Abstract
Background and objective:
Acne is a common dermatologic condition that extends into middle age, particularly among women, and is associated with substantial healthcare resource utilization. Drospirenone (DRSP), a synthetic progestin, has anti-androgenic activity, and women using DRSP 3.0 mg/ethinyl estradiol (EE) 0.02 mg as a 24/4 regimen (DRSP/EE-24/4) for contraception also may use it for treatment of moderate acne. The study used a US national healthcare database to assess acne-related healthcare resource utilization among women aged 18–45 years before (pre-index) and after (post-index) initiation of DRSP/EE-24/4.
Methods:
Resource utilization and costs were evaluated by age group (18–25, 26–35, or 36–45 years) and by type of acne medication (systemic antibiotic, topical, or anti-androgen).
Results:
Data for 1340 women were evaluated. Overall, drug costs, medical costs, and total costs were decreased by 38%, 37%, and 37%, respectively (p < 0.0001 for all) between the pre-index and post-index periods; significant differences were evident across age groups and acne medication categories. Total costs were significantly decreased for patients (41%) and healthcare plans (36%; p < 0.0001 for both) overall and across age groups and drug classes. Acne-related claims and number of days using acne medication were reduced (by 37% each; p < 0.0001 for both).
Study limitations:
The study was retrospective in design and had a limited follow-up period. Database limitations restricted assessment of medication compliance and adherence.
Conclusion:
DRSP/EE-24/4 use was associated with substantial reductions in acne-related healthcare resource utilization, and reductions occurred regardless of age or type of acne medication. DRSP/EE-24/4 therefore represents a cost-effective option for the treatment of acne among women using DRSP/EE-24/4 for oral contraception.
Introduction
Acne is characterized by follicular hyperproliferation, excess sebum production, inflammation, and the proliferation of Propionibacterium acnes, and is the most common dermatologic condition in the US, affecting 40–50 million peopleCitation1,Citation2. Almost 85% of all people have acne at some point in their livesCitation1; acne persists into middle age, with women being affected more frequently than menCitation2,Citation3. In one survey of women from a university clinic, the prevalence of acne was 50.9% for those aged 20–29 years, 35.2% for those aged 30–39 years, and 26.3% for those aged 40–49 yearsCitation2. In a community-based study of people ≥ 25 years old, the overall prevalence of acne among women was 54%, and this rate did not decrease until after the age of 45 yearsCitation3. Acne most often affects the face and can result in a substantial psychosocial burden for the patient, leading to low self-esteem, social isolation, and poor body imageCitation4,Citation5. These consequences of acne particularly affect women who experience a greater level of self-consciousness regarding appearance and negative self-concept relative to men with acneCitation6.
Acne also is associated with a substantial societal economic burden and imposes a financial burden on patients. The total direct cost associated with the treatment of acne, which included substantial costs for prescription ($1.74 billion) and over-the-counter ($319 million) products, was ∼$2.5 billion in 2004Citation7. According to results of the US Medical Expenditure Panel Survey, which included more than 5 million people with acne, nearly 70% use some form of acne medicationCitation8. The survey also showed that medication accounts for ∼36% of total acne-related healthcare costs, and acne-related office visits were correlated with an increase in healthcare costs.
Excess sebum is one of the primary factors contributing to acne development, and androgen hormones, at least in part, regulate sebaceous glands and sebum excretionCitation9,Citation10. Drospirenone (DRSP) is a synthetic progestin with anti-androgenic properties (e.g., reduction in sebum excretion)Citation11,Citation12. In contrast, many combined oral contraceptives—e.g., norgestimate/ethinyl estradiol (Ortho Tri-Cyclen, Ortho-McNeil-Janssen Pharmaceuticals, Inc, Titusville, NJ) and norethindrone acetate/ethinyl estradiol (Estrostep Fe, Warner Chilcott plc, Dublin, Ireland)—contain progestins derived from 19-nortestosterone that have pro-androgenic effects. Inclusion of such androgenic progestins partially negates the favorable effect of the estrogen component on sebum excretionCitation10.
DRSP 3.0 mg/ethinyl estradiol (EE) 0.02 mg as a 24/4 regimen (DRSP/EE-24/4; YAZ, Bayer HealthCare, Wayne, NJ) is an oral contraceptive also indicated in the US for the treatment of moderate acne vulgaris in women who desire an oral contraceptive for birth control. In clinical trials, women using DRSP/EE-24/4 for contraception experienced significant improvements in moderate acne, including decreases in inflammatory, non-inflammatory, and total lesion countsCitation10,Citation11. The present analysis (a retrospective US database review) evaluated whether DRSP/EE-24/4 had an effect on healthcare resource utilization related to acne treatment among women using this product for contraception.
Patients and methods
Patients
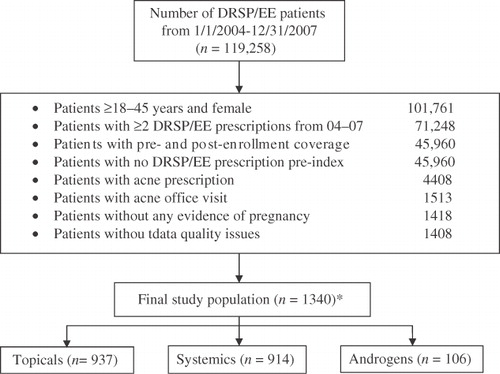
Women aged 18–45 years were eligible if they had newly initiated treatment (i.e., no prescriptions for DRSP/EE during the baseline period) with DRSP/EE-24/4 during the index period, ≥2 prescriptions for DRSP/EE-24/4 (one at the index date and one during the follow-up period), and a minimum cumulative drug supply of 56 days during the follow-up period. Women also were required to have acne vulgaris, defined as patients who had ≥1 healthcare visit for acne (severity not documented) and ≥2 acne prescriptions before the index event (baseline period). Women who became pregnant at any time during the study period were excluded.
Data collection and analysis
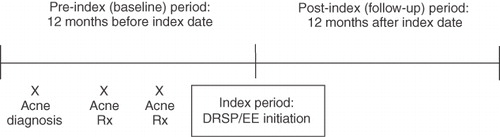
Data collection occurred from January 2003 to December 2008 (). The baseline period comprised the 12 months prior to the index date (pre-index); the index period extended from January 1, 2004, to December 31, 2007; and the follow-up period comprised the 12 months after the index date (post-index), up to December 31, 2008.
Data were derived from Medstat MarketScan (Thomson Reuters (Healthcare) Inc, Ann Arbor, MI), a US national healthcare claims database comprising privately and publicly insured individuals. Healthcare resource utilization was assessed for the pre-index and post-index periods, and differences in costs were calculated. Women were stratified according to age: 18–25, 26–35, or 36–45 years. Women also were stratified according to the class of acne treatment they received: topical agents, systemic antibiotics, and anti-androgens. Because a woman may have received ⩾1 class of acne medication during the study period, individuals may be included in ≥1 acne treatment category. Cost and resource use data were collected by type of acne treatment received and by age classification. Data included drug, medical, and total (drug plus medical) costs; patient and health plan costs; health plan claims; the number of days of acne treatment; and the medication possession ratio (MPR). The MPR captures medication refill history over a period of time and shows how many days of medication a patient obtained during the study period compared with how many days should have been obtained.
Statistical analyses
Mean (standard deviation [SD]) values for all data were calculated. Pre-index vs post-index values for all utilization metrics (comparisons within each acne treatment type) and cost variables were compared using the paired t-test. Health plan claims and days of supply were compared between the pre-index and post-index periods within each acne treatment type using the Wilcoxon signed rank test based on the difference.
Results
Patient demographics
Overall, 119,258 women received DRSP/EE-24/4 between January 1, 2004 and December 31, 2007. Of these, 1340 met study criteria and were included in the analysis (). Baseline demographics and patient characteristics are summarized in . Mean age was 24.6 (SD 7.6) years, and topical agents (70%) or systemic antibiotic agents (68%) for acne were commonly used. Most women (62%) were insured by a preferred provider organization (i.e., a managed healthcare organization composed of healthcare providers that provide healthcare services at a reduced fee).
Table 1. Baseline demographics and characteristics.
Drug costs, medical costs, and total (drug and medical) costs
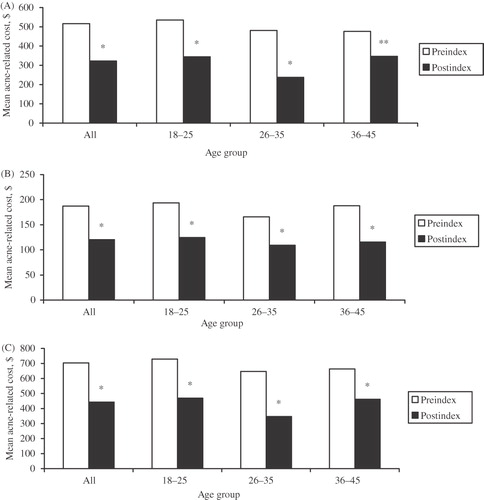
The addition of DRSP/EE-24/4 resulted in a significant reduction in mean drug (), medical (), and total (drug plus medical; ) costs related to acne treatment during the index period.
Figure 3. Mean (A) drug, (B) medical, and (C) total (drug plus medical) costs before and after initiation of DRSP/EE-24/4 therapy. *p < 0.0001 vs pre-index; **p = 0.01 vs pre-index.
Drug costs
Mean drug costs significantly decreased from $516.58 in the pre-index period to $322.84 in the post-index period (38%; p < 0.0001). Significant mean decreases in drug costs were evident across all age groups. For the 18–25, 26–35, and 36–45-year age groups, mean drug costs decreased from $535.60 to $344.63 (36%; p < 0.0001), $481.40 to $237.70 (51%; p < 0.0001), and $475.79 to $346.44 (27%; p = 0.01), respectively. Significant decreases also were evident across all drug classes: systemic antibiotics (40%; p < 0.0001), topical agents (39%; p < 0.0001), and anti-androgens (33%; p = 0.001).
Medical costs
Mean medical costs significantly decreased between the pre-index and post-index periods ($187.14 to $120.36 [37%]; p < 0.0001), with significant decreases across all age groups and all drug classes. For the 18–25, 26–35, and 36–45-year age groups, mean medical costs decreased from $193.60 to $124.81 (36%), $165.91 to $109.16 (34%), and $187.83 to $115.50 (39%), respectively (p < 0.0001 for all). Decreases in mean medical costs were 33% (p < 0.0001) for systemic antibiotics, 36% (p < 0.0001) for topical agents, and 24% (p = 0.03) for anti-androgens.
Total costs
Similarly, mean total costs significantly decreased between the pre-index and post-index periods ($703.72 to $443.20 [37%]; p < 0.0001), with significant decreases across all age groups and all drug classes. For the 18–25, 26–35, and 36–45-year age groups, mean total costs significantly decreased from $729.20 to $469.43 (36%), $647.32 to $346.86 (46%), and $663.62 to $461.95 (30%), respectively (p < 0.0001 for all). Assessed by type of acne treatment, mean decreases in total costs were 37% for both systemic antibiotics and topical agents and 27% for anti-androgens (p < 0.0001 for all).
Patient costs, health plan costs, and claims
The addition of DRSP/EE-24/4 resulted in a significant reduction in mean costs to patients and health plans (), as well as reductions in claims to health plans during the index period.
Table 2. Mean total (drug plus medical) acne-related treatment costs before and after initiation of DRSP/EE-24/4.
Patient costs
outlines the mean total costs of anti-acne medications to patients before (pre-index) and after (post-index) the initiation of DRSP/EE-24/4 treatment, as well as the difference between these periods. Cost data are stratified by acne treatment type and by age group. Among women who added DRSP/EE-24/4 during the index period, total (drug plus medical) patient costs significantly decreased from $166.26 to $98.52 (41%; p < 0.0001) although there was substantial inter-patient variability as evidenced by a large SD. Mean total patient costs also decreased significantly across all age groups and drug classes. For the 18–25, 26–35, and 36–45-year age groups, mean total patient costs decreased from $172.05 to $99.91 (42%), $152.25 to $95.64 (37%), and $159.02 to $96.03 (40%), respectively (p < 0.0001 for all). Mean total patient costs decreased by 41%, 44%, and 40% for women using anti-androgens, topical agents, and systemic antibiotics, respectively (p < 0.0001 for all). Mean patient drug and medical costs were individually reduced significantly across age groups and across drug classes (p < 0.0001 for all; data not shown).
Health plan costs
outlines mean total costs of anti-acne medications to health plans before (pre-index) and after (post-index) the initiation of DRSP/EE-24/4 treatment, as well as the difference between these periods. Cost data are stratified by acne treatment type and by age group. Mean total (drug plus medical) health plan costs significantly decreased from $519.47 to $334.84 (36%; p < 0.0001). Mean total health plan costs significantly decreased across all age groups: $537.33 to $358.40 (33%) for women aged 18–25 years, $497.31 to $250.76 (48%) for women aged 26–35 years, and $492.37 to $347.84 (29%) for those aged 36–45 years (p < 0.0001 for all). Significant mean reductions in total costs also were observed for women using anti-androgens, systemic antibiotics, and topical anti-acne agents (28%, 39%, and 34%, respectively [p < 0.0001 for all]). Mean health plan drug and medical costs also were significantly reduced individually (p ≤ 0.02 for all; data not shown).
Claims
The number of acne-related health plan claims before (pre-index) and after (post-index) the initiation of DRSP/EE-24/4 therapy, as well as the difference between these periods, was evaluated. Claims data are stratified by acne treatment type and by age group. Health plans experienced significant reductions in claims related to acne management, although as for costs there was considerable inter-patient variability. Mean number of claims significantly decreased (5.88 to 3.72 [37%]; p < 0.0001), with significant decreases across all age groups and all drug classes. For the 18–25, 26–35, and 36–45-year age groups, mean number of claims decreased from 6.03 to 3.69 (39%), 5.33 to 3.33 (37%), and 5.94 to 4.51 (24%), respectively (p < 0.0001 for all). Mean number of claims decreased significantly across all drug classes: 3.43 to 1.79 for women using topical agents (48%), 4.23 to 2.45 for women using systemic antibiotics (42%), and 3.65 to 2.1 for women using anti-androgens (40%; p < 0.0001 for all). Significant decreases were evident for type of acne treatment in the individual age group categories, except for anti-androgens in the 36–45-year age group.
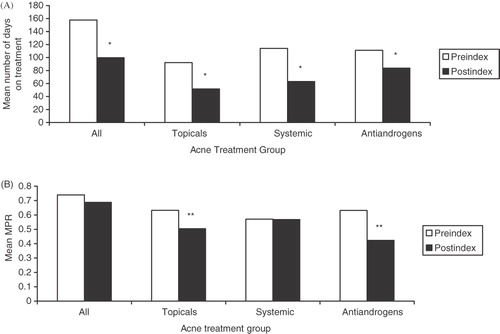
Number of days on acne treatment and MPR
The number of days women used acne treatment drugs and MPRs were reduced after initiation of DRSP/EE-24/4 treatment, with differences between the pre-index and post-index periods reaching statistical significance in all instances except for the sub-group of women aged 36–45 years using androgens. The number of days an acne treatment drug was used significantly decreased from a mean of 157.7 days in the pre-index period to 99.6 days in the post-index period (37%; p < 0.0001; ). The number of days on treatment was significantly decreased across all age groups. Reductions for the 18–25, 26–35, and 36–45-year age groups were from 163.5 to 99.5 days (39%), 137.3 to 84.0 days (39%), and 160.9 to 124.8 days (22%), respectively (p < 0.0001 for all). The mean number of days on treatment also significantly decreased across all drug classes, with mean reductions of 45% for systemic antibiotics, 44% for topical agents, and 25% for anti-androgens (p < 0.0001 for all). The mean MPR decreased from 0.740 in the pre-index period to 0.688 in the post-index period, but the difference was not significant (p = 0.2362; ). However, the decrease in MPR was statistically significant among women using anti-androgens (33%; p = 0.0004) and those using topical agents (20%; p = 0.0005). Stratified by age, the MPR was significantly decreased in only the 18–25-year age group (10%; p = 0.0293); a small increase (4%; p = 0.8622) occurred in the 26–35-year age group and a small decrease (11%; p = 0.0907) was observed in the 36–56-year age group.
Figure 4. Mean (A) number of days on acne treatment and (B) medication possession ratio (MPR) before and after initiation of drospirenone/ethinyl estradiol 24/4 therapy; *p < 0.0001 vs pre-index; **p = 0.001 vs pre-index. A woman may have received >1 class of acne medication during the study period; therefore, women may be included in ≥1 acne treatment category.
Discussion
Acne is associated with substantial economic resource utilizationCitation13. Although data are limited, evidence suggests that effective anti-acne therapy is cost-effectiveCitation14–16. One assessment of topical and oral anti-acne regimens revealed that, because efficacy differences among available treatments are small, cost-effectiveness is driven primarily by drug acquisition costsCitation15,Citation16.
Clinical studies have demonstrated that the combination of DRSP/EE is effective in the treatment of acne vulgaris in women requiring contraceptionCitation10,Citation11. DRSP is an anti-androgenic progestin that reduces sebum excretion while EE increases sex hormone-binding globulin, and thereby reduces free testosterone levels. Results of the present study suggest that the benefits of DRSP/EE-24/4 in women being treated for acne secondary to its use as a contraceptive extend to include significant reductions in many aspects of healthcare resource utilization related to the treatment of their acne. The use of DRSP/EE-24/4 resulted in reductions in overall costs, costs to patients and health plans, the number of acne-related claims submitted to health plans, the number of days women used acne treatments, and the MPR. Importantly, reductions in healthcare resource utilization and associated costs occurred regardless of age and acne treatment being used at baseline.
Although between-group statistical analyses were not performed across age groups and drug classes, several trends were evident. Reductions in drug costs, total costs, and health plan costs tended to be greatest in the 26–35-year age group. Differences between drug classes were less evident, although patients using anti-androgens had slightly lower reductions in drug costs, medical costs, total costs, health plan costs, and days on treatment compared with those using systemic antibiotics or topical agents.
The initiation of Yaz resulted in fewer acne-related claims and, based on MPR, a reduction in the use of acne treatments, particularly anti-androgens and topical agents. The decrease in acne-related healthcare claims and treatment suggests that the cost reductions are not merely a reflection of lower drug costs during the post-index period. In addition, the indirect costs associated with claim processing were not included in the current study. Consequently, the overall healthcare resource reduction may result in correspondingly greater cost reductions than those determined in the current study.
Patient adherence to prescribed therapy is directly correlated with the effectiveness of treatment, with poor adherence resulting in additional healthcare expenditures due to therapy failureCitation17. The healthcare cost of morbidity and mortality due to non-adherence in the US has been estimated to be as much as $300 billionCitation18. Traditional methods of measuring adherence through the use of patient logs, questionnaires, interviews, or pill counting are often unreliable due to patient over-estimation of adherence. With microprocessors to measure and record data such as the date and time of medication events, medication electronic monitoring systems (MEMS) provide a more accurate accounting of medication adherence; among dermatology patients, adherence rates were consistently lower when measured using electronic monitoring systems in comparison to traditional methods of adherence assessmentCitation19. With regard to acne-related medication, MEMS measured adherence to topical acne treatment decreased from 82% to 45% over a 6-week periodCitation20. However, treatment regimen simplification increased adherence; 88% of patients using a dual drug combination topical medication were adherent in comparison to 61% of patients using two separate drugsCitation21. As a result, the use of DRSP/EE-24/4 for both contraception and acne treatment may potentially bring the benefit of higher drug compliance since either indication may serve as a reminder for women to use the drug regularly, resulting in greater medication adherence and potentially lower healthcare costs.
DRSP/EE-24/4 is indicated for the treatment of moderate acne in women who desire an oral contraceptive for birth controlCitation12, an important advantage with respect to cost-effectiveness. Since the primary indication for DRSP/EE-24/4 is contraception, the costs associated with its use for the treatment of acne are ambiguous (i.e., resource savings are not offset by the cost of DRSP/EE-24/4 as acne therapy). Formal cost-effectiveness analyses are needed to quantify the cost savings associated with this dual use. However, the significant reduction in acne-related healthcare resource usage and medication following the initiation of DRSP/EE-24/4 demonstrates the cost benefit associated with its use for both contraception and acne treatment. Other opportunities for further research include evaluating whether DRSP/EE-24/4 provides additional clinical benefits beyond reduced healthcare resource utilization and assessing the difference in resource utilization and costs between DRSP/EE-24/4 and other oral contraceptives that are also indicated for the treatment of acne.
Limitations of the present analysis include the lack of a prospective design and longer term follow-up. In addition, no comparator oral contraceptive was included to determine the effect of alternative oral contraceptives on resource use. Furthermore, an established method was lacking for assessing compliance and adherence to either the acne medications or oral contraceptives.
Conclusion
Results of this retrospective study, which used a US national healthcare database, suggest that use of DRSP/EE-24/4 is associated with substantial reductions in healthcare resource utilization and support that DRSP/EE-24/4 represents a practical treatment option for the large proportion of women using oral contraceptives for birth control who also require treatment for acne.
Transparency
Declaration of funding
This research was funded by Bayer HealthCare Pharmaceuticals, Inc.
Declaration of financial/other relationships
Vijay N. Joish, Susan Boklage, Richard Lynen, and Anja Schmidt are full-time employees of Bayer HealthCare Pharmaceuticals, Inc. Jay Lin is an employee of Novosys Health, which received research funding from Bayer HealthCare Pharmaceuticals, Inc. for the analysis of the data.
Acknowledgments
The authors wish to acknowledge Bret Fulton, RPh, and Maribeth Bogush, PhD, for their editorial assistance, which was funded by Bayer HealthCare Pharmaceuticals.
References
- American Academy of Dermatology. Acne Factsheet. Available at: www.aad.org/media/background/factsheets/fact_acne.html Accessed 20 April 2011
- Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol 2008;58:56‐9
- Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol 1999;41:577‐580
- Fried RG, Wechsler A. Psychological problems in the acne patient. Dermatol Ther 2006;19:237‐240
- Koo JY, Smith LL. Psychologic aspects of acne. Pediatr Dermatol 1991;8:185‐188
- Hassan J, Grogan S, Clark-Carter D, et al. The individual health burden of acne: appearance-related distress in male and female adolescents and adults with back, chest and facial acne. J Health Psychol 2009;14:1105‐1118
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol 2006;55:490‐500
- Balkrishnan R, Kulkarni AS, Cayce K, et al. Predictors of healthcare outcomes and costs related to medication use in patients with acne in the United States. CUTIS 2006;77:251‐255
- Picardo M, Ottaviani M, Camera E, et al. Sebaceous gland lipids. Dermato-endocrinology 2009;1:68‐71
- Maloney JM, Dietze P Jr., Watson D, et al. A randomized controlled trial of a low-dose combined oral contraceptive containing 3 mg drospirenone plus 20 microg ethinylestradiol in the treatment of acne vulgaris: lesion counts, investigator ratings and subject self-assessment. J Drugs Dermatol 2009;8:837‐844
- Koltun W, Lucky AW, Thiboutot D, et al. Efficacy and safety of 3 mg drospirenone/20 mcg ethinylestradiol oral contraceptive administered in 24/4 regimen in the treatment of acne vulgaris: a randomized, double-blind, placebo-controlled trial. Contraception 2008;77:249‐256
- YAZ®. (Drospirenone and ethinyl estradiol) Tablets [prescribing information]. Wayne, NJ: Bayer Healthcare Pharmaceuticals Inc, 2010
- Wessels F, Anderson AN, Kropman K. The cost-effectiveness of isotretinoin in the treatment of acne. Part 3. A cost-minimisation pharmaco-economic model. S Afr Med J 1999;89:791‐794
- Wessels F, Anderson AN, Kropman K. The cost-effectiveness of isotretinoin in the treatment of acne. Part 1. A meta-analysis of effectiveness literature. S Afr Med J 1999;89:780‐784
- Ozolins M, Eady EA, Avery AJ, et al. Comparison of five antimicrobial regimens for treatment of mild to moderate inflammatory facial acne vulgaris in the community: randomised controlled trial. Lancet 2004;364:2188‐2195
- Ozolins M, Eady EA, Avery A, et al. Randomised controlled multiple treatment comparison to provide a cost-effectiveness rationale for the selection of antimicrobial therapy in acne. Health Technol Assess 2005;9:iii‐212
- Koehler AM, Maibach HI. Electronic monitoring in medication adherence measurement. Implications for dermatology. Am J Clin Dermatol 2001;2:7‐12
- DiMatteo MR. Variations in patients' adherence to medical recommendations: a quantitative review of 50 years of research. Med Care 2004;42:200‐209
- Greenlaw SM, Yentzer BA, O'Neill JL, et al. Assessing adherence to dermatology treatments: a review of self-report and electronic measures. Skin Res Technol 2010;16:253‐258
- Yentzer BA, Alikhan A, Teuschler H, et al. An exploratory study of adherence to topical benzoyl peroxide in patients with acne vulgaris. J Am Acad Dermatol 2009;60:879‐880
- Yentzer BA, Ade RA, Fountain JM, et al. Simplifying regimens promotes greater adherence and outcomes with topical acne medications: a randomized controlled trial. Cutis 2010;86:103‐108