Abstract
Objectives:
Invasive aspergillosis (IA) is reported increasingly in non-traditional hosts, typically patients with chronic obstructive pulmonary disease (COPD). Objectives were to describe the excess burden of IA in COPD, including mortality, resource utilization, and costs, as well as to examine the impact of initial antifungal selection on clinical and economic outcomes.
Methods:
This retrospective cohort study used national data from 2005 to 2008, from the Premier Perspective hospital database. IA was identified using proxy ICD-9 codes based on published algorithms. The COPD + IA patient cohort was analyzed using descriptive statistics. Excess resource utilization was analyzed by matching cases (COPD + IA) and controls (COPD patients without aspergillosis) on demographic and clinical variables. Multivariate analyses were used to assess the impact of initial antifungal drug selection on outcomes in COPD + IA.
Results:
In total, 475 COPD + IA patients were identified (mean age 69 years, 50% male, 76% Caucasian). COPD + IA cases had significantly higher costs, length of stay, intensive care unit (ICU) stay, and mortality compared to COPD controls (all p < 0.01). On average, antifungal therapy was initiated on hospital day 6, with mean length of therapy 15 days, and one-third of patients were in the ICU when antifungal treatment was initiated. Most commonly used antifungals were voriconazole, fluconazole, and caspofungin. Patients receiving fluconazole as the initial antifungal, an agent inactive against moulds, had almost two times greater mortality (p = 0.016), two additional hospital days (p = 0.002), and 25% greater costs (p = 0.007), compared to patients receiving voriconazole first-line. Findings were consistent in sub-analyses including ICU patients.
Limitations:
‘Invasive’ form of aspergillosis was identified using proxy ICD-9 codes based on published literature. Additional limitations stem from the study's non-randomized, retrospective design that is typical with any database analyses.
Conclusions:
COPD + IA patients had significantly higher mortality, resource utilization, and costs versus COPD controls. Treatment with an agent active against Aspergillus was associated with better survival and reduced economic burden, therefore this potential etiology of pneumonia should be considered when contemplating antifungal therapy in COPD patients.
Introduction
During the past decade, Aspergillus spp. have become the most prevalent airborne fungal pathogen in developed countries, with a significant increase in invasive aspergillosis (IA) being observedCitation1. This opportunistic disease occurs predominantly in immunocompromised hosts, with the major risk factors being prolonged granulocytopenia and long-term therapy with steroid-containing immunosuppressive regimensCitation2.
Aside from these high-risk groups, many cases of invasive pulmonary aspergillosis are reported in non-traditional hosts, typically patients with chronic obstructive pulmonary disease (COPD). Such patients may have increased susceptibility to invasive fungal infection for several reasons: (1) structural changes in lung architecture related to the pulmonary disease; (2) the common use of long-term or repeated short-term steroids as an additional immunosuppressive factorCitation3; (3) frequent hospitalization and antibiotic treatment, leading to selective pressure, favoring fungal pathogens; and (4) comorbidity factors such as alcoholism, diabetes mellitus, or malnutritionCitation2.
Over the past decade, a considerable expansion in the clinical development of new compounds and strategies targeted against invasive aspergillosis has occurred. The following FDA-approved compounds have in vitro, in vivo, and clinical activity against Aspergillus species and are licensed for the treatment of invasive aspergillosis: amphotericin B, itraconazole, voriconazole, posaconazole, and caspofungin. Voriconazole and amphotericin B are the only compounds licensed in the US for primary treatment of invasive aspergillosis. Itraconazole and caspofungin are approved for salvage therapy, and posaconazole for prophylaxis of invasive aspergillosis in neutropenic patients with leukemia and allogenic hematopoietic stem cell transplantationCitation4.
In a review of 50 studies, COPD was the underlying condition in 26 out of 1941 (1.3%) patients with aspergillosisCitation5. Rodrigues et al.Citation6 reported that COPD patients contribute to 1% of all cases of IA in their institution. However, the true incidence of IA in COPD is probably underestimated due to lack of consistent case definition, absence of surveillance measuresCitation7 and low sensitivity of diagnostic procedures for this diseaseCitation2.
In recent years, several studies based on small samples of local hospital data have focused on the clinical outcomes of IA in COPD patientsCitation2,Citation7–9. Our objective was to expand the existing literature using nationally representative hospital data to specifically describe the outcomes and incremental economic burden of IA in COPD patients, including examination of the treatment patterns, resource utilization, and clinical outcomes for different antifungal strategies.
Methods
Study design and data source
The study was a retrospective hospital database analysis using a case-control study design to analyze health outcomes and resource utilization in patients with COPD and IA (COPD + IA; cases) compared to COPD patients without aspergillosis (controls). The study was conducted from a US payer perspective and did not include indirect costs. Patients were selected from the Premier Perspective database, a large US hospital-based, service-level database providing detailed patient-level resource utilization, diagnoses and procedures from over 500 US hospitalsCitation10. Patient data provided by Premier Perspective was de-identified and thus Institutional Review Board approval was not required. Detailed service-level billing information is available for each hospital day and includes medication information (drug name, strength, quantity dispensed). Patient information includes, but is not limited to, patient demographics, principal and secondary diagnoses/procedures (according to the International Classification of Disease 9th revision [ICD-9 CM]), length of stay (LOS), cost and charge details, and discharge statusCitation10. The study used data from the four most recent years, 2005 to 2008, and cost data were reported in US dollars.
Patient selection
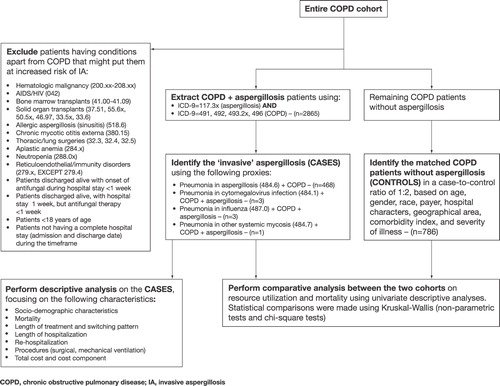
Study population comprised two cohorts, cases and controls. Cases were identified using a three-step process. The first step involved extracting patients ≥ 18 years with primary or non-primary diagnoses of COPD (ICD-9 codes 491.xx, 492.xx, 493.2 x, and 496.xx) and aspergillosis (ICD-9 code 117.3 x). Not all aspergillosis is invasive; hence, the second step identified the ‘invasive’ disease using the following proxies (primary or non-primary codes): pneumonia in aspergillosis (ICD-9 code 484.6), pneumonia in cytomegalovirus infection (ICD-9 code 484.1), pneumonia in influenza (ICD-9 code 487.0), and pneumonia in other systemic mycosis (ICD-9 code 484.7). Furthermore, it was also assumed that if a patient was discharged alive for a clinically invasive case of aspergillosis, the hospital LOS and length of antifungal treatment (all agents) would have been at least 7 days. Patients who died were included irrespective of their length of antifungal therapy. In order to identify invasive cases of aspergillosis in ‘non-traditional hosts’, the third step excluded patients with conditions apart from COPD that might put them at an increased risk of IA. ICD-9 diagnosis and procedure codes for these excluded conditions can be seen in the study design schematic in .
Once the COPD + IA cases were selected, matched controls (i.e., COPD patients without primary or non-primary diagnoses of aspergillosis) were identified using a case-to-control ratio of 1:2. The matching factors included age, gender, race, payer, hospital characteristics, geographical area, comorbidity index, and severity of illness during the hospitalization (). Perfect matching on all controlling variables was possible owing to a large size of the control cohort (>1.25 million). However, matching on the clinical severity of the underlying COPD was not possible because the data did not include information on baseline pulmonary function tests or whether the patient was on baseline oxygen therapy.
Measures
Patient and hospital characteristics
The database contained detailed information on patient and hospital characteristics, such as age, gender, race, payer, discharge status, all patient refined (APR) severity levels, hospital location, teaching status, bed size, and geographic region. An additional data file provided ICD-9 CM codes that were used to identify primary and non-primary diagnoses, comorbidities, and procedures/tests associated with each hospitalization. Charlson Comorbidity Index (CCI) was calculated based on Deyo's method using ICD-9 CM diagnosis codesCitation11,Citation12.
Resource utilization by COPD + IA patients
LOS for each hospitalization was provided with the data, whereas length of intensive care unit (ICU) stay and mechanical ventilation (MV) were computed using the standardized charge codes within billing records. A dummy variable for re-hospitalization due to aspergillosis within 4 months of discharge was generated using a unique patient identifier obtained with the dataset. However, this variable only allowed re-hospitalizations to be captured for the same hospital. Total patient costs were provided with the dataset, and costs incurred among different hospital departments were generated using billing records. The matched case-control cohort was analyzed to determine the excess resources utilized by cases compared to controls. Resources examined included total costs, cost by departments, LOS, ICU stay, days of MV, re-hospitalization and mortality rates.
Antifungal treatment patterns
Standardized charge codes in billing records were used to identify the following antifungal drugs administered during hospitalization: voriconazole, echinocandins (caspofungin, micafungin, and anidulafungin), itraconazole, fluconazole, posaconazole, and amphotericin B. The first use of an antifungal during the hospital stay was identified as the ‘index event’. Length of therapy was computed for each patient as the number of days on antifungal treatment. Antifungal therapies were categorized under ‘concomitant uses’ and ‘non-concomitant uses’, and under each category, first-line, second-line, and third-line treatments were examined. In addition, antifungal doses dispensed (loading and maintenance)Citation13 and switching patterns were examined in detail.
Statistical analyses
Patient and hospital characteristics, resource utilization, and antifungal treatment patterns were described using simple descriptive statistics. Mean, standard deviation (SD) and range was calculated for continuous variables; frequencies and proportions for categorical variables. Statistical comparisons were conducted between the different antifungal treatments using chi-square tests for categorical variables, analysis of variance (ANOVA) for continuous variables, and Kruskal–Wallis test for variables with skewed distribution. A two-sided p-value of <0.05 was considered statistically significant. All tests were conducted using SAS (v.9.1) software.
Since cases and controls were matched on all controlling variables, resources utilized by the two groups were assessed using univariate descriptive statistics (mean and SD; frequencies and proportions). Excess resource utilization by cases (COPD + IA) compared to controls was tested using non-parametric (Kruskal–Wallis) and chi-square tests.
For the cases, impact of index antifungal (first antifungal dispensed) was assessed on the total costs incurred and LOS using generalized linear models (GLMs); and on mortality and re-hospitalizations using binary logistic regression. The models were controlled for key patient demographics, hospital characteristics, and severity of illness. Clinically relevant comorbidities, such as diabetes and acute renal/hepatic disease, were not included since they did not improve the model fit significantly. Furthermore, the APR severity of illness score was included, which is calculated in part based on these key comorbidities.
To test the robustness of findings in the overall cohort, additional subgroup analyses were conducted in: ICU patients; patients receiving a diagnostic test (as not all patients with a 117.3 ICD-9 diagnosis code had a billing record for bronchoscopy; needle biopsy; or antibody Aspergillus test); and patients with both an ICU stay and a diagnostic test.
Results
Of ∼10,000 unique patient hospitalizations with aspergillosis in the dataset, 3365 were identified as having COPD. After applying the exclusion and ‘invasive’ criteria, 475 unique patient hospitalizations were extracted as the COPD + IA cohort. The major exclusions were due to hematologic malignancies, lung surgeries (a potential marker of aspergilloma), aplastic anemia, and for those discharged alive, LOS < 1 week and length of antifungal treatment < 1 week. Only about two-thirds of the 475 patients (63.4%) had a billing code for a diagnostic test, with bronchoscopy, needle biopsy and antibody Aspergillus tests performed in 54.8%, 3.8%, and 4.8% patients, respectively. Therefore, to account for this uncertainty around the clinical diagnosis we tested important outcomes in three subsets: COPD + IA patients with a diagnostic test, with an ICU stay, and with both.
Patient characteristics
Patient demographics and hospital characteristics are presented in , while patient clinical and comorbidity characteristics are presented in . Mean CCI score was 2.7, and the majority of the patients (83.6%) had an extreme level of APR severity of illness. On average, antifungal therapy was initiated (index event) on the sixth day of hospital stay (median 4.5), with a mean length of therapy being 15 (SD 10.1) days. Approximately 31% of the patients were identified to be in the ICU at their antifungal therapy initiation, and 29% of patients died in the hospital. Mortality rates were lower than 50% in all cohorts, whether it was patients with a diagnostic test, patients with an ICU stay, or patients with both.
Table 1. Demographic and hospital characteristics of COPD + IA patients.
Table 2. Clinical characteristics of COPD + IA patients.
Resource utilization
shows the key resource utilization items, re-hospitalization, and mortality rates of COPD + IA patients and controls. More than half (56%) of the COPD + IA patients had an ICU stay, and 35% of the patients required MV support. Mean total costs incurred per COPD + IA patient were US$48,827 (53,377). Room and board ($22,368 [24,497]) and pharmacy department costs ($9584 [12,482]) accounted for the largest proportions of the total cost. According to the cost file in this database, the average cost per day were $550 for general ward bed, $1515 for ICU stay, and $198 for mechanical ventilator use.
Figure 2. Excess burden of COPD + IA (n = 457 cases [COPD + IA] vs. n = 786 matched controls [COPD only]).
![Figure 2. Excess burden of COPD + IA (n = 457 cases [COPD + IA] vs. n = 786 matched controls [COPD only]).](/cms/asset/32f1e231-fcaa-4b1c-a147-2e90c4d9f327/ijme_a_564246_f0002_b.jpg)
COPD + IA cases had significantly greater resource utilization than similar patients without aspergillosis (). Compared to the controls, cases incurred greater total costs, ($48,163 vs. $30,210; p < 0.001); and longer LOS (23.2 vs. 13.6 days; p < 0.001), ICU stay (13.8 vs. 7.2 days; p < 0.001), and MV support (16.1 vs. 9.0 days; p < 0.001). Frequency of mortality (28.9 vs. 22.8%; p = 0.016) and re-hospitalizations (5.7 vs. 1%; p < 0.001) were also significantly higher in cases.
Antifungal treatment pattern
Overall 76% of the patients were given voriconazole, 29% caspofungin, 34% fluconazole, and 14% itraconazole at least once during their treatment. The vast majority of patients (418 of 475) received single, non-concomitant antifungal use (). Approximately 12% of patients (57 of 475) had some concomitant antifungal therapy, of which the majority (77%) was on a combination of voriconazole and caspofungin with a mean length of therapy of 7 days.
Table 3. Antifungal drug use description (without concomitant use).
Intravenous (IV) to oral conversion on voriconazole was common, with 48% of first-line voriconazole IV patients switching to voriconazole oral with an average conversion on day 4. Switching antifungal agents was commonly observed among fluconazole patients, with 37% switching to voriconazole and 20% to echinocandins.
shows data for average daily dose on day 1 and day ≥2. Loading doses were administered in only 22% of first-line voriconazole (mean 861 [SD 154] mg), 50% of first-line caspofungin patients (mean 73 [SD 11] mg), and 20% of first-line fluconazole (mean 467 [SD 132] mg IV; 513 [SD 165] mg oral).
Table 4. Antifungal therapy: average doses.
The GLMs and logistic regression model confirmed that the index antifungal had a significant impact on total costs, LOS, and mortality after controlling for several key variables (). Patients on index fluconazole and itraconazole incurred 25% and 54% greater costs, respectively, than index voriconazole patients. Moreover, index fluconazole patients had two additional hospital days and twice the risk of death compared with index voriconazole patients.
Table 5. Descriptive analysis based on the key index antifungal therapies.
Table 6. Impact of index antifungal on total costs, controlling for key variables (n = 465).
Table 7. Impact of index antifungal on length of stay, controlling for key variables (n = 467).
Table 8. Impact of index antifungal on mortality, controlling for key variables (n = 475).
Additional analyses were conducted for different patient subgroups to determine whether the impact of index antifungal was consistent. shows significantly increased mortality rates with index fluconazole compared to index voriconazole in ICU patients, diagnostic test patients, and ICU + diagnostic test patients.
Table 9. Mortality comparisons in COPD + IA patient subsets.
Discussion
This is the first nationally representative US hospital database analysis to describe the clinical and economic outcomes of IA in COPD. Our findings demonstrate that COPD + IA patients are extremely ill, having significantly higher mortality, resource utilization, and costs than control COPD cases without IA. More specifically, the COPD + IA patients required longer hospital stays, longer in ICU, MV, and antifungal therapy.
Known risk factors for developing IA in COPD include high-dose corticosteroids, ICU stay, and comorbidities that increase susceptibility to fungal infections, such as diabetes. Our analysis found that approximately 85% of the COPD + IA study cohort was on high-dose corticosteroid therapy, most patients for a duration of ≥14 days. These numbers support the existing belief that high doses and long duration of corticosteroid use increases the susceptibility to IA. Approximately one-third of patients were in the ICU on the day of antifungal therapy initiation. Hence, ICU stay can be argued as a risk factor, or marker, for encountering IA infections.
The occurrence rates from our study data demonstrated that 0.7% of hospitalized COPD patients on high-dose corticosteroids for ≥14 days potentially encounter IA. This increased to 1.2% when additional risk factors, such as ICU stay and mechanical ventilation support, were accounted for. Nevertheless, the occurrence estimates from a hospital database analysis could be an underestimation because antemortem diagnoses were used for including the patients. Comparisons of IA diagnoses in live patients with those established by autopsy reveal that the incidence of IA is often missed, potentially due to the low sensitivity (up to 50%) and specificity of microbiological tests of cultured respiratory specimensCitation14–17. Furthermore, underlying patient pathologies and conditions in the ICU can obscure the characteristic clinical and radiological signs of IA, rendering this and other fungal infections the most commonly under-diagnosed condition in ICU patientsCitation16,Citation17.
While the hospital database did not provide clinical information about laboratory test results used to diagnose IA, the billing data revealed that around two-thirds of the patients had a bronchoscopy, needle biopsy, and/or antibody Aspergillus test in the hospital. For the remaining one-third, the basis of the diagnosis remains unclear, and may have contributed to lower mortality rates seen in this database analysis compared to previous single-hospital studiesCitation2,Citation7–9 where medical chart and postmortem data were available to confirm invasive disease.
Voriconazole was the most widely used antifungal with almost half the patients receiving it as first-line treatment. Our analysis found that fluconazole, which does not have an indication for aspergillosis, was used as the first-line treatment in almost one-third of patients. The lack of adequate antifungal coverage in these patients is evidenced by the study results. Switching antifungal therapies was commonly observed, with the majority of fluconazole patients being switched to either voriconazole or caspofungin after few days. This indicates that physicians often start COPD patients on fluconazole as a general antifungal and are not specifically considering antifungal coverage for aspergillosis, and once the symptoms deteriorate they switch to other recommended antifungals.
Loading doses on day 1 were dispensed to a relatively small proportion of patients, which was concerning from the perspective of antifungal dosing practices because increasing compliance with dosing guidelines could, arguably, lead to better outcomes.
The study analyzed mortality rates in three patient subsets: patients with an ICU stay, patients with a diagnostic test, and patients with both. The findings revealed a mortality rate less than 50% in all three subsets. Several authors have reported a very high mortality rate (80–100%) in COPD patients with occurrence of IA, although these studies generally pre-date the availability of the newer antifungals studied in this analysisCitation2,Citation8,Citation14,Citation18. Literature also suggests that an early diagnosis is crucial to improve prognosis and reduce the mortality rateCitation19. Our findings expand upon the existing knowledge that in addition to early diagnosis, the initial antifungal selection is crucial for patient outcome. The regression model results demonstrated that patients do better with index voriconazole or itraconazole therapy, which are active against moulds, compared to index fluconazole, which is not active.
Our study includes limitations that are typical with database analyses that must rely on diagnosis coding and lack of clinical details from patient medical charts. More specifically, there were no clearly defined ICD-9 codes to identify the clinically ‘invasive’ form of aspergillosis. Hence, the inclusion criteria were developed primarily based on clinical opinion and published literatureCitation20, with most of our COPD + IA cohort having ‘pneumonia in aspergillosis’, so it is possible that we may have excluded some patients that had IA. In addition, the ICD-9 coding of aspergillosis in the database may not have represented the clinical reality, as only two-thirds of the patients had a billing code for a diagnostic test. As a result, one may argue the generalizability of the study and the potential inaccuracies in estimates derived from this sample. To test for this uncertainty around the clinical diagnosis, we created three subsets: COPD + IA patients with a diagnostic test, with an ICU stay, and with both. Mortality rates varied between the subgroups as expected; however, the impact of index antifungal selection was consistent across all groups. These findings suggest that the results overall are robust.
Additional limitations include the study's retrospective, non-randomized design and the fact that the database does not capture a patient's activity outside the Premier Perspective group of hospitals. The analysis was based on the cost file from the billing data, and not actual clinical records, which may lead to bias due to missing data or measurement error. Finally, the cost values range over 4 years, and no adjustments were made to report them for one baseline year.
Conclusion
Hospitalized COPD patients with IA incurred significantly higher costs and used more resources compared to similar patients without aspergillosis. Clinical and economic outcomes were significantly impacted by the initial antifungal selection, with index fluconazole patients incurring greater costs and mortality risk compared to their counterparts initially started on an Aspergillus-active treatment. This suggests that appropriate initial treatment with an agent active against Aspergillus might reduce mortality and economic burden. Clinicians should consider the likelihood of this potential etiology of pneumonia when contemplating antifungal therapy in COPD patients.
Transparency
Declaration of funding
This research and preparation of this manuscript were funded by Pfizer Inc.
Declaration of financial/other relationships
D.P., X.G., and J.S. were employees of Pharmerit North America, who were paid consultants to Pfizer in connection with this research and the development of this manuscript. M.T. and M.F. were employed by Pfizer Inc, the manufacturer of antifungal agents.
The work was performed at Pharmerit North America LLC, Bethesda, MD, USA, and was sponsored by Pfizer Inc.
Acknowledgments
All authors contributed to project design and methodology, review and interpretation of results, manuscript writing and revisions, and approved the final manuscript. D.A. Patel and X. Gao conducted the programming and statistical analysis.
Editorial assistance was provided by Dean Clarke and Cath Bryant at Complete Medical Communications, and was funded by Pfizer Inc.
References
- Steinbach WJ, Stevens DA, Denning DW, et al. Advances against aspergillosis. Clin Infect Dis 2003;37(Suppl 3):S155-6
- Ader F, Nseir S, Le Berre R, et al. Invasive pulmonary aspergillosis in chronic obstructive pulmonary disease: an emerging fungal pathogen. Clin Microbiol Infect 2005;11:427-9
- McEvoy CE, Niewoehner DE. Adverse effects of corticosteroid therapy for COPD. A critical review. Chest 1997;111:732-43
- Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis 2008; 46:327-60
- Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis 2001;32:358-66
- Rodrigues J, Niederman MS, Fein AM, et al. Nonresolving pneumonia in steroid-treated patients with obstructive lung disease. Am J Med 1992;93:29-34
- Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J 2007;30:782-800
- Bulpa PA, Dive AM, Garrino MG, et al. Chronic obstructive pulmonary disease patients with invasive pulmonary aspergillosis: benefits of intensive care? Intensive. Care Med 2001;27:59-67
- Franquet T, Muller NL, Gimenez A, et al. Semiinvasive pulmonary aspergillosis in chronic obstructive pulmonary disease: radiologic and pathologic findings in nine patients. AJR Am J Roentgenol 2000;174:51-6
- Chandwani S, Wentworth C, Burke TA, et al. Utilization and dosage pattern of echinocandins for treatment of fungal infections in US hospital practice. Curr Med Res Opin 2009;25:385-93
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83
- RxList - WebMD, 2009. Antifungal dosage and administration. Available at: www.rxlist.com [Last accessed 30 June 2009]
- Dimopoulos G, Piagnerelli M, Berre J, et al. Disseminated aspergillosis in intensive care unit patients: an autopsy study. J Chemother 2003;15:71-5
- Groll AH, Shah PM, Mentzel C, et al. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect 1996;33:23-32
- Meersseman W, Lagrou K, Maertens J, et al. Invasive aspergillosis in the intensive care unit. Clin Infect Dis 2007;45:205-16
- Klont RR, Meis JF, Verweij PE. Critical assessment of issues in the diagnosis of invasive aspergillosis. Clin Microbiol Infect 2001;7(Suppl 2):32-7
- Rello J, Esandi ME, Mariscal D, et al. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease: report of eight cases and review. Clin Infect Dis 1998;26:1473-5
- von Eiff M, Roos N, Schulten R, et al. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 1995;62:341-7
- Chang D, Burwell L, Lyon G, et al. Comparison of the use of administrative data and an active system for surveillance of invasive aspergillosis. Infect Control Hosp Epidemiol 2008; 29:25-30