Abstract
Objective:
A Markov model was used to assess the impact of RV5, a pentavalent (G1, G2, G3, G4, P1A[8]) human bovine (WC3 strain) reassortant rotavirus vaccine, on reducing the healthcare burden and cost associated with rotavirus gastroenteritis (RGE) in Taiwan. Other cost-effectiveness analyses for rotavirus vaccination in industrialized countries have produced varying results depending on the input parameters assumed.
Methods:
Vaccination with RV5 is compared to no vaccination in a hypothetical cohort of Taiwanese children during their first 5 years of life to determine the per dose prices at which vaccination would be cost neutral or provide good value based on established standards from the healthcare (direct medical care costs only) and societal (all RGE-related costs) perspectives. The effects of vaccination on RGE healthcare utilization and days of parental work loss missed are based on results from the Rotavirus Efficacy and Safety Trial.
Results:
Without vaccination there would be 122,526 symptomatic episodes of RGE. Universal vaccination would reduce RGE-related deaths, hospitalizations, emergency department, and outpatient visits by 91.7%, 92.1%, 83.7%, and 73.4%, respectively. The price per dose at which vaccination would be cost-neutral is US$ 21.80 (688 NTD) and US$ 26.20 (827 NTD) from the healthcare and societal perspectives, respectively. At $25 per dose, the cost per QALY gained is US$ 2261 (71,335 NTD) from the healthcare perspective and cost saving from the societal perspective.
Key limitations:
The model only assesses the effect of RV5 on vaccinated children and does not account for herd immunity. However, given that high levels of coverage are anticipated in Taiwan, the effects of herd immunity are likely to be short-term.
Conclusion:
A pentavalent rotavirus vaccination program is likely to substantially reduce the healthcare burden associated with rotavirus gastroenteritis at a cost per QALY ratio within the range defined as cost-effective.
Introduction
Rotavirus gastroenteritis (RGE) is the leading cause of severe diarrheal disease worldwide in children under 5 years of ageCitation1. Children with RGE usually experience a combination of diarrhea, vomiting, and/or fever, often leading to dehydration in severe casesCitation2,Citation3. In industrialized countries deaths due to RGE are relatively rare but healthcare costs are substantial. In Taiwan, estimates of RGE-related annual healthcare costs range from US$10–18.5 millionCitation4,Citation5.
Currently there are two rotavirus vaccines licensed in Taiwan. One is a pentavalent (G1, G2, G3, G4, P1A[8]) human bovine (WC3 strain) reassortant rotavirus vaccine (RV5; RotaTeq®, Merck & Co., Inc., Whitehouse Station, NJ, USA). The other is a monovalent G1P1A[8] human rotavirus vaccine (RV1; Rotarix®, GlaxoSmithKline Biologicals, Rixensart, Belgium)Citation6. Until now, vaccine use has been limited to the private sector and studies are currently being conducted in preparation for public sector reimbursementCitation7,Citation8. Government reimbursement would significantly help to increase the coverage rates in Taiwan because nearly all children are covered by the Taiwanese government’s national health insurance programCitation4,Citation9,Citation10.
The purpose of this study is to assess the potential cost-effectiveness of universal vaccination with RV5 in Taiwan from the healthcare and societal perspectives. The healthcare perspective is limited to direct medical care costs and the cost of the immunization program. The societal perspective encompasses all RGE-related costs including travel to healthcare facilities, oral rehydration therapies given at home, extra diapers, and parental work days missed to care for sick children.
Other cost-effectiveness analyses have been published for high and middle-income countries throughout North America, South America, Europe, Australia, New Zealand, and AsiaCitation11–20. Many of these cost-effectiveness analyses have demonstrated that rotavirus vaccination is cost-effective and should be added to the immunization scheduleCitation12,Citation13,Citation15,Citation16,Citation18,Citation19. These results are supported by post-marketing surveillance studies that have reported dramatic reductions in RGE-related healthcare utilization since rotavirus vaccination was added to the immunization scheduleCitation21–25. However, there are studies that have concluded rotavirus vaccination is not cost-effectiveCitation17,Citation20. The differences in study results, often within the same country, are largely due to differences in the input parameters assumedCitation26. Although another cost-effectiveness analysis for rotavirus vaccination was published in Taiwan, the estimates of hospitalizations and ambulatory visits included in the base case in that study were lower than other published studies in TaiwanCitation3–5,Citation10. In addition, the study did not include the cost per quality adjusted life year (QALY) as an outcome measure.
The healthcare utilization and cost estimates for the base case scenario in this study are derived from a recently published prospective, cross-sectional surveillance study for rotavirus gastroenteritis conducted in hospitals and outpatient pediatric clinics in TaiwanCitation8. These estimates are then varied based on other published studies from TaiwanCitation3–5,Citation10. The results are presented for the prices at which universal vaccination would be cost-neutral as well as the cost per quality adjusted life years gained for prices ranging from US$20–30 per dose.
Methods
Model design
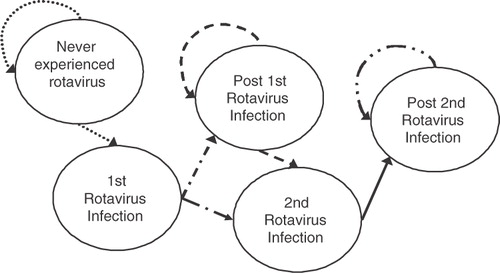
The cost-effectiveness analysis is based on a Markov model using EXCEL software to assess the cost-effectiveness of universal vaccination with RV5 against RGE for a hypothetical birth cohort of 220,260 Taiwanese children during their first 5 years of life (). Epidemiology studies have shown that although children may develop multiple episodes of RGE, the first two infections are generally the most severe, and subsequent infections are generally very mild or asymptomatic because heterotypic protection develops with successive episodes of RGECitation27. This is why we allow children to contract up to two symptomatic episodes in the model.
Children may move between different health states over a discrete time period known as a Markov cycleCitation28. The different health states reflect whether the child has ever experienced a rotavirus infection, the number of prior rotavirus infections, the serotypes causing the episodes, and whether the child survived or died of the illness. Although deaths due to rotavirus gastroenteritis are relatively rare in developed countries, cost-effectiveness analyses should capture all possible outcomes. The number of deaths assumed should reflect that rotavirus deaths are uncommon. During the first 6 months of life, monthly cycles are used to allow flexibility in the vaccination schedule. Quarterly cycles are employed after 6 months to track a child’s health state. The transition probabilities, or the likelihood that the child will move from one health state to another, reflect the age-specific probability of developing first and second episodes of rotavirus and the percentage of the episodes that are symptomatic. These data are based on a prospective, population-based study in Mexico that closely monitored children for the development of symptomatic and asymptomatic episodesCitation27,Citation29. This study was unique because study personnel collected stool specimens from all children born in the catchment area during the study period regardless of symptoms every 2 weeks for the first 2 years of life. Almost all other rotavirus studies were conducted in specific settings and usually once the children became symptomatic. The age distribution of episodes involving deaths and healthcare encounters by type are based on data from Taiwan in which the cumulative frequency of rotavirus is 78% and 73% for pediatric and hospital settings, respectively, by 36 months of ageCitation8.
Analytic perspectives and outcome measures
This cost-effectiveness analysis compares infants receiving RV5 as a 3-dose series to no vaccination in Taiwan. All of the scenarios are evaluated with per dose prices ranging from US$20–30. The QALY data is based on a Canadian study that includes the impact on the child as well as the caregiver where the utility weights for children with rotavirus gastroenteritis are based on the HUI2 and the Visual Analog Scale. The utility weights for the parents are based on the EQ-5D and the Visual Analog ScaleCitation30. Given that there are no established benchmarks in Taiwan on which to evaluate the cost-effectiveness of an intervention, we use the World Health Organization’s benchmark based on recommendations from the WHO Commission on Macroeconomics and HealthCitation31,Citation32. This is consistent with the criteria used in other cost-effectiveness analyses conducted in TaiwanCitation5,Citation33. Interventions are classified as highly cost-effective if the cost per QALY gained is lower than the per capita GDP, may be cost-effective if the cost is less than 2–3-times the per capita GDP, and are not cost-effective if the cost is higher than 3-times the per capita GDP. In Taiwan, the per capita GDP was US$18,303 for 2010Citation3Citation4.
Model inputs
General overview
lists the key model inputs along with the values for the base case scenario. All medical and non-medical care costs are presented in US dollars (US$) and adjusted for inflation to 2010 dollars using the Consumer Price Index for TaiwanCitation35. Costs are given in US dollars as well as new Taiwanese dollars (NTD) shown in parentheses based on an exchange rate of US$1 = NTD 31.55 in 2010Citation3Citation6. RV5 is administered as a 3-dose vaccine at 2, 4, and 6 months of age, which is consistent with the schedule for DTwP in TaiwanCitation37. The base case scenario assumed that 96% of children would receive three doses of RV5, 2% would receive two doses, and 2% would receive one dose based on the immunization coverage level for DTP3 reported in the National Immunization Information System in Taiwan, and this assumption is varied in sensitivity analysesCitation37.
Table 1. Summary of key model inputs for base case scenario.
The effect of vaccination on reducing the rate of hospitalizations, emergency department (ED) visits, office visits, and days of parental work loss missed due to RGE for those receiving all three doses are based on results from the Rotavirus Efficacy and Safety Trial (REST), a large-scale, placebo-controlled, Phase III trial that enrolled nearly 70,000 childrenCitation38. The REST trial provided efficacy data for the first 2 years of life and different assumptions regarding efficacy were extended for the 3rd–5th years of life for different scenarios. Since there are no efficacy data against death, the effect of vaccination on hospitalizations is also applied to RGE deaths. The efficacy prior to completion of the 3rd dose shown in is consistent with post-hoc analyses of the clinical trial data from RESTCitation39. Given that there is limited data on the efficacy of RotaTeq® for children not completing the three dose regimen, we assume the efficacy is 25% and 50% of a 3-dose regimen for one and two doses, respectively, to be conservative. These assumptions are then varied in sensitivity analyses based on available effectiveness data of the vaccine after administration of only one and only two dosesCitation40,Citation41. The efficacy estimates are applied to all episodes of RGE because efficacy has been established against all of the major serotypes circulating in TaiwanCitation8.
The cost of vaccination is based on the price per dose of RV5 with no additional administration fees charged by physicians in the public sector. Although there is a required registration fee that parents must pay for a physical examination prior to the administration of vaccines, RV5 is given on the same schedule as DTwP and the registration fee is already being paid for that vaccine. Therefore, it is not considered a marginal cost associated with RV5. There are also no costs associated with the adverse effects of vaccination since the clinical trial results found RV5 to be generally safe and well toleratedCitation38,Citation42.
Base case scenario
For the base case scenario, we rely on the surveillance study conducted by Mast et al.Citation8 for the average hospital length of stay, the average number of days missed from work and the percentage of parents missing time from work to care for sick children as well as the average medical costs for hospitalizations and outpatient visits (). We assume that, among children with RGE involving hospitalization or death, 56% of working parents miss an average of 4 days of work. For episodes involving ED visits and office visits, 21% of working parents miss an average of 1.3 days of workCitation8. The cost estimate for an ED visit is based on the estimate used in the Wu et al.Citation5 cost-effectiveness analysis.
The number of ambulatory visits is based on an estimate provided in the Mast et al.Citation8 study. The number of hospitalizations is based on the results from the Asian Rotavirus Surveillance NetworkCitation10. The number of deaths is consistent with Wu et al.Citation5 In the Wu et al. cost-effectiveness analysis, the number of emergency department visits is 8.6% of all ambulatory care visits. This percentage is applied to the total number of ambulatory visits estimated in the Mast et al. study to distinguish between outpatient visits and emergency department visits for RGE. We also apply the Basic Wage Rate in Taiwan for 2007 to be consistent with the Wu et al. cost-effectiveness analysis and the Basic Wage rate has not been adjusted for inflation since 2007Citation43.
Finally, we use the utility weight based on the Visual Analog Scale to estimate the QALY loss for the child and the utility weight based on the EQ-5D for the parents in the base case scenario and then vary these values in the sensitivity analysis.
Sensitivity analyses
These sensitivity analyses are designed to evaluate the impact of using different assumptions for healthcare utilization, vaccine coverage and efficacy as well as the QALY loss associated with episodes of rotavirus gastroenteritis. The estimates for the number of deaths, hospitalizations, ambulatory care visits, and work days missed are based on various published RGE surveillance studies in Taiwan. The number of annual deaths due to rotavirus varies from 1–22Citation11. For inpatient care two different types of sensitivity analyses are performed. In one set of scenarios, all of the assumptions in the base case scenario remain the same except the number of hospitalizations. In another set of scenarios a three-way sensitivity analysis varies the number of hospitalizations, the average hospital length of stay, and the cost per day in the hospital to reflect the assumptions in two published studies, with the lowest and highest number of hospitalizations for RGE reported in TaiwanCitation4,Citation5.
Similarly, in the sensitivity analyses for outpatient care all of the assumptions in the base case scenario remain the same except the number of ambulatory care visits including emergency department (ED) and outpatient visits. In another set of scenarios the sensitivity analysis varies the number of ambulatory care visits and the cost of an outpatient visit based on the two published studies with the lowest and highest number of outpatient visits. The cost estimate for the ED visit does not change because only Wu et al.Citation5 provided a cost estimate for ED visits. Lu et al.Citation4 estimated that the number of RGE hospitalizations varies from 21,800–28,500, and the number of ambulatory care visits ranges from 255,600–596,400. The 25,150 hospitalizations and the 426,000 ambulatory visits (36,636 ED visits and 389,364 outpatient visits) in the sensitivity analysis reflect the average of these ranges. We again assume that 8.6% of the ambulatory visits are ED visits and 91.4% are physician outpatient visitsCitation5.
Another set of sensitivity analyses is designed to evaluate the effect of the number of doses administered, the efficacy of receiving only one or two doses, and the duration of protection. In one set of sensitivity analyses the coverage is not changed but the efficacy of only one and only two dose regimens are 81% and 69%, respectively, for hospitalizations and emergency department visitsCitation40. In another scenario the coverage with three doses is reduced to 90%, with another 5% receiving two doses and 5% receiving one dose where the efficacy of an incomplete regimen is 0.
In the base case scenario we extend the efficacy of the vaccine in the 2nd year after vaccination up to 5 years of age. In the sensitivity analysis we assume a 10% drop in the relative rate reduction each year for healthcare encounters in the 3rd, 4th, and 5th years after vaccination.
We also vary the value of a day missed from work and the utility loss associated with episodes of RGE. In one scenario we increase the value of a day missed from work to US$58.16 (1897 NTD). This reflects the average monthly earnings and monthly working hours of industrial and service-sector employees as reported by the government of TaiwanCitation8.
Finally, we use the utility weight based on the HUI2 for the child with no QALY loss assumed for the parents as a conservative assumption and then use the VAS for two parents as well as the child as an upper bound.
Results
Base case scenario
The model predicts that in the absence of vaccination there would be 122,526 symptomatic episodes of RGE in a single birth cohort over 5 years with rotavirus related costs of US$20.5 million (646.4 million NTD) including US$16.6 million (525.5 million NTD) in direct medical care costs of which 42% are for hospitalizations (). When the price per dose is US$25 (789 NTD), the cost of vaccinating the birth cohort with a three dose regimen of RV5 would be US$16.2 million (510.7 million NTD).
Table 2. Effect of vaccination on the healthcare burden and medical care costs of RGE at a cost of US$25 per dose for the base case scenario.
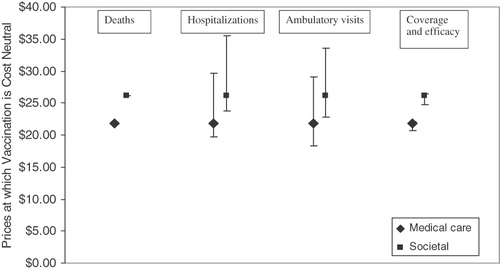
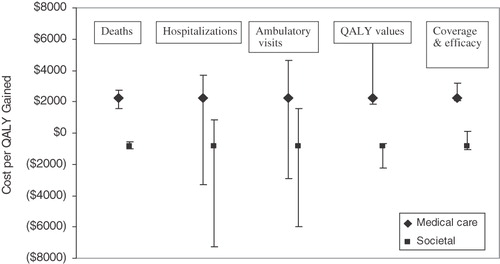
Universal vaccination would result in reductions of 91.7%, 92.1%, 83.7%, and 73.4% for deaths, hospitalizations, emergency department, and outpatient visits, respectively. The price per dose at which vaccination would be cost-neutral is US$21.80 (688 NTD) and US$26.20 (827 NTD) from the healthcare and societal perspectives, respectively. At $25 per dose, the cost per QALY gained would be US$2261 (71,335 NTD) from the healthcare perspective and cost saving from the societal perspective.
Sensitivity analyses
When the price per dose ranges from US$20–30, universal vaccination with RV5 would either be cost saving or highly cost-effective under all the scenarios examined (). From the healthcare perspective, the results are most sensitive to the number of hospitalizations and ambulatory visits ( and ). When 12,900 hospitalizations are assumed, universal vaccination is cost neutral at US$19.75 (623 NTD). When 25,150 hospitalizations are assumed, universal vaccination is cost neutral at US$29.70 (937 NTD.). Similarly, the prices at which universal vaccination would be cost neutral ranges from US$18.30 (577 NTD) when 148,325 ambulatory visits are assumed to US$29.15 (920 NTD) when 426,000 ambulatory visits are assumed.
Table 3. Sensitivity analyses for incremental cost-effectiveness ratios with costs of vaccination ranging from US$20–30.
The utility weights assumed do not affect the prices at which vaccination is cost neutral, but they do affect the cost/QALY gained. If we limit the quality-of-life impact of rotavirus gastroenteritis to the sick child without considering the impact on the parents and use the utility weight derived from the HUI2, vaccination is again cost saving at US$20 per dose and still highly cost-effective at US$30 per dose. The incremental cost-effectiveness ratios are US$15,702 (495,398 NTD) and US$7,279 (229,653 NTD) from the healthcare and societal perspectives, respectively. If we include the effect of rotavirus gastroenteritis on the sick child as well as the child’s parents and use the utility weights derived from the Visual Analog Scale the incremental cost-effectiveness ratios are US$4,748 (149,800 NTD) and US$2,201 (69,442 NTD) from the healthcare and societal perspectives. Nevertheless, vaccination is expected to be cost-effective or cost-saving regardless of the utility weights assumed at prices ranging from US$20–30.
It should also be noted that if high levels of coverage are expected in Taiwan, the efficacy associated with incomplete regimens has little effect on the results. In a worst case scenario, if 90% of the population receives three doses, with the remaining 10% receiving only one or two doses, and we assume no efficacy for partial vaccination, the price at which vaccination is cost neutral is US$20.65 (652 NTD) and US$24.80 (782 NTD) from the healthcare and societal perspectives, respectively.
Finally, we also conducted a series of 2- and 3-way sensitivity analyses because the study that reported the lowest healthcare utilization also assumed the highest unit costs and the study with the lowest unit costs assumed the highest rates of healthcare utilizationCitation4,Citation5. We therefore wanted to compare the total burden for hospitalizations and ambulatory visits from each of these studies. When the number of hospitalizations, the average hospital length of stay, and the average cost per day in the hospital are changed at the same time to reflect the assumptions in the Wu et al. cost-effectiveness analysis, universal vaccination is cost neutral at US$22.70 (716 NTD) () and at US$25 per dose, the cost per QALY gained is US$1,577 (49,754 NTD) from the healthcare perspective ()Citation5. In comparison, if we use the average of the range given for the number of RGE hospitalizations found in the Lu et al.Citation4 study, universal vaccination is cost neutral at US$22.00 (694 NTD) and at US$25 per dose, the cost per QALY gained is US$2,109 (66,539 NTD) from the healthcare perspective. Both scenarios would be cost saving from the societal perspective. When the number of ambulatory visits and the cost of an outpatient visit are changed at the same time to reflect the assumptions in the Wu et al.Citation5 cost-effectiveness analysis, universal vaccination is cost neutral at US$17.30 (546 NTD) and at US$25 per dose, the cost per QALY gained is US$5,395 (170,212 NTD). If we use the average of the range given for the number of ambulatory visits found in the Lu et al.Citation4 study, universal vaccination is cost neutral at US$20.20 (637 NTD) and at US$25 per dose, the cost per QALY gained is US$3,331 (105,093 NTD).
Table 4. Sensitivity analyses for costs of vaccination at which universal vaccination is cost neutral.
Discussion
Like many industrialized countries, Taiwan needs to balance access to quality healthcare with cost. Nearly the entire population is covered by national health insurance and healthcare costs are rising rapidly. Many new products are being developed and the government needs to select which technologies will contribute most to the overall health of the population within budgetary constraints in order to contain healthcare costs.
This cost-effectiveness analysis demonstrates that universal vaccination with RV5 will most likely be cost saving or highly cost-effective at prices ranging from US$20–30 per dose in Taiwan. (From the healthcare perspective, a cost-saving strategy would lower the total healthcare expenditure by the national health insurance, as the decrease in costs associated with RGE would cover the cost of the vaccine program. Even if there is an increase in cost to the national health insurance program, vaccination might be cost-saving from the societal perspective when offsets in cost to the parents are also included.)
Although it is unlikely all children will receive three doses of RV5, very high levels of coverage are expected in Taiwan if the vaccine is publicly reimbursed based on the percentage of children receiving three doses of DTwP at 2, 4, and 6 months of ageCitation37. At high levels of coverage with ≥90% completing the three dose regimen, the assumptions regarding the efficacy of incomplete regimens has little effect on the results.
There are several published studies on the RGE burden in Taiwan, with varying estimates of the number of hospitalizations, ambulatory care visits, and medical care costs. The assumptions related to healthcare resource utilization in the base case scenario are conservative, but they are between the highest and lowest estimates reported in the literature. The base case scenario assumed that in the absence of vaccination there would be 122,526 symptomatic episodes of RGE in a single birth cohort followed for the first 5 years of life with 15,400 hospitalizations and 236,300 ambulatory visits. In comparison, Wu et al.Citation5 assumed 185,149 symptomatic episodes of RGE. Based on other published studies, the estimates of RGE hospitalizations ranged from 12,900–28,492Citation4,Citation5 and the estimates of RGE ambulatory visits ranged from 148,325–596,400Citation4,Citation5.
The results demonstrate that when the healthcare utilization and cost estimates from the published studies are applied together in multi-way sensitivity analyses, the higher number of hospitalizations and ambulatory visits in the Lu et al.Citation4 study are partially offset by much lower cost estimates for medical care. Conversely, the lower number of hospitalizations is offset by higher cost estimates in the Wu et al.Citation5 cost-effectiveness analysis. As a result, the incremental cost-effectiveness ratios and the cost neutral prices for the 2- and 3-way sensitivity analyses are closer to the results for the base case scenario than varying the number of hospitalizations and ambulatory care visits without changing the cost estimates.
This study also provides the cost per QALY gained in addition to the price at which vaccination is cost neutral because the latter measure does not allow us to evaluate the value of the product if the cost of vaccination exceeds the benefits. Most new technologies are cost additive, but they may represent good value for the money spent. The QALY measure assesses this and provides a common metric by which decision makers can compare different interventions. However, the derivation of utility weights for young children is particularly challenging, especially since they are often not able to answer questions for themselves and their cognitive abilities change over timeCitation44,Citation45. The issue of whether to include the impact of a condition on caregivers is also controversialCitation44. The results are sensitive to these choices and each of the methods to derive utility weights has different strengths and weaknesses. The HUI2 and the EQ-5D are choice-based valuation methods consistent with economic theory, but they are focused on functional limitations that may not apply to young children. Studies that ask parents to complete the HUI2 for their young children describe parental difficulties in interpreting the meaning of items given the child’s developmental ageCitation4,Citation5. In contrast, the VAS is not based on economic theory, but it is also not limited to specific functional limitations and parents have more freedom to determine how the manifestations of rotavirus gastroenteritis apply to their situation.
Although the results are not dramatically different from the results of the Wu et al.Citation5 cost-effectiveness analysis, the per dose prices evaluated in this study are lower than those evaluated in the earlier publication and it includes an extensive sensitivity analysis to account for the range of healthcare utilization estimates derived from published studies. Like the Wu et al.Citation5 cost effectiveness analysis, our study does not account for herd immunity, an indirect effect of vaccination whereby children are less susceptible to an infectious condition due to an overall decrease in the amount of circulating virus in the environment. However, given that high levels of coverage are anticipated in Taiwan, the effects of herd immunity are likely to be short-term because nearly all children will be protected through vaccination.
Conclusion
Universal vaccination would most likely result in substantial reductions in morbidity and healthcare costs in Taiwan. At a price of US$20 per dose, vaccination with RV5 is likely to be cost-saving from the healthcare and societal perspectives. Although vaccination may not be cost saving at prices ranging from US$25–30 per dose from the healthcare perspective, a pentavalent rotavirus vaccination program is likely to reduce the healthcare burden associated with rotavirus gastroenteritis at a cost per QALY ratio within the range defined as cost-effective.
Transparency
Declaration of funding
This study was funded by Merck & Co., Inc., North Wales, Pennsylvania, USA.
Declaration of financial/other relationships
R.F.I., A.C.E., and J.R.C. are employees of and own Merck & Co. stock. C.L. is an employee of MSD, Taipei, Taiwan. P.Y.C. was an investigator who enrolled subjects at the study sites and coordinated the clinic sites for the Mast et al. 2010 study. As an investigator, he was compensated by Merck & Co. for all activities related to the execution of the study.
Acknowledgments
The authors would like to thank Karen Collins of JK Associates, Inc., Conshohocken, PA, for assistance with manuscript preparation on behalf of Merck & Co., Inc.
References
- Parashar UD, Hummelman EG, Bresee JS. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003;9:565–72
- Staat MA, Azimi PH, Berke T, et al. Clinical presentations of rotavirus infection among hospitalized children. Pediatr Infect Dis J 2002;21:221–7
- Chen KT, Chen PY, Tang RB, et al. Sentinel hospital surveillance for rotavirus diarrhea in Taiwan, 2001–2003. J Infect Dis 2005;92(1 Suppl):S44–8
- Lu CY, Lauderdale TL, Fang YH, et al. Disease burden and related medical costs of rotavirus infections in Taiwan. BMC Infect Dis 2006;6:176
- Wu CL, Yang YC, Huang LM. Cost-effectiveness of childhood rotavirus vaccination in Taiwan. Vaccine 2009;27:1492–9
- Centers for Disease Control. Prevention of rotavirus gastroenteritis among infants and children: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2009;58:RR2
- Chang CC, Chang MH, Lin TY et al. Experience of pentavalent human-bovine reassortant rotavirus vaccine among healthy infants in Taiwan. J Formos Med Assoc 2009; 108:4; 280–5
- Mast TC, Chen PY, Lu KC, et al. Epidemiology and economic burden of rotavirus gastroenteritis in hospitals and pediatric clinics in Taiwan, 2005–2006. Vaccine 2010;28:3008–13
- National Health Insurance Annual Statistical Report: Taipei [in Chinese]. Bureau of National Health Insurance. Taiwan: Bureau of National Health Insurance, 2003
- Chen KT, Fan SF, Tang RB, et al. Hospital-based study of the economic burden associated with rotavirus diarrhea in Taiwan. Vaccine 2007;25:4266–72
- Podewils IJ, Antil L, Hummelman E, et al. Projected cost-effectiveness of rotavirus vaccination for children in Asia. J Infect Dis 2005;192(1 Suppl):S133–45
- Lorgelly PK, Joshi D, Iturriza Gomara M, et al. Exploring the cost-effectiveness of an immunization programme for rotavirus gastroenteritis in the UK. Epidemiol Infect 2007;136:1, 44–55
- Newall AT, Beutels P, MaCartney K, et al. The cost-effectiveness of rotavirus vaccination in Australia. Vaccine 2007;25:8851–60
- Rheingans RD, Constenla D, Antil L, et al. Potential cost-effectiveness of vaccination for rotavirus gastroenteritis in 8 Latin American and Caribbean countries. Rev Panam Salud Publica 2007;21:205–16
- Widdowson MA, Meltzer MI, Zhang X, et al. Cost-effectiveness and potential impact of rotavirus vaccination in the US. Pediatrics 2007;119:684–97
- Goossens LM, Standaert B, Hartwig N, et al. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine 2008;26:1118–27
- Melliez H, Levybruhl D, Boelle PY, et al. Cost and cost-effectiveness of childhood vaccination against rotavirus in France. Vaccine 2008;26:706–15
- Ho AM, Nelson EA, Walker DG. Rotavirus vaccination for Hong Kong children: an economic evaluation from the Hong Kong government perspective. Arch Dis Child 2008;93:52–8
- Milne RJ, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value in Health 2009;12:888–98
- Jit M, Blicke J, Mangen MJ, et al. The cost-effectiveness of rotavirus vaccination: comparative analyses for 5 European countries and transferability in Europe. Vaccine 2009;27:6121–28
- Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med S Aust 2009;191:157–60
- Wang FT, Mast TC, Glass RJ, et al. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010;125:e208–13
- Zeller M, Rahman M, Heylen E, et al. Rotavirus incidence and genotype distribution before and after national rotavirus vaccine introduction in Belgium. Vacine 2010;28:7507–13
- Buttery JP, Lambert SB, Grimwood K, et al. Reduction in rotavirus-associated acute gastroenteritis following the introduction of the rotavirus vaccine into Australia's national childhood vaccine schedule. Pediatr Inf Dis J 2011;30(1 Suppl):S25–9
- Patel MM, Steele D, Gentsch JR, et al. Real world impact of rotavirus vaccination. Pediatr Inf Dis J 2011;30(1 Suppl):S1–5
- Blicke J, Beutels P. Reviewing the cost-effectiveness of rotavirus vaccination. Pharmacoeconomics 2009;27:281–97
- Velázquez FR, Matson DO, Calva JJ, et al. Rotavirus infection in infants as protection against subsequent infections. N Engl J Med 1996;335:1022–8
- Briggs A, Sculpher M. An introduction to Markov modelling for economic evaluation. Pharmacoeconomics 1998;13:397–409
- Velázquez FR, Calva JJ, Guerrero ML, et al. Cohort study of rotavirus serotype patterns in symptomatic and asymptomatic infections in Mexican children. Pediatr Infect Dis J 1993;12:54–61
- Brisson M, Senecal M, Drolet M et al. Health-related quality of life lost to rotavirus-associated gastroenteritis in children and their parents: a Canadian prospective study. PIDJ 2010;29(1):73–75
- World Health Organization. The World Health Report. 2002. Reducing risks, promoting healthy life. Available at: http://www.who.int/whr/2002/en. Accessed 15 March 2011
- World Health Organization. Tables of costs and prices used in WHO-CHOICE analysis. 2007. World Health Organization Web site. Available at: www.who.int/choice/costs/CER_levels/en/index.html. Accessed 2 July 2009
- Dasbach EJ, Insinga RP, Yang YC, et al. The cost-effectiveness of a quadrivalent human papillomavirus vaccine in Taiwan. Asian Pacific J of CancerPrev 2008;9:1–8
- International Monetary Fund. List of countries by GDP (nominal) per capita. Available at: http://en.wikipedia.org/wiki/List_of_countries_by_GDP_(nominal)_per_capita. Accessed 15 March 2011
- Statistical Yearbook of the Republic of China 2009. Table 151: Indices of Consumer Price by Commodity Quality. Available at: http://eng.stat.gov.tw/public/data/dgbas03/bs2/yearbook_eng/y151.pdf. Accessed March 28, 2011.
- IRS.gov. Yearly Average Exchange Rates for Converting Foreign Currencies into U.S. Dollars, 2010. Available at: http://www.irs.gov/businesses/small/international/article/0,,id=206089,00.html. Accessed 15 March 2011
- Current Immunization Schedule in Taiwan. Centers for Disease Control, ROC (Taiwan). Available at: http://www.cdc.gov.tw/lp.asp?ctNode=157&CtUnit=134&BaseDSD=7&mp=1. Accessed 29 December 2009
- Vesikari T, Matson DO, Dennehy P, et al. Rotavirus efficacy and safety trial (REST) study team. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med 2006;354:23–33
- Dennehy PH, Vesikari T, Matson DO. Efficacy of a pentavalent rotavirus vaccine, RotaTeq® (RV5), between doses of a 3-dose series: potential benefits of early protection. Human Vaccines 2011;7:563–8
- Boom JA, Tate JE, Sahni LC, et al. Effectiveness of pentavalent rotavirus vaccine in a large urban population in the United States. Pediatrics 2010;125:e199–207
- Payne D, Staat MA, Donauer S, et al. Postlicensure effectiveness of RotaTeq vaccine in US children. Abstract presented at: 5th International Conference on Vaccines for Enteric Diseases (VED); September 2009; Malaga, Spain
- Dennehy P, Goveia M, Dallas M, et al. The integrated Phase III safety profile of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine. Int J Infect Dis 2007;11:36–42
- Council of Labor Affairs, Government of Taiwan. Adjustment of the Basic Wage. Available at: http://www.cla.gov.tw/cgi-bin/siteMaker/SM_theme?page=48eaf6bd. Accessed 2 January 2010
- Griebsch I, Coast J, Brown J. Quality-adjusted life-years lack quality in pediatric care: a critical review of published cost-utility studies in child health. Pediatrics 2005;115:e600–8
- Grange A, Bekker H, Noyes J, et al. Adequacy of health-related quality of life measures in children under 5 years old: systematic review. J Adv Nursing 2007;59:197–220
- Statistical Yearbook of the Republic of China 2009. Table 18- Live Births by Age of Mother. Available at: http://eng.dgbas.gov.tw/public/data/dgbas03/bs2/yearbook_eng/y018.pdf. Accessed 28 March 2011