Abstract
Background:
Total hip and total knee replacement (THR/TKR) patients are at increased risk of developing venous thromboembolism (VTE). VTE prevention using anticoagulation therapy increases the risk of bleeding. Therefore, any assessment of the cost of VTE and its prevention should also take into consideration risks and costs of bleeding.
Objective:
To assess the risks of developing VTE and bleeding in patients after THR or TKR given real-world use of thromboprophylaxis, and to quantify the incremental cost associated with each.
Methods:
Analyses of insurance healthcare claims from the Ingenix IMPACT National Managed Care DatabaseTM from January 2004 to December 2008 were conducted. Subjects were ≥18 years and had ≥1 procedure code for THR or TKR. Patients had to have ≥180 days of observation prior to surgery and were observed for ≤3 months after THR or TKR. VTE was defined as ≥1 diagnosis code for deep vein thrombosis or pulmonary embolism. Bleeding events were classified as major or non-major. Risks of VTE or bleeding events were calculated as number of patients with an event divided by number of patients with the procedure. Incremental all-cause healthcare costs associated with VTE or bleeding were calculated as the difference between cohorts of patients without VTE or bleeding matched 1:1 to patients with VTE or bleeding.
Results:
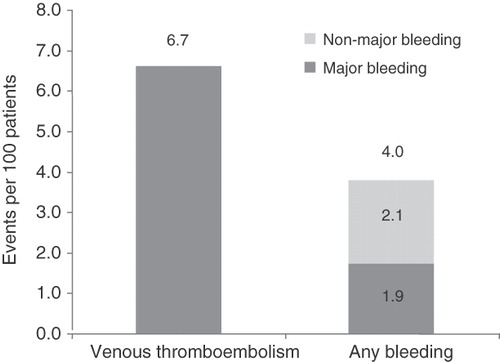
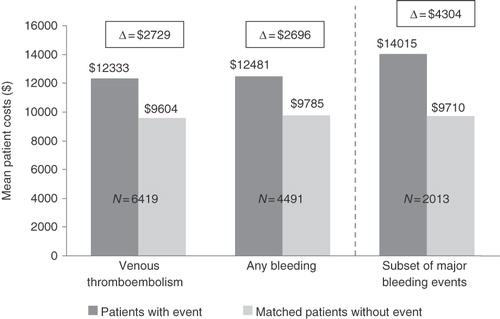
Of 119,729 patients (43,670 THR and 76,059 TKR), 7974 had a VTE event and 4849 had a bleeding event (2216 major bleeding [a subset of ‘any bleeding’]). The risks of VTE, any bleeding, and major bleeding were 6.7, 4.0, and 1.9 events, respectively, per 100 patients. Up to 3 months after THR/TKR, mean incremental all-cause healthcare costs per patient per month associated with VTE, bleeding, and major bleeding were $2729, $2696, and $4304, respectively. Total monthly costs versus matched controls over 3 months were: VTE: $12,333 vs. $9604; any bleeding: $12,481 vs. $9785; major bleeding: $14,015 vs. $9710; p < 0.001 for all.
Limitations:
Key limitations included potential inaccuracies or omissions in procedures, diagnoses, or costs of claims data; lack of information on the amount of blood transfused or decreases in the hemoglobin level to evaluate the severity of a bleeding event; and potential biases due to the observational design of the study.
Conclusion:
From the managed-care population perspective, in THR/TKR patients the greater incidence of VTE compared to any bleeding and major bleeding translated into a higher cumulative cost burden.
Introduction
Deep vein thrombosis (DVT) and pulmonary embolism (PE) comprise venous thromboembolism (VTE) and together are responsible for approximately 600,000 hospitalizations each yearCitation1. Moreover, approximately 300,000 deaths each year – 10% of all hospital deathsCitation2 – are attributed to PECitation1–3, and 25% of all PE cases result in sudden deathCitation4–6.
Among major surgeries, total hip and total knee replacement (TKR/THR) are associated with the highest risk of VTE eventsCitation7,Citation8. Approximately 50% of all patients who undergo THR or TKR surgery without prophylaxis develop DVT, and 1–2% develop PECitation6. Even in patients receiving standard thromboprophylaxis, between 1.4 and 2.8% develop DVT and 0.4–1.2% will experience PECitation9.
Few data exist on the economic burden associated with VTE following THR/TKR. One study by Ollendorf and colleagues found that among patients who underwent orthopedic surgery, the estimated average inpatient cost was $9345 for patients with no post-operative VTE complications, compared with $17,114 and $18,521 for those experiencing DVT and PE, respectivelyCitation6. In 2007, costs for the more than 1 million hospital discharges with a primary diagnosis of knee or hip replacement totaled over $15.6 billionCitation10.
Moreover, VTE has a significantly high rate of recurrence: patients who develop DVT or PE have a 7–14% chance of experiencing a second DVT or PECitation3. The majority of these recurrent thromboembolic events occur within the first 3 months following the initial eventCitation7,Citation8. The true financial burden of VTE complications therefore goes beyond the cost of treating the incident event. It has been estimated that the per-event cost after THR is $15,000Citation11, while the average discounted lifetime cost of DVT complications after THR, including long-term sequelae of DVT, such as post-thrombotic syndrome, or of PE, such as chronic pulmonary arterial hypertension, is $3069Citation4.
Assessment of the risk and cost of VTE and its prevention must include bleeding risks and associated costs. Depending on the anticoagulant used, management of bleeding events has been estimated to represent up to 57% of the average per-patient cost of thromboprophylaxis in patients undergoing hip replacementCitation12. The goal of this study was to assess the all-cause risk of developing VTE and/or bleeding in managed-care patients up to 3 months after THR or TKR surgery, and to quantify the all-cause healthcare costs of patients who underwent THR or TKR surgery, as well as the incremental cost associated with VTE, any bleeding, and major bleeding events.
Patients and methods
Data sources
Health insurance claims data from the Ingenix (IMPACT) National Managed Care DatabaseTM between January 2004 and December 2008 were used to conduct the analysis. This large national database was designed to support benchmarking projects, healthcare outcomes research, and other research initiatives. The IMPACT database includes complete medical and pharmacy claims for more than 80 million managed-care lives from more than 45 health plans, covering all census regions of the United States. Data elements used in the present analysis included health plan enrollment records, patient demographics, inpatient and outpatient medical services, and outpatient prescription drug dispensing records. In order to account for differences in provider contracting and other pricing variations across health plans, costs were standardized to reflect allowed payments among all provider services. Finally, any data in the IMPACT database that might allow individual identification were removed in order to preserve patient anonymity and confidentiality and comply with the Health Insurance Portability and Accountability Act of 1996 (HIPAA).
Study design
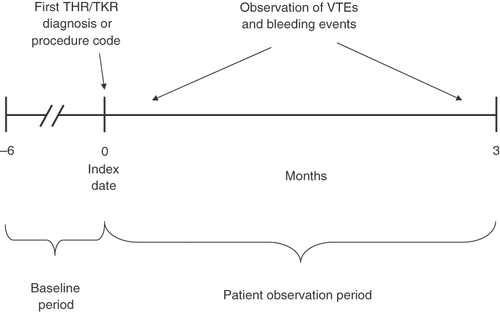
A retrospective cohort design was used to evaluate the risk of developing VTE or bleeding, including a subset of major bleeding events, in patients undergoing THR or TKR, while a matched-cohort design was used to quantify increased costs associated with VTE or bleeding. To be included in the study sample, patients were required to (1) have at least one claim with a procedure code for THR or TKR (International Classification of Disease, Ninth Revision, Clinical Modification [ICD-9-CM] procedure codes: 00.70, 81.51, 00.80, 00.84, 81.54, 81.55; Current Procedural Terminology, Fourth Edition [CPT-4] codes: 01214, 01215, 27130, 27132, 27134, 01402, 27447, 27486, 27487); (2) be ≥18 years of age as of the date of the THR/TKR procedure; and (3) have continuous enrollment with the same health plan for ≥6 months prior to THR/TKR (baseline period). Patient observation period spanned from the date of the first THR/TKR (index date), up to 3 months after the index date, disenrollment, or end of data availability (December 2008), whichever occurred earlier (). A 3-month cutoff was chosen because it has been reported that 49–81% of all cases of VTE occur during the first 3 months following hip or knee replacementCitation8,Citation13–15.
Outcome measures
The main outcomes of the study included the risk of and total and incremental healthcare costs associated with VTE and bleeding regardless of anticoagulant therapy. VTE events were defined as one or more diagnosis code(s) for DVT (DVT – ICD-9-CM codes: 451.1x, 451.2, 453.4x, 453.8, 453.9) or PE (PE – ICD-9-CM code: 415.1x).
Bleeding was defined as one or more diagnosis code(s) for bleeding (see Appendix 1 for complete list of ICD-9-CM codes for bleeding). Because diagnosis codes for bleeding are less specific than those for VTE, bleeding events could vary widely depending on the definition applied. In order to control for this lack of specificity, outcomes were also studied using a more stringent definition for bleeding events limited to major bleeding, which included intracerebral hemorrhage (Appendix 1).
Statistical analyses
The all-cause risk of VTE and bleeding complications among patients undergoing THR and TKR was evaluated using the absolute risk of VTE, any bleeding (major and non-major), and the subset of major bleeding events. Absolute risk was calculated as the number of patients with an event during the study period divided by the total number of patients undergoing THR or TKR. Binomial distribution was used to construct 95% confidence intervals for the absolute risk of events over the 3 months following THR/TKR surgery.
To quantify the incremental costs associated with VTE or bleeding, patients with VTE or bleeding events were matched 1:1 with control THR or TKR patients who did not have a diagnosis of VTE or bleeding events. Cases and controls were matched based on both (1) exact matching factors and (2) propensity scores using a caliper of 5%. The exact matching factors included age (5-year intervals), gender, degree of illness severity (Deyo–Charlson comorbidity index)Citation16, risk factors for VTE (trauma, major surgery, and history of VTE – for the VTE group), risk factors for bleeding (anemia, major surgery, and prior bleeding events – for the bleeding group), and use of anticoagulants during the baseline period. The propensity score was generated using probability estimates from a logistic regression model in which VTE or bleeding assignment was the binary dependent variable and baseline covariates were used as predictors of VTE and bleeding.
Descriptive univariate analyses were conducted to determine the incremental all-cause healthcare costs associated with VTE relative to no VTE, bleeding relative to no bleeding, and major bleeding relative to no bleeding. Incremental cost was defined as the weighted average monthly cost per patient of the VTE, bleeding, and major bleeding groups, minus the weighted average monthly cost per patient of the no-VTE, no-bleeding, and non-major bleeding groups, respectively. We used the observation period of each patient as the weight; therefore, the normalized per-patient cost represents the mathematical equivalent of a standard per-patient-per-month value (i.e., aggregated costs divided by aggregated months, with both values summed across all patients). This approach produced a value for each patient, allowing for statistical testing.
Results
A total of 119,729 patients who underwent THR/TKR (43,670 THR and 76,059 TKR) met the entry criteria and formed the study population. describes the baseline characteristics of the study population, stratified by type of event developed. Among all patients with THR/TKR, 7974 (6.66%) experienced VTE and 4849 (4.05%) had a bleeding event, including 2216 (1.85%) patients with a major bleeding event. Some patients had both VTE and bleeding events. The bleeding group was older (62.8 vs. 61.8 years, p < 0.001) and included a lower proportion of women (48.9 vs. 56.1%, p < 0.001), compared with the VTE group.
Table 1. Characteristics of patients with THR/TKR stratified by type of event developed.
Mean length of stay for the index THR/TKR hospitalization for patients with VTE was 4.6 days, and for those with a bleeding event, it was 4.5 days (). While outpatient service costs at baseline were similar between the two groups (VTE: $4919; bleeding: $5120; p = 0.3699), inpatient services and pharmacy costs were both more expensive in the bleeding group compared with the VTE group (inpatient services: $2471 vs. $2057, p = 0.0194; pharmacy: $1541 vs. $1432, p = 0.0059) ().
VTE and bleeding events
A total of 7054 DVT and 1599 PE events were identified (). Gastrointestinal bleeding was the most frequent major bleeding event (1268 events identified) and hematuria the most frequent non-major bleeding event (1829 events identified; ).
Table 2. Distribution of VTE and bleeding events.
All-cause risk of VTE and bleeding complications and associated total all-cause healthcare costs
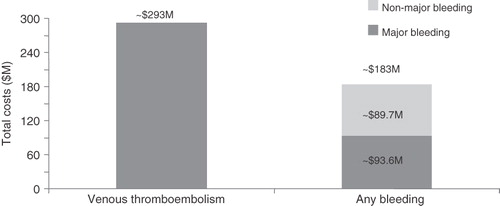
For the overall THR/TKR population, the absolute risk (95% confidence interval) of VTE, any bleeding, and the subset of major bleeding was 6.7 (6.5–6.8), 4.0 (3.9–4.2), and 1.9 (1.8–1.9) events per 100 patients, respectively (). The corresponding cumulative all-cause healthcare costs were $292,690,000 for the 7974 patients who developed a VTE event, and $183,254,000 for the 4849 patients who had a bleeding event (). The corresponding cumulative healthcare cost for the subset of 2216 patients with major bleeding events (i.e., out of 4849 any bleeding events) was $93,570,000.
Incremental cost associated with VTE, bleeding, and major bleeding
From the initial populations of 7974 and 4849 patients who developed VTE and any bleeding events, respectively, 6419 (80.5%) and 4491 (92.6%) were matched with a THR/TKR control patient without VTE or bleeding events. Characteristics of the case-control populations for each type of event are presented in and .
Table 3. (a) Characteristics of matched VTE and control (no VTE) cohorts; (b) Characteristics of matched bleeding and control (no bleeding) cohorts.
After matching the VTE and bleeding cohorts with comparable control patients, both the VTE and bleeding groups had increased healthcare costs compared with the no-VTE and no-bleeding control groups (). The mean incremental all-cause healthcare costs per patient per month associated with VTE, any bleeding, and the subset of major bleeding events were $2729, $2696, and $4304, respectively (VTE: $12,333 vs. $9604 for no-VTE controls; bleeding: $12,481 vs. $9785 for no-bleeding controls: major bleeding: $14,015 vs. $9710 for no-major bleeding controls; p < 0.001 for all).
Discussion
This large retrospective study based on health insurance claims from the IMPACT National Managed Care DatabaseTM was conducted to assess the all-cause risks and cost burden of VTE and bleeding in patients after THR/TKR. A total of 119,729 THR/TKR patients were analyzed. Both the real-world risk and corresponding cumulative all-cause healthcare costs were greater for VTE than for any bleeding. Although specific cost data cannot be compared directly between the VTE and bleeding cohorts (i.e., different populations with different risk factors), the overall real-world healthcare costs for VTE did represent a larger cost burden to managed-care organizations than the cost burden associated with bleeding.
The objective of the current study was to evaluate the risk and costs of VTE and bleeding from a managed-care perspective. While anticoagulation therapy is highly effective for VTE prophylaxis following THR/TKR, its benefits are counterbalanced by an increased risk of bleeding, both at the surgical site and elsewhere. It was beyond the scope of this study to assess whether thromboprophylaxis is beneficial and cost saving or whether it leads to increased costs due to bleeding, or to evaluate the impact of dosage and duration of thromboprophylaxis on these outcomes. Therefore, our study was conducted on all patients who underwent orthopedic surgery regardless of thromboprophylaxis and was not specifically focused on a comparison of orthopedic patients treated with thromboprophylaxis versus no thromboprophylaxis. Further research is warranted to evaluate the potential cost benefit or cost saving associated with VTE prophylaxis in orthopedic surgery patients.
After matching the VTE and any bleeding cohorts with comparable control patients, the increased healthcare cost associated with a VTE event was comparable to that of a bleeding event ($2729 vs. $2696), but substantially lower than a major bleeding event ($2729 vs. $4304). However, the overall short-term (3-month) risk of any bleeding was lower (4.0%) compared to the risk of VTE (6.7%), and even if major bleeding events were more costly, the risk of major bleeding (1.9%) was less than one-third the risk of VTE events following THR or TKR. Consequently, from a managed-care population perspective, the greater incremental cost associated with major bleeding compared to VTE was offset by its lower incidence. This resulted in higher total costs associated with VTE compared to bleeding.
In patients undergoing THR, Hitos et al. evaluated the incidence of VTE at 3.8% up to 3 months following hospital dischargeCitation17. Samama and colleagues found the risk of VTE in patients hospitalized for THR and TKR to be 1.8% at 3 monthsCitation18. In contrast, in our study the observed risk of VTE up to 3 months following the initial THR/TKR hospitalization was 6.7%. Various factors can explain the higher proportion of patients developing VTE events observed in the current study, including patients’ risk-factor profile and the extent of thromboprophylaxis.
The risk of bleeding events has been less extensively studied than that of VTE. Hitos et al.Citation17 found that the risk of major bleeding up to 3 months following THR was 1.6%, which is comparable to the risk of major bleeding of 1.9% found in this study. On the other hand, Samama et al. found that the risk of major bleeding 3 months after lower limb joint replacement was 1%Citation18. This estimate is four times lower than the risk of any bleeding and approximately half the risk of major bleeding observed in the current study for the same follow-up period. Unlike VTE, however, comparisons of the risks of bleeding between studies must be considered cautiously, given the more subjective assessment of bleeding events and especially of a major or significant bleeding event. Thus, the higher risk estimate observed in the current study is likely due to differences in the identification of bleeding events. In our study, bleeding events were identified solely based on ICD-9-CM codes, whereas in the study by Samama, the definition was based on detailed clinical information on the site of bleeding and requirements for blood transfusionCitation18. Lefebvre and colleagues, using a definition of bleeding events based on ICD-9-CM codes, estimated the inpatient risk of any bleeding and major bleeding at 0.90 and 0.33 events per 100 inpatient stays, respectivelyCitation19,Citation20.
MacDougall et al. estimated that the annual total all-cause healthcare costs for any patients with PE and DVT were, $47,200 and $53,100, respectively, compared with $3400 for controls selected from the general population without PE or DVTCitation3. Although not directly comparable, considering the different observation periods and the fact that VTE patients in the current study all had THR/TKR, these estimates are in line with our finding that VTE patients incurred healthcare costs of $36,999 on average for the period up to 3 months following THR/TKR. Of note, one would expect the period immediately following THR or TKR to be the most cost-intensive, given the increased risk of VTECitation8. In fact, Lefebvre et al. reported an average cost per inpatient stay of $21,646 for patients developing VTE during THR or TKR hospitalizationCitation19.
This study is subject to several limitations. First, claims databases may contain inaccuracies or omissions in procedures, diagnoses, or costs. Second, the study evaluated only direct medical costs. Information to determine the indirect costs of VTE and bleeding, such as work productivity loss and reduced quality of life, was not available. Third, the observational design was susceptible to various biases, such as information or classification bias (e.g., false-positive identification of VTE or bleeding events). It is also possible that VTE and bleeding events were undercoded (i.e., false negative). However, well-designed observational studies with appropriate statistical and matching techniques adjusting for potential confounding factors provide valuable information, with real-life scenarios and high generalizability. Fourth, in addition to the site of bleeding, the severity of a bleeding event is often determined by the amount of blood that needs to be transfused or the decrease in the hemoglobin level. However, the databases used in the present study did not specify the amount of blood transfused and did not include laboratory data. Therefore, the identification of major bleeding events relied solely on the site of bleeding as specified by diagnosis codes, which excluded the surgical site. Finally, given current use of thromboprophylaxis, the risk and costs of VTE and bleeding were evaluated from a managed-care perspective. It was beyond the scope of this study to assess whether thromboprophylaxis is beneficial or cost saving or whether it leads to increased costs due to bleeding.
Conclusion
Results of this systematic assessment of the risk and healthcare costs associated with VTE and bleeding in THR/TKR patients suggest that while the incremental healthcare costs associated with VTE were similar to those associated with bleeding (but lower compared with major bleeding), the risk of VTE was greater than the risk of bleeding. From a population perspective, the greater incidence of VTE translates into higher costs compared to costs due to any bleeding or major bleeding events.
Transparency
Declaration of funding
This research was funded by Ortho-McNeil Janssen Scientific Affairs, LLC, Raritan, NJ, USA.
Declaration of financial relationships
Five of the authors (F.V., F.L., M.S.D., K.D., and P.L.) are employees of Analysis Group, Inc., a consulting company that has received research grants from Ortho-McNeil Janssen, and four of the authors (J.C.L., B.K.B., J.S., and W.H.O.) were employees of Ortho-McNeil Janssen at the time that the study was conducted. Four of the authors own stock in Johnson & Johnson (J.S., B.K.B., J.C.L., W.H.O.). E.N. received research grants from Ortho-McNeil Janssen Scientific Affairs, LLC, Raritan, NJ, USA.
This contribution represents original work, has not been previously published or simultaneously submitted for publication elsewhere. This manuscript has been read and approved by all the authors, and all conditions stated by the International Committee of Medical Journal Editors have been met.
Acknowledgments
The authors would like to acknowledge Ruth Sussman, PhD, for editorial assistance in the preparation of this manuscript with funding from Ortho-McNeil Janssen Scientific Affairs, LLC.
Parts of this work were presented as posters at the Academy of Managed Care Pharmacy (AMCP) 22nd Annual Meeting and Showcase, April 7–10, 2010, San Diego, California and the 2010 Summer Meeting of the American Society of Health-System Pharmacists, June 6–9, 2010 Tampa, Florida, USA.
References
- Heit JA, Cohen AT, Anderson FA Jr, et al. Estimated annual number of incident and recurrent, non-fatal and fatal venous thromboembolism (VTE) events in the US. Blood 2005;106:267a. Abstract 910
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126:338-400S
- MacDougall DA, Feliu AL, Boccuzzi SJ, et al. Economic burden of deep-vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm 2006;63:S5-15
- Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6:59-74
- Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002;162:1245-8
- Ollendorf DA, Vera-Llonch M, Oster G. Cost of venous thromboembolism following major orthopedic surgery in hospitalized patients. Am J Health Syst Pharm 2002;59:1750-4
- White RH, Gettner S, Newman JM, et al. Predictors of rehospitalization for symptomatic venous thromboembolism after total hip arthroplasty. N Engl J Med 2000;343:1758-64
- White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998;158:1525-31
- Edelsberg J, Ollendorf D, Oster G. Venous thromboembolism following major orthopedic surgery: review of epidemiology and economics. Am J Health Syst Pharm 2001;58(Suppl 2):S413
- Costs for hospital discharges with a primary diagnosis of knee or hip replacement. Rockville, MD: U.S. Department of Health & Human Services, 2010. Available at: http://www.hcupnet.ahrq.gov. [Last accessed 29 October 2010]
- Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism event during hospitalization. J Manag Care Pharm 2005;11:663-73
- Saunders ME, Grant RE. Cost effectiveness of low-molecular weight heparin versus warfarin following hip replacement surgery. J Natl Med Assoc 1998;90:677-80
- Colwell CW Jr, Collis DK, Paulson R, et al. Comparison of enoxaparin and warfarin for the prevention of venous thromboembolic disease after total hip arthroplasty. Evaluation during hospitalization and three months after discharge. J Bone Joint Surg Am 1999;81:932-40
- Leclerc JR, Gent M, Hirsh J, et al. The incidence of symptomatic venous thromboembolism during and after prophylaxis with enoxaparin: a multi-institutional cohort study of patients who underwent hip or knee arthroplasty. Canadian Collaborative Group. Arch Intern Med 1998;158:873-8
- Oster G, Ollendorf DA, Vera-Llonch M, et al. Economic consequences of venous thromboembolism following major orthopedic surgery. Ann Pharmacother 2004;38:377-82
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613-19
- Hitos K, Fletcher JP. Venous thromboembolism following primary total hip arthroplasty. Int Angiol 2009;28:215-21
- Samama CM, Ravaud P, Parent F, et al. Epidemiology of venous thromboembolism after lower limb arthroplasty: the FOTO study. J Thromb Haemost 2007;5:2360-7
- Lefebvre P, Vekeman F, Nutescu EA, et al. Risk and costs of bleeding events versus risk and costs of VTE for patients undergoing total hip or total knee arthroplasty. Presented at the 30th Annual Congress of the National Association of Orthopaedic Nurses; May 15–19, 2010; Seattle, WA. Abstract 464
- Vekeman F, Bookhart BK, Lefebvre P, et al. Incidences and associated costs for venous thromboembolism and bleeding events in patients undergoing total hip or total knee arthroplasty. Presented at the Summer Meeting of the American Society of Health System Pharmacists; June 6–9, 2010; Tampa, FL. Abstract 15-T
Appendix
Appendix 1. Diagnosis codes for bleed events.