical care costs in patients chronically infected with hepatitis C virus.
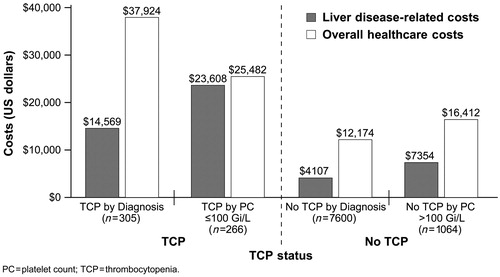
Overall healthcare costs and liver disease-related costs were modelled among HCV patients with complete laboratory data and results, age and gender matched in a ratio of 1 : 4, with or without thrombocytopenia diagnoses. Overall healthcare costs and liver disease-related costs were significantly higher in HCV patients with thrombocytopenia diagnoses ($25,482 and $23,608, respectively) compared with HCV patients without thrombocytopenia diagnoses ($16,412 and $7354, respectively; p < 0.001 for both comparisons). Statistically significant factors contributing to these differences include age, region, heart disease, GI disorders, dosing of ribavirin as part of the antiviral regimen, and medical resource use during the baseline period.
Discussion
Thrombocytopenia was diagnosed in a relatively small percentage (3.9%) of adult patients with HCV. However, HCV patients diagnosed with thrombocytopenia had a disproportionately greater rate of certain comorbidities or sequelae (anaemia, neutropenia, serious bleeding events, liver cancer), medical interventions (IFN treatment, platelet transfusion, liver transplantation), and medical resource utilisation compared with HCV patients without diagnosed thrombocytopenia. HCV patients with thrombocytopenia diagnoses had 2.8-fold more bleeding events, a greater than 36-fold higher incidence of platelet transfusions, and a significantly greater number of ambulatory visits, ER visits, and inpatient hospital stays during the 1-year study period compared with HCV patients without thrombocytopenia diagnoses. Because these results were derived retrospectively from a large, inclusive, heterogenous patient population, they likely reflect what can be anticipated in clinical practice.
The percentage of HCV patients diagnosed with thrombocytopenia in the present study is relatively low compared with other studies where prevalence of thrombocytopenia in HCV-positive individuals ranged from 10% (based on a platelet count <100,000/µL) to 41% (platelet count <150,000/µL)Citation12,Citation14. The evaluable subset of 2500 HCV patients in the study for whom platelet counts were available (data not reported) had a thrombocytopenia prevalence of 10.8% when a clinical threshold of thrombocytopenia was defined as platelet counts ≤100,000/µL at any time during the study period. This suggests that the 3.9% overall prevalence reported here may underestimate the true extent of thrombocytopenia as an HCV complication or comorbidity in the study population. Based on this work, patients with no thrombocytopenia diagnosis are not likely to have platelets <100,000/µL (concordance 92–93%); patients with thrombocytopenia diagnosis are likely to have platelets <100,000/µL (concordance 65%). It is likely that some patients with laboratory data indicating thrombocytopenia were seeing physicians for reasons unrelated to thrombocytopenia and therefore thrombocytopenia is not coded. The entire HCV population including those with and without thrombocytopenia had a mean platelet count of 218,721/μL. In patients with laboratory results, thrombocytopenia was defined as platelet counts <100,000/µL, a more clinically relevant standard than the lower limit of the normal range of 150,000/µL. For HCV patients with diagnosed thrombocytopenia and complete lab results, the average platelets were 112,900/µL. Not only mean platelet counts but distributions were quite different between patients with or without thrombocytopenia. Frequency distributions of patient platelet counts both at the index date and averaged across multiple dates were normally distributed. However, the curves peaked at different platelet counts depending upon evidence of thrombocytopenia. Index date platelet counts for HCV patients with or without thrombocytopenia were most frequently between 50,000/µL and 150,000/µL and between 150,000/µL and 300,000/µL, respectively. Mean counts for HCV patients with or without thrombocytopenia were most frequently between 50,000/µL and 150,000/µL, and between 150,000/µL and 250,000/µL, respectively.
Anaemia, neutropenia, liver cancer, and liver transplantation were more prevalent among thrombocytopenic HCV patients than HCV patients without thrombocytopenia diagnoses. These outcomes are consistent with the positive correlation of HCV-related thrombocytopenia with haematology disorders and more serious hepatopathies, including fibrosis, cirrhosis, and HCC, as reported in other studiesCitation12,Citation25,Citation26. Patients with these more advanced forms of CLD would be expected to incur proportionately greater medical resources regardless of their thrombocytopenia status because of disease progression. However, the presence of thrombocytopenia might further increase medical resource use.
Thrombocytopenia has been associated with an increased bleeding risk in other studies of patients with platelet counts ≤60,000/μLCitation15,Citation24 and platelet transfusions are a standard treatment for severe thrombocytopenia (platelet counts <10,000–20,000/µL)Citation27,Citation28. Thus, the greater incidence of bleeding events and platelet transfusions in HCV patients with thrombocytopenia diagnoses observed in the study was not unexpected. Approximately 10% of patients with HCV who had a bleeding event were not diagnosed with thrombocytopenia and had platelet counts >223,000/µL (). This indicates that bleeding risk factors other than thrombocytopenia were involved (e.g., alcohol consumption, NSAIDs use, ulcers, varices). This result also indicates that in a broadly mixed HCV population, even patients with normal platelet levels have some risk of bleeding.
Three in ten HCV patients with thrombocytopenia diagnoses received IFN treatment, nearly double the rate for HCV patients without thrombocytopenia diagnoses. The increase in IFN treatment in HCV patients with thrombocytopenia could be attributed to more advanced fibrosisCitation12 or development of thrombocytopenia during treatment with IFN, a common side-effect of this antiviral therapy ()Citation21.
IFN is an expensive therapy and would be a contributing factor to higher short-term medical care costs. However, a careful pharmacoeconomic analysis has clearly shown that IFN treatment is cost effective in patients with HCV and contributes to improved quality of life and life expectancyCitation29. Although IFN treatment has been shown to have a significant benefit as antiviral therapy in patients with HCVCitation19,Citation20,Citation29,Citation30, when either the standard IFN dosage or duration of treatment is reduced, likelihood of sustained virological response as determined by elimination of HCV-RNA is impaired. One study showed that reducing the cumulative PEG-IFN/RBV dose to ≤60% during the first 20 weeks of treatment reduced the SVR at week 20 by nearly two-thirdsCitation30. Less than 80% adherence to a full PEG-IFN/RBV dose regimen in patients with HCV appears to significantly reduce the achievement of an SVRCitation20,Citation29. Thus, even a relatively modest overall IFN dose reduction can significantly detract from treatment response in patients with HCV. The benefit of IFN as standard HCV therapy was indirectly corroborated in this study by the estimation of significantly lower rates of most medical care services, except liver disease-related ER visits, in HCV patients who were given a full course of IFN therapy compared with those receiving less than a full course of IFN. The 3.8-fold greater number of estimated outpatient visits among HCV patients during therapy may reflect more intensive monitoring or routine treatment of less severe medical problems in these individuals. However, this service use corresponds to statistically greater cost among those receiving a full course of IFN compared to those with limited or no IFN therapy (generalised linear regression modelling controlling for baseline MRU, data not shown).
The rate of IFN discontinuance and dose reduction was high in both groups but not significantly different (except when anaemia was also present). IFN treatment was discontinued in nearly half of HCV patients with thrombocytopenia and in 40% of HCV patients without thrombocytopenia. The reason for IFN discontinuance is not known with certainty, but adverse events, including cytopenias, may have contributed to the discontinuation rateCitation31. Although individual practice may vary, initiation of IFN treatment is discouraged by product label if platelet counts are <75,000/µL to 100,000/µLCitation23. For adults, dose reductions are recommended if platelet counts decline to <50,000/µL, and IFN is suspended if platelet counts fall below 25,000/µL, depending on the type of IFN being usedCitation23,Citation31. Patients requiring a dose reduction or discontinuation due to thrombocytopenia are less likely to achieve a sustained virological response to IFN treatment and are likely to have long-term consequences due to chronic infection with HCVCitation32. Indeed, prescribing information for two IFN agents cautions that HCV patients in need of antiviral therapy may be denied IFN if their baseline platelet counts are too lowCitation33,Citation34.
Other studies have confirmed that HCV-related medical resource utilisation and costs in the United States are substantial despite a two-decade drop in disease incidenceCitation1,Citation32. The presence of thrombocytopenia appears to substantially increase medical resource utilisation and healthcare costs in patients with HCV.
Cost-intensive measures and conditions such as liver cancer, liver transplantation, and platelet transfusion were more common in HCV patients with thrombocytopenia. Novel and improved thrombocytopenia therapies that would enable IFN treatment without dose reductions or interruptions could fill an unmet medical need and possibly result in decreased overall healthcare costs in the longer term.
Limitations
A medical claims database is an important, readily available, and relatively low-cost source for medical resource utilisation analysis, but certain inherent limitations should be noted. Administrative claims data are collected for purposes of payment, not research, and are subject to coding errors. Unrecorded and undiagnosed thrombocytopenia leads to exclusion of relevant HCV patients from analysis cohorts. This in turn leads to limitations in the ability to generalise findings to an individual health plan and across health insurance plans. In addition, thrombocytopenia influences medical management, whether or not a diagnosis of low platelets is recorded or coded in the administrative record, so that its impact may not be recognised in an administrative claims database.
The population of patients with chronic HCV in this study may not be completely representative of the chronic HCV population because it represents only those seeking medical care; and, the prevalence of HCV in this database is much lower than national prevalence (per The National Health and Nutrition Examination Survey [NHANES] dataCitation35). For example, medical claims related to a bleeding diagnosis may be limited to those that require medical intervention. Medical care is also influenced by insurance benefits’ design. The HCV population with commercial coverage may look different from that receiving care from the Veterans Administration, for example.
The HCV population is associated with certain characteristics not typical of the population in general, including illicit drug use, high-risk sexual behaviour, and lower socioeconomic classCitation2–4,Citation36. These characteristics are not evident from the medical claims database, but should be considered in evaluating risk and prospects for long-term success of treatment programs. In addition, although the data reported are from 2001 through 2003, relative differences between groups were analysed, not absolute differences. Because treatments for HCV have not changed in recent years, an analysis from 2010 would likely show the same relationship.
Conclusions
Diagnosis of thrombocytopenia in patients with HCV is associated with increased incidence of certain comorbidities, complications, and medical interventions, including anaemia, neutropenia, liver cancer, liver transplant, platelet transfusion, and need for IFN treatment. The presence of thrombocytopenia in patients with HCV is also associated with significantly increased medical resource utilisation. Additional study is recommended to determine whether an effective thrombocytopenia therapy, which would permit use of IFN without dose reductions or dose interruptions and could potentially enhance viral clearance and improve management of HCV, would thereby reduce long-term medical resource utilisation and its associated costs.
Transparency
Declaration of funding
This study was funded by GlaxoSmithKline (GSK). The funder played no role in the preparation of this article other than that described below.
Declaration of financial/other relationships
F.P has no relationship to declare. D.T is an employee of and holds stock in GSK. J.S. receives honoraria from GSK, and is employed by i3 Innovus where she provided consultancy. K.G. is an employee of GSK.
Acknowledgements
A summary of this study was presented at Digestive Disease Week, May 2008. The authors thank Mark Dana and AOI Communications, L.P., for assistance in preparing the manuscript. The authors thank Mehul Dalal, PhD, for assistance in the analyses.
References
- Centers for Disease Control and Prevention. Viral hepatitis surveillance: estimates of disease burden from viral hepatitis. Available at: http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Accessed October 20, 2010
- Wasley A, Grytdal S, Gallagher K. Surveillance for acute viral hepatitis-United States, 2006. MMWR Surveill Summ 2008;57:1-24
- Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999;341:556-62
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006;144:705-14
- Centers for Disease Control and Prevention. National hepatitis C prevention strategy. Summer; 2001: http://www.cdc.gov/hepatitis/hcv/Strategy/PDFs/NatHepCPrevStrategy.pdf. Accessed November 8, 2010
- Centers for Disease Control and Prevention. Hepatitis C: fact sheet. June 2010: http://www.cdc.gov/hepatitis/hcv/pdfs/hepcgeneralfactsheet.pdf. Accessed November 8, 2010
- Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology 1997;26(3 Suppl 1):15-20S
- Xu J, Kochanek KD, Murphy SL, et al. Deaths: final data for 2007. Natl Vital Stat Rep 2010;58:1-135
- Shiffman ML, Saab S, Feng S, et al. Liver and intestine transplantation in the United States, 1995-2004. Am J Transplant 2006;6:1170-87
- Bashour FN, Teran JC, Mullen KD. Prevalence of peripheral blood cytopenias (hypersplenism) in patients with nonalcoholic chronic liver disease. Am J Gastroenterol 2000;95:2936-9
- Bordin G, Ballare M, Zigrossi P, et al. A laboratory and thrombokinetic study of HCV-associated thrombocytopenia: a direct role of HCV in bone marrow exhaustion? Clin Exp Rheumatol 1995;13(Suppl 13):S39-43
- Wang CS, Yao WJ, Wang ST, et al. Strong association of hepatitis C virus (HCV) infection and thrombocytopenia: implications from a survey of a community with hyperendemic HCV infection. Clin Infect Dis 2004;39:790-6
- Giannini E, Borro P, Botta F, et al. Serum thrombopoietin levels are linked to liver function in untreated patients with hepatitis C virus-related chronic hepatitis. J Hepatol 2002;37:572-7
- Nagamine T, Ohtuka T, Takehara K, et al. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol 1996;24:135-40
- Sharma P, McDonald GB, Banaji M. The risk of bleeding after percutaneous liver biopsy: relation to platelet count. J Clin Gastroenterol 1982;4:451-3
- Liangpunsakul S, Ulmer BJ, Chalasani N. Predictors and implications of severe hypersplenism in patients with cirrhosis. Am J Med Sci 2003;326:111-16
- McVay PA, Toy PT. Lack of increased bleeding after liver biopsy in patients with mild hemostatic abnormalities. Am J Clin Pathol 1990;94:747-53
- Williford SK, Salisbury PL 3rd, Peacock JE Jr., et al. The safety of dental extractions in patients with hematologic malignancies. J Clin Oncol 1989;7:798-802
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82
- Shimakami T, Lanford RE, Lemon SM. Hepatitis C: recent successes and continuing challenges in the development of improved treatment modalities. Curr Opin Pharmacol 2009;9:537-44
- Ong JP, Younossi ZM. Managing the hematologic side effects of antiviral therapy for chronic hepatitis C: anemia, neutropenia, and thrombocytopenia. Cleve Clin J Med 2004;71(Suppl 3):S17-21
- Strader DB, Wright T, Thomas DL, et al. Diagnosis, management, and treatment of hepatitis C. Hepatology 2004;39:1147-71
- Poordad F. Thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther. 2007;26(Suppl 1):5-11
- Weksler BB. The pathophysiology of thrombocytopenia in hepatitis C virus infection and chronic liver disease. Aliment Pharmacol Ther 2007;26(Suppl 1):13-19
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep [May 2; 2001:http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9790221. Accessed April 29, 2008
- Dieterich DT, Spivak JL. Hematologic disorders associated with hepatitis C virus infection and their management. Clin Infect Dis 2003;37:533-41
- McCullough J. Current issues with platelet transfusion in patients with cancer. Semin Hematol 2000;37(2 Suppl 4):3-10
- Rinder HM, Arbini AA, Snyder EL. Optimal dosing and triggers for prophylactic use of platelet transfusions. Curr Opin Hematol 1999;6:437-41
- McHutchison JG, Manns M, Patel K, et al. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology 2002;123:1061-9
- Shiffman ML, Ghany MG, Morgan TR, et al. Impact of reducing peginterferon alfa-2a and ribavirin dose during retreatment in patients with chronic hepatitis C. Gastroenterology 2007;132:103-12
- Dienstag JL, McHutchison JG. American Gastroenterological Association technical review on the management of hepatitis C. Gastroenterology 2006;130:231-64; quiz 214-37
- Brown Jr RS. A pharmacoeconomic analysis of thrombocytopenia in chronic liver disease. Aliment Pharmacol Ther 2007;26(Suppl 1):41-8
- Pegasys® (peginterferon alfa-2a). Full prescribing information. Hoffmann-La Roche Inc. Nutley, NJ, 2008
- PegIntron® (peginterferon alfa-2b). Full prescribing information. Schering-Plough Corporation. Kenilworth, NJ, 2009
- Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705-14
- Wasley A, Miller JT, Finelli L. Surveillance for acute viral hepatitis—United States, 2005. MMWR Surveill 2007;56:1-24