Abstract
Objective:
To compare the effectiveness of budesonide/formoterol fumarate dihydrate (BFC) and fluticasone propionate/salmeterol (FSC), two combination inhaled corticosteroid/long-acting beta-agonist (ICS/LABA) products approved for the treatment of chronic obstructive pulmonary disease (COPD) in the US with respect to cost, therapy adherence, and related healthcare utilization. The effectiveness of these two treatments has not previously been compared in a US COPD population.
Methods:
A retrospective cohort study assessed COPD-related outcomes using administrative claims data among ICS/LABA-naïve patients. Patients initiating BFC were propensity matched to FSC patients. Cost and effectiveness were measured as total healthcare expenditures, exacerbation events (hospitalizations, emergency department visits, or outpatient visits associated with oral corticosteroid or antibiotic prescription fills), and treatment medication adherence. Differences in COPD symptom control were assessed via proxy measure through claims for rescue medications and outpatient encounters.
Results:
Of the 6770 patients (3385 BFC and 3385 FSC), fewer BFC patients had claims for short-acting beta agonists (SABA) (34.7% vs 39.5%; p < 0.001) and ipratropium (7.8% vs 9.8%, p < 0.005) than FSC patients, but no substantial differences were seen in other clinical outcomes including tiotropium or nebulized SABA claims, COPD-related outpatient visits, or exacerbation events. There were no significant differences in total COPD-related medical costs in the 6-month period after initiation of combination therapy.
Limitations:
This was a retrospective observational study using claims data and accuracy of COPD diagnoses could not be verified, nor was information available on severity of disease. The results and conclusions of this study are limited to the population observed and the operational definitions of the study variables.
Conclusions:
For most outcomes of interest, BFC and FSC showed comparable real-world effectiveness.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease characterized by deterioration in lung function due to airway obstruction and inflammation. An estimated 24 million Americans suffer from COPD, the third leading cause of death in the USCitation1. In 2000, COPD accounted for 1.5 million emergency department (ED) visits and 726,000 hospitalizations in the US; since 1990, hospitalizations for COPD patients across all age groups have increased significantlyCitation2. In 2004, COPD-related illnesses were estimated to cost $37.2 billion per year, $20.9 billion attributed to direct medical costsCitation2. Hospitalization costs are estimated to account for up to 70% of the total healthcare costs associated with the diseaseCitation3–7. Acute exacerbations are a major cause of poor health status among COPD patients and generate high demand for healthcare servicesCitation7,Citation8. The majority of exacerbations, 50–70%, are due to respiratory infectionsCitation9. Because of their tremendous negative impact on patients and the healthcare systemCitation10, current guidelines site the prevention and treatment of exacerbations as one of the primary goals of COPD managementCitation11. Pharmacotherapy for COPD patients is used to decrease respiratory symptoms and reduce the risk for exacerbationsCitation12. Inhalation therapy is recommended for all with moderate to very severe disease, and long-acting inhaled bronchodilators are considered more effective and convenient than short-acting treatment therapiesCitation12,Citation13. There is good evidence that long-acting inhaled anti-cholinergics, β2-agonists, and corticosteroids are similarly effective in reducing exacerbationsCitation13, and that inhaled corticosteroid (ICS) and long acting β2-agonist (LABA) combination therapy allows for treatment optimization and is superior to either aloneCitation14–16. The results of several observational retrospective studies have found that the combination of an ICS and the LABA salmeterol are associated with significantly lower risk of COPD-related hospitalizations compared with ipratropium (IPR) among various populations of COPD patientsCitation17–20.
The benefits of using either of the leading ICS/LABA combination therapies, budesonide/formoterol (BFC) or fluticasone propionate/salmeterol (FSC) in comparison with monotherapy have been summarized elsewhereCitation14,Citation15,Citation21,Citation22. FSC (Advair Diskus 250/50, GlaxoSmithKline, Research Triangle Park, NC) is a dry powder inhaler (DPI) that was approved for use in the US in November 2003 for COPD associated with chronic bronchitis, and, in April 2008, further indicated for maintenance treatment of airflow obstruction and reducing exacerbations in patients with COPD. BFC (Symbicort, AstraZeneca LP, Wilmington, DE), a pressurized meter dose inhaler (pMDI), was approved for use in the US for the treatment of asthma in June 2007 and, more recently, for maintenance treatment of airflow obstruction in patients with COPD in February 2009, but not for reducing exacerbations. Few studies have compared healthcare respiratory-related utilization and associated costs among individuals with COPD treated with these two now widely used combination treatment therapiesCitation23. This analysis assesses differences in outcomes, including exacerbations and associated healthcare service use, adherence to prescribed treatment regimen, healthcare costs, the use of rescue inhalers, and pneumonia events.
Methods
This retrospective, 2-group, intent to treat cohort analysis used the PharMetrics Integrated Database (IMS, Norwalk, CT) consisting of HIPAA compliant, de-identified healthcare claims for more than 55 million patients and 90 US health insurance plans. The dataset includes International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes, dates of service, billed charges information, and participating plan payments. Procedure, facility, and pharmacy costs for entire episodes of care are included and estimated as actual cost from the health insurance carrier perspective. Paid costs as contained in the database are the basis for cost measures in the analysis. PharMetrics imputes payments for claims with missing payment information because of capitation arrangementsCitation24.
Sample
This analysis included patients with a diagnosis of COPD (primary or secondary ICD-9-CM codes 491.xx, 492.xx, or 496) associated with an ED visit, hospitalization, or outpatient visit. These were defined as COPD-related encounters. Patients were aged ≥40, a commonly used demarcation point for higher COPD prevalenceCitation11,Citation12, and identified as new users of either BFC or FSC. New users had no evidence of previous BFC or FSC use in the 6 months before the first prescription claim for either agent (the index date). Patients were required to have continuous health insurance coverage for the 6 months before and 3−6 months after the index date. Index dates ranged from January 1, 2007 to January 31, 2009. Patients must have had ≥1 prescription claim during the study period.
Oral corticosteroid (OCS)-dependent patients, defined as those with a medication possession ratio (MPR) for OCS ≥ 0.50 in the 6 months before the index date, were excluded, as were patients with a medical claim indicating respiratory tract cancer (ICD-9-CM codes 161, 161.x, 162, 163, 231, or 231.x).
To reduce potential patient selection bias from the non-random nature of an observational study, we used a propensity matching method (greedy match algorithm) to match BFC patients to FSC patients using baseline characteristicsCitation25,Citation26. The greedy match algorithm uses logistic regression modeling to create matched samples based on each patient’s predicted probability (propensity) of assignment to treatment. Patients are matched using the ‘nearest available pair’ (or ‘nearest-neighbor’) matching method. Once a match is made, the greedy algorithm does not reconsider the match. Patients were matched based on probability of receiving BFC, conditional on age category, sex, geographic region, treatment initiation year, comorbid conditions, months of follow-up, and pre-index utilization for any COPD-related hospitalization, any pneumonia-related hospitalization (associated with a pneumonia ICD-9 code), any COPD-related emergency department (ED) visit, any OCS fill, any antibiotic fill, use of short-acting β2-agonist (SABA)/nebulized SABA with 0, 1, 2, or ≥3 fills, and IPR use with 0, 1, or ≥2 fills.
Comorbidities were identified based on Elixhauser et al.Citation27 classifications: asthma, obstructive sleep apnea, heart disease (rheumatic heart failure, hypertensive heart disease, ischemic heart disease, and cardiovascular heart disease). The Elixhauser measure has the advantage of being able to provide information on relationships between individual comorbidities and study outcomes, as opposed to an index comorbidity score like the Charlson measureCitation28. To further comparatively characterize patients, the existence of other serious lung conditions (primary or secondary diagnosis of bronchiectasis, cystic fibrosis, coal worker pneumoconiosis, asbestosis, pneumoconiosis due to other silica, inorganic dust, inhalation of other dust, or unspecified, respiratory conditions due to chemical fumes and vapors or other unspecified external agents, post-inflammatory pulmonary fibrosis, other alveolar and parietoalveolar pneumonopathy, lung involvement in conditions classified elsewhere, other respiratory diseases, lung cancer, TB, extrinsic allergic alveolitis, lipid pneumonia, detergent asthma, or other diseases of the lungCitation29 was also included (). Patients were not matched on use of ICS or LABA monotherapy or use of tiotropium (TIO).
Table 1. After propensity matching—comparison of baseline characteristics.
Measures
Main outcome measures of interest were utilization and costs categorized by COPD-related and pneumonia-related medical service events and COPD-related pharmacy prescriptions. Utilization was measured for exacerbation events (hospitalizations, ED visits, antibiotic claims, and OCS claims) and for outpatient visits. Procedure, facility, and pharmacy costs for entire episodes of care (e.g., hospitalizations, ED visits) were defined as amounts paid by the health plan as stated in the database; costs paid by other sources were not included. Outcomes also included treatment medication adherence and measures demonstrating lack of COPD symptom control (post-index use of ipratropium [ipratropium alone or in combination with albuterol (IPR)], SABAs/nebulized SABAs, and outpatient visits). MPR, defined as days in possession of the treatment drug divided by the number of days reviewed (varied from 3–6 months), was used to measure adherence.
Statistical analysis
Pre-index baseline demographic and utilization characteristics before (not shown) and after propensity matching ( and ) and outcomes post-initiation of therapy for the two cohorts were compiled and compared using Pearson χ2 test for categorical variables and Student’s t-test for continuous variables. The focus of our cost analyses was on observed differences in total costs between the two treatment groups. Our cost data, like most cost data, was non-normal and right-skewed. Though this has been noted to not be an issue in analyses using large datasetsCitation30, as sensitivity analyses, cost differences were also analyzed using a log transformation; any zero cost values were first set equal to 0.0001.
Table 2. After propensity matching—comparison of baseline costs prior to controller.
All statistical tests were 2-sided with a level of significance of 0.01 (lower due to multiple outcome comparison tests) and were conducted with SAS v 9.1.3 for Windows (SAS Institute, Cary, NC).
Results
Before matching, 3390 patients met inclusion criteria for BFC initiation and 90,070 for FSC initiation. The pool of potential FSC patients for matching with BFC patients was extremely large and consequently almost all BFC patients were matched; matching resulted in two cohorts of 3385 each. After matching, no significant demographic, baseline utilization measures, or post-index follow-up time differences emerged between the BFC and FSC cohorts (). Before matching, patients initiating with FSC were slightly older (mean age: FSC, 62.2 years; BFC, 61.6 years), and fewer had evidence of comorbid conditions with the exception of arrhythmia, congestive heart failure, complicated diabetes, and fluid disorders, any COPD-related utilization (except for hospitalization [BFC, 6.3%; FSC, 7.5%]), or any SABA use, but a greater number had at least one pneumonia-related hospitalization (data not shown).
The most prevalent comorbidities in the baseline period were uncomplicated hypertension, asthma, heart disease, and other lung conditions. Among other lung conditions, the majority (BFC, 70%; FSC, 74%) were other diseases of the lung (ICD-9-CM 518.x) and the remaining portion of other lung conditions was comprised mainly of post-inflammatory pulmonary fibrosis and bronchiectasis. During the baseline period, more than 65% of each cohort had an exacerbation event. Approximately 1/3 of patients in each cohort had ≥1 SABA prescription fill during the baseline period (). These propensity-matched groups differed only in their use of ICS, LABA, and TIO, with a larger percentage of BFC patients having any use of these medications. There were no significant differences for COPD-related or pneumonia-related utilization costs in the baseline period (, also shown are p-values for sensitivity analyses using log-transformed costs). It was noticed that there was one very significant hospital cost outlier in the FSC group and results are also presented with this outlier excluded (). There was a slight difference in mean costs incurred by each group for rescue inhalers (BFC, $65.99; FSC, $54.64; p = 0.01).
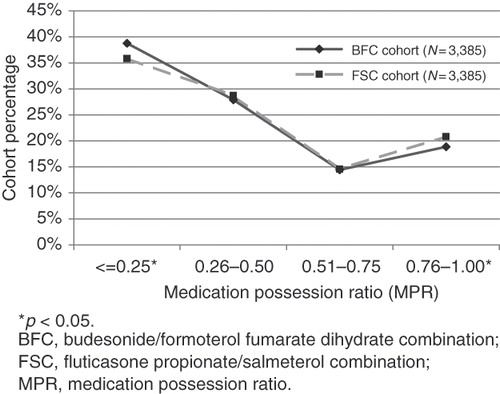
Patients were followed for up to 6 months. After-initiation, a lower proportion of BFC patients had claims for SABAs (34.7% vs 39.5%; p < 0.001) and IPR (7.8% vs 9.8%, p < 0.005) compared with the FSC patients. While in the baseline period, differences existed for use of ICS, LABA, and TIO; after initiation there was no significant difference between BFC and FSC patients. There was no difference in the percentage of patients with any COPD-related outpatient visits (29.5%, 31.3%; p = 0.11) or any exacerbation events (hospitalizations (4.1%, 3.5%; p = 0.14), any ED visits (1.3%, 1.4%; p = 0.67), any OCS claims (29.2%, 28.7%; p = 0.68), and any antibiotic claims (56.2%, 55.9%; p = 0.78)). The percentage filling a prescription for the alternate treatment medication during the follow-up period was slightly higher for the BFC group (). There was minimal cross-over between treatments: 1.2% of the FSC group also used BFC, 3.8% of the BFC group also used FSC (p < 0.001). Adherence was similar for both cohorts, with the FSC cohort having a marginally higher percentage of patients with an MPR > 0.25. ()
Table 3. Utilization outcomes post-initiation of controller therapy.
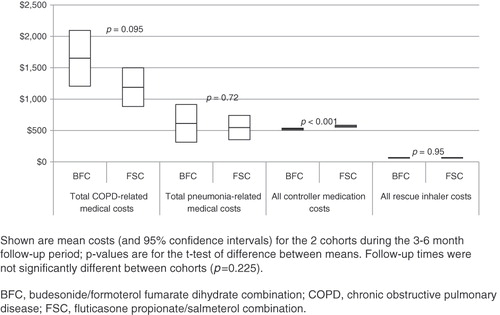
There were no significant differences in total average COPD-related medical services costs () during the follow-up period, or for total average rescue inhaler costs. Controller medication costs were ∼$43 higher for FSC patients ($566.10 vs 522.76, p < 0.001) and could be attributed to higher mean MPR among FSC patients.
Discussion
To our knowledge, this is the first US study to compare the two leading COPD ICS/LABA combination treatments, BFC and FSC, in a real-world use study on COPD outcomes measuring exacerbations and associated healthcare use, adherence to prescribed treatment regimen, healthcare costs, use of rescue inhalers, and pneumonia events. In addition to similar demographics, prior to initiation of combination treatment, groups were similar in percentages of patients with any COPD or pneumonia-related hospitalization or ED visit, as well as any SABA or IPR prescription claim. Post-initiation, this study did not find differences in percentages of patients with a COPD-related or pneumonia-related hospitalization or ED visit. Differences were found between BFC and FSC in the percentages filling a prescription for IPR, and for SABAs in the follow-up period, with significantly fewer BFC patients filling prescriptions for these relief medications. There was no difference, however, between groups for mean SABA or ipratropium claims. While GOLD guidelines do suggest increased use of SABAs during exacerbations as part of a home-management strategy to possibly avoid hospitalizations, they do not suggest that increased SABA use can avoid exacerbations. In fact, they encourage increased use of long-acting bronchodilators at baseline to reduce the risk for exacerbations and thus the need for SABAs, based on evidence from several randomized clinical trialsCitation11.
Not surprisingly, use of ICS and LABA monotherapy decreased considerably for both treatment groups after initiating combination therapy. Within each treatment group, 25% had ≥1 prescription claim for tiotropium, a percentage greatly increased from the baseline period use, and may warrant further study. No cost differences were found between the two groups for use of medical services (hospitalization, ED visit, outpatient visit) or for rescue medications; however, costs for controller medications for the FSC group were slightly higher ($43).
Interestingly, the pneumonia rate went down after initiation of either BFC or FSC, which has been a concern among ICS therapies due to a slight increase in pneumonia rates observed in some clinical trialsCitation22. The incidence of COPD hospitalizations fell from 6.3% to 4.1% in the BFC cohort and from 6.1% to 3.5% in the FSC cohort, suggesting that these treatments are having the favorable impact on severe exacerbations seen in combination therapy trials that included exacerbations as a primary or secondary end-pointCitation22. However, no change was seen in COPD-related ED or outpatient visits.
A recently published Canadian population study compared COPD-related exacerbation events after initiation of a BFC or FSC combination therapy and found that BFC-initiating patients experienced a significantly lower relative risk for COPD-related hospitalizations (95% CI = 0.47–0.81) and ED visits (95% CI = 0.58–0.97), prescription claims for tiotropium (95% CI = 0.57–0.89) and had on average fewer doses per day for ipratropium bromideCitation23. In that study, there was no difference between groups for average dose per day for SABAs. The observation periods before and after treatment initiation were longer in the Canadian analysis of adults with COPD—1 year before and after treatment, compared to 6 months before and 3–6 months after in this study. There are also indications that the COPD population in the Canadian study had more severe COPD. In the Canadian study, before treatment initiation, more than 50% of patients in each treatment group had ≥1 SABA claim, 30% had ≥1 ipratropium claimCitation23. In comparison, our study showed less than 35% of patients in each group had ≥1 SABA claim, 7.5% had a claim for nebulized SABA, and slightly >9% had a claim for IPR. COPD-related hospitalizations and ED visits were lower in our population compared with the Canadian, with ∼4% of each group experiencing a hospitalization vs 8.6% of Canadian BFC patients and 12.4% of Canadian FSC patients, and less than 1.5% experiencing an ED visit vs more than 10% in the Canadian studyCitation23. Furthermore, medication adherence in our study was lower, with 2/3 or more of each group having an MPR less than 50% () compared with an MPR average of slightly more than 50% in the Canadian study, and the Canadian population appears to have been an older cohort, 78% were aged 65 or olderCitation23. Lastly, 44% of patients in our study were male vs 52% in the Canadian study. There were similarities in percentages of OCS or tiotropium prescriptions. We found that 25% of each treatment group had ≥1 claim for tiotropium; in the Canadian study, 21.4% of BFC and 23.4% of FSC patients did.
This study was not without limitations. Our study is a retrospective database study and is not designed to examine causal effects between treatment and outcomes. Further, since the accuracy of COPD diagnoses could not be verified, the results and conclusions of this study are limited to the population observed and the operational definitions of the study variables. The lack of disease severity detail in encounter data may have also limited our findings regarding resource utilization and costs. The 6-month follow-up timeframe was relatively short in this study; a longer follow-up analysis period may allow further examination of the effect of patients gaining proficiency in maintenance of treatment regimens to more effectively control COPD symptoms and exacerbations.
Conclusion
We found that BFC and FSC showed comparable real-world effectiveness as measured by the number of COPD exacerbation and pneumonia events during the observation period. There were no significant differences observed in overall adherence rates and treatment costs between BFC and FSC, although BFC patients had lower rescue medication use, suggesting a potentially higher level of symptom control.
Transparency
Declaration of funding
Funding for this study was provided by AstraZeneca LP (Wilmington, DE).
Declaration of interest
Ms Roberts was employed at Lovelace Respiratory Research Institute (LRRI) when this study was performed, and is now employed by Lovelace Clinic Foundation dba/LCF Research. Ms Roberts and Mr Petersen, through employment at LRRI, have worked on studies funded by GlaxoSmithKline, AstraZeneca LP, Sepracor, Novartis, and Merck. Dr Mapel has received research funding from and served as a consultant to AstraZeneca LP, GlaxoSmithKline, and Pfizer Pharmaceuticals. Dr Blanchette was employed by LRRI at the time of this study and has received research funding from and served as a consultant to GlaxoSmithKline, AstraZeneca LP, Sepracor, Novartis, and Merck during the time of this study. Dr Ramachandran is currently and was employed by AstraZeneca LP at the time of the study.
Acknowledgments
Susan R. Berry, MSW, of Lovelace Respiratory Research Institute assisted in manuscript preparation by providing medical writing and editorial services funded by AstraZeneca LP.
Author contributions
Ms Roberts contributed to the study concept and design, analysis, and/or interpretation of data, drafting of the manuscript, statistical analysis, critical revision of the manuscript for important intellectual content, final approval of the manuscript, and administrative, technical, or material support. She had full access to all data in the study and takes full responsibility for the integrity of the data and the accuracy of the data analysis.
Dr Mapel contributed to the study concept and design, analysis, and/or interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript.
Mr Petersen contributed to the study concept and design, acquisition of data, analysis and/or interpretation of data, and critical revision of the manuscript for important intellectual content. He had full access to all data in the study and final approval of the manuscript.
Dr Blanchette contributed to the study concept and design, analysis and/or interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and obtaining funding. He had full access to all data in the study and final approval of the manuscript.
Dr Ramachandran contributed to the analysis and/or interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the manuscript.
References
- U.S. Department of Health & Human Services. What Is COPD? National Institutes of Health. National Heart Lung and Blood Institute. Available at: http://www.nhlbi.nih.gov/health/dci/Diseases/Copd/Copd_WhatIs.html. Accessed April 8, 2011
- Mannino DM, Braman S. The epidemiology and economics of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:502-6
- Connors AFJr ., Dawson NV, Thomas C, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments). Am J Respir Crit Care Med 1996;154:959-67
- Halpern MT, Stanford RH, Borker R. The burden of COPD in the USA: results from the Confronting COPD survey. Respir Med 2003;97:S81-S9
- Mapel DW, Hurley JS, Frost FJ, et al. Health care utilization in chronic obstructive pulmonary disease - A case-control study in a health maintenance organization. Arch Intern Med 2000;160:2653-8
- Strassels SA, Smith DH, Sullivan SD, et al. The costs of treating COPD in the United States. Chest 2001;119:344-52
- Miravitlles M, Murio C, Guerrero T, et al. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest 2002;121:1449-55
- Seemungal TAR, Donaldson GC, Paul EA, et al. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998;157:1418-22
- Ball P. Epidemiology and treatment of chronic bronchitis and its exacerbations. Chest 1995;108:43S-52S
- Mannino DM, Homa DM, Ford ES, et al. Chronic obstructive pulmonary disease surveillance– United States, 1971–2000. MMWR 2002;51:1
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Available from: http://www.goldcopd.org/. [Last accessed 19 August 2011]
- Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease -- GOLD executive summary. Am J Respir Crit Care Med 2007;176:532-55
- Wilt TJ, Niewoehner D, MacDonald R, et al. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med 2007;147:639-53
- Giembycz MA, Kaur M, Leigh R, et al. A Holy Grail of asthma management: toward understanding how long-acting beta(2)-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids. Br J Pharmacol 2008;153:1090-104
- Miller-Larsson A, Selroos O. Advances in asthma and COPD treatment: combination therapy with inhaled corticosteroids and long-acting beta(2)-agonists. Curr Pharm Des 2006;12:3261-79
- Welte T. Optimising treatment for COPD - new strategies for combination therapy. Int J Clin Prac Aug 2009;63:1136-49
- Akazawa M, Hayflinger DC, Stanford RH, et al. Economic assessment of initial maintenance therapy for chronic obstructive pulmonary disease. Am J Manag Care 2008;14:438-48
- Blanchette CM, Akazawa M, Dalal A, et al. Risk of hospitalizations/emergency department visits and treatment costs associated with initial maintenance therapy using fluticasone propionate 500 mu g/salmeterol 50 mu g compared with ipratropium for chronic obstructive pulmonary disease in older adults. Am J Geriatr Pharmacother 2008;6:138-46
- Rascati KL, Akazawa M, Johnsrud M, et al. Comparison of hospitalizations, emergency department visits, and costs in a historical cohort of Texas Medicaid patients with chronic obstructive pulmonary disease, by initial medication regimen. Clin Ther 2007;29:1203-13
- Rascati KL, Stanford RH, Borker R. A comparison of the risk of hospitalizations due to chronicobstructive pulmonary disease in medicaid patients with various medication regimens, including ipratropium, inhaled corticosteroids, salmeterol, or their combination. Clin Ther 2005;27:346-54
- Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Resp J 2003;22:912-9
- Mapel D, Hurley JS, Dalal AA, et al. The role of combination inhaled corticosteroid/long-acting beta-agonist therapy in COPD management. Prim Care Respir J 2010;19:93-103
- Blais L, Forget A, Ramachandran S. Relative effectiveness of budesonide/formoterol and fluticasone propionate/salmeterol in a 1-year, population-based, matched cohort study of patients with chronic obstructive pulmonary disease (COPD): effect on COPD-related exacerbations, emergency department visits, and hospitalizations, medication utilization, and treatment adherence. Clin Ther 2010;32:1320-8
- Jilinskaia E, Norton S, Johnson C. Comparison of methods for imputation of paid amounts in media insurance data. In: 2002 ASA Proceedings, Joint statistical meetings. New York, New York: American Statistical Association, 2002. Available at http://www.amstat.org/sections/srms/Proceedings/y2002/files/JSM2002-000171.pdf. [Last accessed 10 April 2011]
- D'Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stats Med 1998;17:2265-81
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. SUGI 26 Proceedings (Proceedings of the 26th Annual SAS User Group International Conference) 2001;Paper 214–26
- Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8-27
- Baldwin LM, Klabunde CN, Green P, et al. In search of the perfect comorbidity measure for use with administrative claims data -- Does it exist? Med Care 2006;44:745-53
- Mapel DW, Robinson SB, Lydick E. A comparison of health-care costs in patients with chronic obstructive pulmonary disease using lightweight portable oxygen systems versus traditional compressed-oxygen systems. Respir Care 2008;53:1169-75
- Diehr P, Yanez D, Ash A, et al. Methods for analyzing health care utilization and costs. Annu Rev Public Health 1999;20:125-44