Summary
Objective:
This study aims to compute the budget impact of lacosamide, a new adjunctive therapy for partial-onset seizures in epilepsy patients from 16 years of age who are uncontrolled and having previously used at least three anti-epileptic drugs from a Belgian healthcare payer perspective.
Methods:
The budget impact analysis compared the ‘world with lacosamide’ to the ‘world without lacosamide’ and calculated how a change in the mix of anti-epileptic drugs used to treat uncontrolled epilepsy would impact drug spending from 2008 to 2013. Data on the number of patients and on the market shares of anti-epileptic drugs were taken from Belgian sources and from the literature. Unit costs of anti-epileptic drugs originated from Belgian sources. The budget impact was calculated from two scenarios about the market uptake of lacosamide.
Results:
The Belgian target population is expected to increase from 5333 patients in 2008 to 5522 patients in 2013. Assuming that the market share of lacosamide increases linearly over time and is taken evenly from all other anti-epileptic drugs (AEDs), the budget impact of adopting adjunctive therapy with lacosamide increases from €5249 (0.1% of reference drug budget) in 2008 to €242,700 (4.7% of reference drug budget) in 2013. Assuming that 10% of patients use standard AED therapy plus lacosamide, the budget impact of adopting adjunctive therapy with lacosamide is around €800,000–900,000 per year (or 16.7% of the reference drug budget).
Conclusions:
Adjunctive therapy with lacosamide would raise drug spending for this patient population by as much as 16.7% per year. However, this budget impact analysis did not consider the fact that lacosamide reduces costs of seizure management and withdrawal. The literature suggests that, if savings in other healthcare costs are taken into account, adjunctive therapy with lacosamide may be cost saving.
Introduction
Epilepsy is a neurological disorder that disrupts the normal transmission of electrical signals in the brain and is characterised by abnormal electrical neuronal activity resulting clinically in unprovoked recurring seizures. Partial-onset seizures are those that involve only a portion of the brain at seizure onset. The prevalence of epilepsy varies between 4 and 8 cases per 1000 individuals in developed countriesCitation1, and partial seizures are the predominant type of epileptic seizureCitation2.
Epileptic seizures are associated with significant morbidity, impaired quality of life, mortality, and are a primary driver of hospital admissions and healthcare costs. A literature review reported an increased mortality risk for people with epilepsy as compared with the general populationCitation3. A bottom-up, prevalence-based, cost-of-illness analysis estimated the costs of epilepsy from a societal perspective in the 25 European Union member countries, plus Iceland, Norway, and SwitzerlandCitation4. The estimated total cost of the disease in Europe was €15.5 billion in 2004, indirect costs related to productivity loss being the single most important cost category (€8.6 billion). The total cost per case was €2000–11,500 and the estimated cost per European individual was €33.
The management of epilepsy tends to begin with monotherapy with an anti-epileptic drug (AED). Given that around 30% of patients are not controlled with monotherapy regimensCitation5, treatment of refractory epilepsy will move to polytherapy with regimens consisting of two or more anti-epileptic drugs. As the objective of seizure freedom within refractory patients can be difficult, a more realistic goal in this patient population may be to achieve a significant reduction in seizure frequencyCitation6. For patients who experience refractory epilepsy on monotherapy, the newer anti-epileptic drugs are added to the monotherapy regimens (adjunctive therapy). A literature review of systematic reviews reported that newer anti-epileptic drugs were effective as adjunctive therapy for refractory epilepsy as compared with placeboCitation7.
Several factors inform the choice of the most appropriate treatment regimen in patients requiring adjunctive therapy. Of key importance is that adjunctive therapy for uncontrolled epilepsy is effective in achieving seizure freedom or reduction in a group of patients who have continued to experience a high seizure burden despite initial treatment for their epilepsy. Given the chronic nature of epilepsy and the requirement for life-long management in many cases, it is important that treatments are tolerable. This is particularly important in the adjunctive treatment of uncontrolled patients as these individuals will be treated with several drugs, all of which may be associated with distinct adverse events. Also, when patients are being treated as part of a polytherapy regimen, they are at an increased risk of experiencing drug–drug interactions. The ease of use and convenience of the drug also need to be considered. Key positive attributes are treatments that do not require multiple administrations a day and those with flexible dosing options.
There is an unmet need for treatment options for uncontrolled patients with partial-onset epilepsy. Lacosamide (Vimpat, UCB Pharma, Brussels, Belgium) is a new AED which is indicated as adjunctive therapy for partial-onset seizures with or without secondary generalisation in patients from 16 years of age in Europe. The pivotal multi-centre, double-blinded, placebo-controlled trials 667, 754 and 755 have demonstrated the efficacy of lacosamide in approximately 1300 adults with partial-onset seizuresCitation8–10. An intention-to-treat analysis of pooled data showed that patients randomised to lacosamide had a significant reduction in seizure frequency per 28 days and significantly higher ≥50% responder rates as compared to patients randomised to placebo.
With a view to assessing a drug reimbursement application, regulatory agencies in an increasing number of countries require data about, amongst other things, the budgetary impact of the drug on national, regional or local budgetsCitation11. A budget impact analysis examines the financial impact of the adoption and diffusion of a drug within a particular setting and, thus, considers the affordability of a drug. Specifically, a budget impact analysis explores how a change in the current mix of treatment strategies by the introduction of a new drug will impact spending on a disease. However, to date, budget impact analyses have rarely been published in the international literatureCitation12.
In a context of spiralling healthcare costs and limited resources, policy makers and healthcare payers are concerned about the budget impact of lacosamide and other AEDs. Therefore, the aim of this study is to compute the budget impact of adjunctive therapy with lacosamide as a treatment for partial-onset seizures with or without secondary generalisation in epilepsy patients from 16 years who are uncontrolled having previously used at least three AEDs from the perspective of the healthcare payer in Belgium.
Patients and methods
Analytic technique
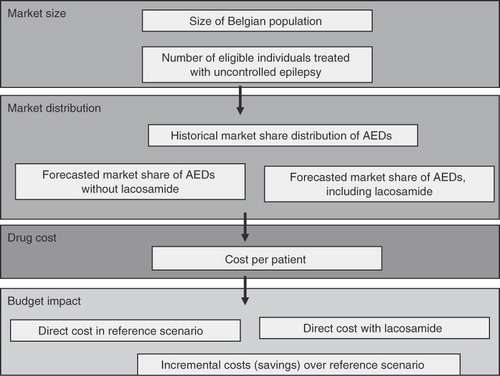
The methodology of budget impact analysis is still developing, although principles of good practice for budget impact analysis have recently been proposedCitation13. The budget impact analysis compared the ‘world with lacosamide’ to the ‘world without lacosamide’ and calculated how a change in the mix of AEDs used to treat uncontrolled epilepsy would impact the trajectory of drug spending on this condition. The general model for conducting the budget impact analysis of lacosamide is outlined in .
The budget impact analysis consisted of four modules. First, the Market Size was estimated by identifying the total number of patients eligible to receive adjunctive therapy with lacosamide. Second, the Market Distribution module estimated the market share of lacosamide as well as the impact of the adoption of lacosamide on the market shares of other AEDs. Third, the Drug Costs scenario calculated daily AED costs per patient. Fourth, the Budget Impact module calculated total drug costs in the reference scenario (‘world without lacosamide’) and the new drug scenario (‘world with lacosamide’). The cost difference between these two scenarios revealed the budget impact of adopting adjunctive therapy with lacosamide. The time horizon of the budget impact analysis was 5 years from 2008 until 2012.
Market size
The total Belgian population was estimated to be 10,666,866 people in 2008Citation1Citation4. A total of 82% of the Belgian population was adults, amounting to 8,746,830 adults. Using a prevalence rate of epilepsy of 0.6%Citation15, this generated 57,399 adults with epilepsy. Using a prevalence rate of partial-onset seizures of 57%Citation16, 32,718 epileptic adults suffered from partial-onset seizures. Finally, as around 16% of these patients have uncontrolled disease after having used three AEDsCitation17, the target population of epileptic patients from 16 years with partial-onset seizures who are uncontrolled on current treatment with at least two AEDs was estimated at 5333 patients in 2008. Estimates for the other years of the time horizon were derived in a similar way.
Market distribution
As lacosamide has been approved as adjunctive therapy in partial-onset epilepsy, the relevant reference scenario is standard AED therapy alone. Given that there were no publicly available data on the Belgian AED market, the various AEDs included in the standard therapy arm were extracted from the ‘placebo’ arm of the pivotal trials 754 and 755. The most frequently used AEDs in these studies were carbamazepine (23%), lamotrigine (22%), levetiracetam (19%), topiramate (17%) and valproate (19%)Citation9,Citation10,Citation18.
In the absence of Belgian market data on lacosamide, two scenarios were applied to model the ‘world with lacosamide’. The first scenario was based on data used by the Scottish Medicines Consortium indicating that the market share of lacosamide would increase linearly from 0.2% in 2008 to 7% in 2013Citation1Citation9, and that lacosamide would take market share evenly from all other AEDs. A second scenario was tested in the sensitivity analysis assuming that 10% of patients with partial-onset epilepsy would use standard AED therapy plus lacosamide.
The associated patient numbers were calculated by multiplying the estimated market share of each AED by the size of the target population.
Drug costs
The daily AED cost per patient is presented in . The daily AED cost per patient was calculated based on the defined daily dose and the average cost per mg for that drug. The average cost per mg of a drug was calculated based on the different strengths and pack sizes available for a particular AED. Trial data demonstrated the efficacy of lacosamide in patients receiving lacosamide 200–600 mg/dayCitation8–10. The dose of 300 mg/day was used in the budget impact analysis as this corresponds with the defined daily dose. Additionally, a sensitivity analysis considered the budget impact of lacosamide 400 mg/day. In accordance with Belgian guidelines for pharmaco-economic studiesCitation20, the reference price was used when generic products existed, even though generic products are used infrequently to treat epilepsy in Belgium.
Table 1. Daily anti-epileptic drug costs per patient.
Budget impact
Annual costs for a specific AED were calculated by multiplying the number of patients taking that AED by the daily cost of that AED and by 365 days. Summing annual costs over all AEDs generates total drug costs in the reference scenario (‘world without lacosamide’) and in the new drug scenario (‘world with lacosamide’).
Results
The Belgian target population of epileptic patients from 16 years with partial-onset seizures who are uncontrolled on current treatment with at least two AEDs was expected to increase from 5,333 patients in 2008; 5,387 patients in 2009; 5,443 patients in 2010; 5,498 patients in 2011; 5,554 patients in 2012; to 5,522 patients in 2013.
Assuming that the market share of lacosamide increases linearly over time, shows total drug costs in the reference scenario (‘world without lacosamide’) and the new drug scenario (‘world with lacosamide’), respectively. In the reference scenario, total drug costs are expected to increase from €4.9 million in 2008 to €5.1 million in 2013. In the new drug scenario, total drug costs would rise from €4.9 million in 2008 to €5.4 million in 2013. The estimated budget impact of treatment with lacosamide is the difference in total drug costs between the new drug scenario and the reference scenario. demonstrates that the absolute budget impact of adopting adjunctive therapy with lacosamide increases to €242,700 in 2013. Overall, treatment with lacosamide raises the 2008–2013 budget by €858,771 (or 2.8% of the reference drug budget).
Table 2. Budget impact of adopting lacosamide, 2008–2013 – market share of lacosamide increases linearly over time.
presents the reference scenario and the new drug scenario assuming that 10% of patients use standard AED therapy plus lacosamide. The number of patients with partial-onset epilepsy who take standard AED therapy plus lacosamide would be expected to rise from 533 patients in 2008 to 552 patients in 2013. As a result, the budget impact of adopting adjunctive therapy with lacosamide is around €800,000–900,000 per year. Adjunctive therapy with lacosamide increases the 2008–2013 budget by a total of €5,090,276 (or 16.7% of the reference drug budget).
Table 3. Budget impact of adopting lacosamide, 2008–2013 – 10% of patients use standard AED therapy plus lacosamide.
When the maximum registered dose of lacosamide 400 mg/day was used in a sensitivity analysis, the budget impact of adopting adjunctive therapy with lacosamide increased to 5.2% of the reference drug budget (assuming that the market share of lacosamide increases linearly over time) or to 22.3% of the reference drug budget.
Discussion
The budget impact analysis compared the world with lacosamide to the world without lacosamide. The analysis took into account the target population, the market share of AEDs and AED costs with a view to estimating the budget impact of adjunctive therapy with lacosamide.
The results of the budget impact analysis depended critically on the scenarios about the market uptake of lacosamide. A first scenario reflected a linear increase over time in the market share of lacosamide to the detriment of the market share of other AEDs. Under this scenario, the adoption of lacosamide would raise the drug budget for this patient population by 0.1–4.7% per year. Looking at the total AED budget associated with epilepsy management of €28,092,000 in 2008, the maximum additional impact of adjunctive therapy with lacosamide of €242,700 would raise the annual budget by 0.9%. The budget impact analysis took into account a maximum impact as we assumed that all patients started on lacosamide will continue their treatment with lacosamide, although in reality there will be withdrawals. A second scenario assumed that 10% of patients with partial-onset epilepsy would use standard AED therapy plus lacosamide. This scenario implied that the adoption of adjunctive therapy with lacosamide would increase the drug budget for this patient population by 16.7% per year. This increase resulted from the fact that around 500–600 patients per year would be treated with lacosamide in addition to standard AED therapy.
This study is subject to several methodological limitations. In the absence of Belgian data on epilepsy prevalence or the AED market, the analysis drew on published sources or on assumptions. Data on epilepsy prevalence taken from the literature were relatively old and did not consider that the prevalence of partial-onset epilepsy has now likely increased due to the higher number of elderly inhabitants in the population. Therefore, the analysis underestimated the budget impact of lacosamide. To account for the uncertainty surrounding the Belgian AED market, the analysis explored the budget impact of two scenarios relating to the ‘world with lacosamide’.
The budget impact analysis of adjunctive therapy with lacosamide was restricted to drug costs only as such an approach is sufficient for reimbursement purposes in Belgium. This means that the analysis did not take into account the impact of lacosamide on other healthcare costs, such as the costs of hospitalisation, A&E visits, physician visits, practice nurse visits, or laboratory tests. A Belgian economic evaluation demonstrated that the higher drug costs of standard AED therapy plus lacosamide were more than offset by lower costs of seizure management and withdrawal, thus resulting in overall savings to the Belgian healthcare payerCitation21. In particular, standard AED therapy plus lacosamide was associated with savings in other healthcare costs amounting to €4168 per patient per year as compared with standard AED therapy alone. These savings exceeded the annual drug costs of lacosamide of €1555 per patient.
The results presented in this manuscript are in line with those reported in the literature. A Finnish budget impact analysis calculated the budget impact of lacosamide from the healthcare payer perspectiveCitation22. Data on the number of patients, AED market shares and unit costs were taken from Finnish sources. The authors applied the conservative assumption of using generic drug prices in the analysis. It should be noted that this analysis focused on drug costs only and did not consider other healthcare costs. The results indicated that the introduction of lacosamide would increase the annual drug budget by 2.23% in 2012. Three economic evaluations used a similar design to determine the cost effectiveness of lacosamide from the healthcare payer perspective in Sweden, Finland and BelgiumCitation21–23. These studies showed that standard AED therapy plus lacosamide is likely to constitute a cost-effective alternative or is even cost saving. However, studies derived efficacy estimates from short-term trials; there were few head-to-head comparisons of the efficacy of anti-epileptic drugs; and there were few data on utility values associated with epileptic health states.
Conclusions
The budget impact analysis indicated that the adoption of adjunctive therapy with lacosamide would raise the drug budget for this patient population by as much as 16.7% per year. However, this budget impact analysis did not consider the fact that lacosamide reduces costs of seizure management and withdrawal. The literature suggests that, if savings in other healthcare costs are taken into account, adjunctive therapy with lacosamide may be cost saving.
Transparency
Declaration of funding
The budget impact analysis was requested by the National Institute for Health and Disability Insurance (i.e., the Belgian third-party payer) and paid for by UCB Pharma.
Declaration of financial/other relationships
The author has no conflicts of interest that are relevant to this manuscript.
Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Hauser W. Epilepsy: Frequency, Causes, and Consequences. New York: Demos Press, 1990
- Kotsopoulos IA, van Merode T, Kessels FG, et al. Systematic review and meta-analysis of incidence studies of epilepsy and unprovoked seizures. Epilepsia 2002;43:1402-9
- Hitiris N, Mohanraj R, Norrie J, et al. Mortality in epilepsy. Epilepsy Behav 2007;10:363-76
- Pugliatti M, Beghi E, Forsgren L, et al. Estimating the cost of epilepsy in Europe: a review with economic modeling. Epilepsia 2007;48:2224-33
- Brodie MJ. Epilepsy: randomised trials and genetic tribulations. Lancet Neurol 2008;7:7-8
- Leppik I, Morrell M, Godfroid P, et al. Seizure-free days observed in randomized placebo-controlled add-on trials with levetiracetam in partial epilepsy. Epilepsia 2003;44:1350-52
- Wilby J, Kainth A, Hawkins N, et al. Clinical effectiveness, tolerability and cost-effectiveness of newer drugs for epilepsy in adults: a systematic review and economic evaluation. Health Technol Assess 2005;9:1-157, iii–iv
- Ben-Menachem E, Biton V, Jatuzis D, et al. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia 2007;48:1308-17
- Chung S, Sperling MR, Biton V, et al. Lacosamide as adjunctive therapy for partial-onset seizures: a randomized controlled trial. Epilepsia 2010;51:958-67
- Halasz P, Kalviainen R, Mazurkiewicz-Beldzinska M, et al. Lacosamide: efficacy and safety as oral adjunctive therapy in adults with partial seizures. Epilepsia 2006;47(Suppl 4):3
- Cohen J, Stolk E, Niezen M. The increasingly complex fourth hurdle for pharmaceuticals. Pharmacoeconomics 2007;25:727-34
- Mauskopf JA, Earnshaw S, Mullins CD. Budget impact analysis: review of the state of the art. Expert Rev Pharmacoecon Outcomes Res 2005;5:65-79
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR task force on good research practices – budget impact analysis. Value Health 2007;10:336-47
- National Statistics of Belgium. Available at: http://www.statbel.fgov.be/ [Last accessed 6 September 2010]
- Boon P, De Deyn PP, Hauman H, et al. Epidemiologie van epileptische toevallen in Vlaanderen. Tijdschr Geneeskund 1996;52:47
- Hauser WA. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia 1993;34:453-68
- Schiller Y, Najjar Y. Quantifying the response to antiepileptic drugs: effect of past treatment history. Neurology 2008;70:54-65
- National Institute for Health and Clinical Excellence. Newer drugs for epilepsy in adults. London: National Institute for Health and Clinical Excellence, 2004
- Scottish Medicines Consortium. Lacosamide. Edinburgh: Scottish Medicines Consortium, 2009
- Cleemput I, Van Wilder P, Vrijens F, et al. Richtlijnen voor farmacoeconomische evaluaties in België. Brussel: Federaal Kenniscentrum voor de Gezondheidszorg, 2008
- Simoens S, Dedeken P, Benhaddi H. Cost-utility analysis of lacosamide adjunctive therapy in the treatment of partial-onset seizures in epileptic patients in Belgium. Presentation at the ISPOR 13th Annual European Congress. Prague, Czech Republic, 6–9th November 2010
- Soini E, Martikainen J, Vanoli A. Cost-effectiveness and budget impact modelling of lacosamide in the treatment of partial-onset seizures in Finland. Value Health 2009;12:A367
- Bolin K, Berggren F, Forsgren L. Lacosamide as treatment of epileptic seizures -- cost utility results for Sweden. Acta Neurol Scand 2010;121:406-12