Abstract
Objective:
The objective of this analysis was the evaluation of the outcomes and costs associated with rivaroxaban and enoxaparin for the prevention of postsurgical venous thromboembolism (VTE) in patients undergoing total hip replacement (THR) and total knee replacement (TKR) from the US payer perspective.
Methods:
VTE event rates have been reported in three Phase III clinical trials that compared rivaroxaban and enoxaparin for VTE prevention after orthopedic surgery during the prophylaxis (≤35 days for THR patients and 10–14 days for TKR patients) and post-prophylaxis periods (≤90 days following surgery). These data were used in this decision-analytic model to estimate and compare health outcomes and costs associated with rivaroxaban and enoxaparin. The base-case analysis considered the number and costs of symptomatic VTE events during the prophylaxis period only. A 90-day horizon was considered in the sensitivity analysis.
Results:
Following THR, when extended durations of prophylaxis (35 days) were compared, rivaroxaban was associated with lower costs than enoxaparin, with total saving costs of $695/patient. When an extended duration of rivaroxaban prophylaxis (35 days) was compared with a short duration (10–14 days) of enoxaparin prophylaxis, rivaroxaban was estimated to prevent 9.9 additional symptomatic VTE events per 1000 patients, while saving $244/patient (rate/1000 patients). In the TKR population, short duration of rivaroxaban prophylaxis was estimated to prevent 13.1 additional symptomatic VTE events per 1000 patients. It was also less costly than short duration enoxaparin prophylaxis, with a saving of $411/patient (rate/1000 patients).
Limitations:
Only statistically significant differences were captured in the base-case economic analysis, and, therefore, differences in pulmonary embolism (PE) and bleeding events were not captured.
Conclusions:
In this model, rivaroxaban reduced total treatment payer costs vs enoxaparin for the prevention of VTE in THR or TKR patients.
Introduction
Major orthopedic surgery, such as total hip replacement (THR) and total knee replacement (TKR), is associated with an increased risk of venous thromboembolism (VTE). Estimates indicate that the incidence of hospital-acquired deep vein thrombosis (DVT) is ∼40–60% following major orthopedic surgeryCitation1. Pulmonary embolism (PE), in which a blood clot leaves a deep vein and becomes lodged in blood vessels in the lungs, accounts for ∼10% of all in-hospital deathsCitation1,Citation2. In the US, the reported numbers of elective THR and TKR procedures were 276,528 and 615,716, respectively, in 2008Citation3.
The American College of Chest Physicians (ACCP) guidelines recommend that patients undergoing THR receive prophylaxis to reduce the risk of VTE events for no less than 10 days, and that patients considered to be at high risk of developing VTE receive it for up to a maximum of 35 days following surgeryCitation1. For patients undergoing TKR, the recommended duration of prophylaxis is at least 10 daysCitation1. Low molecular-weight heparins (LMWHs), vitamin K antagonists (VKAs), and fondaparinux are the recommended forms of prophylaxis for the prevention of VTE in the US following THR or TKRCitation1. However, these current options for thromboprophylaxis have limitations.
LMWHs reduce thromboembolic events but necessitate subcutaneous (SC) administration, and therefore they require patients or caregivers to inject the medication. VKAs, such as warfarin, have unpredictable pharmacologic effects and numerous food and drug interactions and require frequent monitoring—and are therefore difficult to manageCitation1. Furthermore, there is evidence to suggest that the incidence of major bleeding events is greater with VKAs than with LMWHs given after THR.
Enoxaparin is an LMWH that has been approved by the US Food and Drug Administration (FDA) for use in patients at risk of DVT, patients undergoing hip and knee replacement surgery, patients undergoing abdominal surgery who are at risk for thromboembolic complications, and medical patients who are at risk for thromboembolic complications due to severely restricted mobility during acute illness. Enoxaparin binds to and potentiates the action of antithrombin and inhibits coagulation Factors XIa, IXa, Xa, and IIa (thrombin), thereby preventing the formation of blood clots. Compared with unfractionated heparin (UFH), enoxaparin has many advantages in terms of its pharmacodynamic profile and its efficacyCitation4. However, given the need for SC administration with enoxaparin and the trend for shorter hospital stays, a simple, effective oral anti-coagulant regimen for use in an outpatient setting would be beneficial.
Rivaroxaban is an orally bioavailable Factor Xa inhibitor that selectively blocks the active site of Factor Xa and does not require a co-factor (such as antithrombin III) for activity. Activation of Factor X to Factor Xa via the intrinsic and extrinsic pathways plays a central role in the cascade of blood coagulationCitation4. Rivaroxaban was evaluated in the Phase III REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism (RECORD) program, which included four randomized controlled trials in more than 12,500 patients. The aim of the program was to compare oral, once-daily rivaroxaban with SC enoxaparin for the prevention of VTE after THR and TKR surgery. Data from the RECORD studies, which demonstrated that rivaroxaban is associated with fewer VTE events than enoxaparin after THR or TKRCitation5–8, were used as part of a decision-analytical model to estimate the costs and health outcomes of rivaroxaban compared with enoxaparin for the prevention of VTE from a US payer’s perspective.
A recent study conducted in the US concluded that patients developing VTE during hospitalization had a higher likelihood of mortality, bleeding, rehospitalization, and extended duration of hospitalization and incurred higher costs than patients without VTECitation9. Previous studies have addressed the costs and health outcomes of interventions in these patient groupsCitation10–14; however, this is the first economic analysis of rivaroxaban in the US for prevention of post-surgical VTE.
Methods
Model period
A decision-analytic model was developed to estimate the costs and health outcomes of rivaroxaban compared with enoxaparin for the prevention of VTE. It was divided into two periods: the prophylaxis period, based on the duration of the respective clinical trials analysed (≤35 days for THR patients and 10–14 days for TKR patients), and the post-prophylaxis period (≤90 days following surgery, because evidence shows that patients remain at risk of VTE for that period of time)Citation2,Citation15.
Clinical trials data included in models
The model was populated using the event rates from three of the Phase III RECORD trials comparing rivaroxaban with commonly used enoxaparin regimens in the US. Rivaroxaban was administered at the dose of 10 mg once daily and enoxaparin at the dose of 40 mg once daily in all three studies: RECORD1–3. In the RECORD1 study, extended therapy with rivaroxaban (35 days) was compared to extended therapy with enoxaparin (35 days) in 4541 THR patientsCitation5. The mean age of the patients enrolled in the trial was 63.2 years (range, 18–91 years). In the RECORD2 trial, extended therapy with rivaroxaban (35 days) was compared with short-term enoxaparin (10–14 days) followed by placebo in 2509 patients undergoing THRCitation6. The mean age of patients in this study was 61.5 years (range, 18–93 years). In RECORD3, short-term rivaroxaban (10–14 days) was compared with short-term enoxaparin (10–14 days) in 2531 TKR patients. Patients’ mean age was 67.6 years (range, 28–91 years)Citation7.
RECORD4 compared rivaroxaban 10 mg once daily vs enoxaparin 30 mg twice daily, initiated post-operatively, for 10–14 days in 1742 patients undergoing TKRCitation8. However, because rivaroxaban was approved for use in orthopedic patients in the US based on the first three RECORD trials, RECORD4 was considered only as part of the sensitivity analysis.
The primary and secondary efficacy outcomes and safety outcomes of the RECORD1–3 trials have been described in detail elsewhereCitation5–7. Results of these trials consistently showed a significant reduction in total VTE with rivaroxaban, with no significant differences in rates of major bleeding. The prophylaxis- and post-prophylaxis-period event rates from three of the four published Phase III RECORD trials included in this model are presented in Citation5,Citation6,Citation8,Citation16,Citation17.
Table 1. Event rates in the prophylaxis and post-prophylaxis periods.
Outcome measures
The model included seven different endpoints: VTE, symptomatic VTE, asymptomatic VTE, fatal PE, non-fatal PE, symptomatic DVT, and prophylaxis-related major bleeding events. Symptomatic VTE events included symptomatic DVT and PE in addition to venographically detected DVT. The number of asymptomatic VTE events was estimated by subtracting the number of symptomatic VTE events from the total number of VTE events. Symptomatic DVT events were defined as the number of symptomatic VTE events that were not PE events. The primary outcome measure in the model was a composite of DVT (symptomatic or asymptomatic), non-fatal PE, and all-cause mortality. For the RECORD1–3 studies, the primary efficacy endpoint of total VTE was the composite of any DVT, non-fatal PE, and all-cause mortality and was similar in all three studies. Therefore, this primary outcome was chosen for the present study. In the RECORD1–3 studies, the pre-specified primary efficacy outcome was defined as the representation of the clinical components of total VTE.
Data sources for resource use and healthcare cost estimates
The model included estimated healthcare costs related to the duration of VTE prophylaxis and treatment. These were obtained using the assumed duration of hospitalization described above and the duration of post-discharge prophylaxis used in the RECORD1, 2, and 3 trials. The medical care component of the Consumer Price IndexCitation18 was used to adjust associated costs to 2010 values where necessary. Costs were estimated from the perspective of the healthcare payer.
Medicare reimbursement rates were used to estimate the costs of diagnosis of DVT and PE and were based on resource use data reported by McGarry et al.Citation19. Non-fatal prophylaxis-related bleeding events were assigned the cost of a major bleeding event occurring in the hospital setting in patients undergoing THR or TKR. This cost was estimated by calculating the difference between the cost weights associated with the Medicare Severity–Diagnosis Related Groups for major joint replacements with and without major complicationsCitation20 in order to ascertain the cost of complications. The costs associated with the inpatient treatment of VTE events were based on data from Spyropoulos and LinCitation21, whereas the follow-up costs associated with the treatment of VTE events were based on results from McGarry et al.Citation19.
Base-case deterministic analysis assumptions
In the base-case deterministic analysis, the model assumed parity between rivaroxaban and enoxaparin for endpoints in which the trial did not find statistically significant differences between the arms. The risk of death among the cohort was assumed to be equal to the background (all-cause) mortality in the USCitation22; this risk was assumed to be the same for all individuals in the model. Patients in the model were assumed to be 64 years of age, based on the mean age observed in the four Phase III RECORD trials of rivaroxabanCitation5–8, and the mortality rate used reflected this.
Resource use and healthcare cost assumptions
For resource use and healthcare costs, it was assumed that:
Patients treated with rivaroxaban would not incur any administration fees, given its oral formulation.
Patients treated with LMWH would require nurse assistance during the first administration of the drug while in hospital; however, these costs are relatively minor ($23/day).
There would be no additional costs prior to discharge associated with training patients on self-injecting LMWH.
Two-thirds of post-surgery discharged patients who subsequently developed a DVT would be readmitted, with the remainder being treated on an outpatient basis (based on the DVT FREE registry of 5451 patients with ultrasound-confirmed DVT)Citation23.
All patients developing a PE would be treated on an inpatient basis.
Duration of hospitalization would be 3 days following THR and 4 days following TKR (based on the US registry database in orthopedic surgery)Citation15.
The likelihood of an asymptomatic event becoming symptomatic would be the same in both treatment arms when extrapolating events beyond the trial period.
Sensitivity analyses
Sensitivity analyses are conducted to define the sensitivity of the model to anticipated variations in the values of input parameters. In the current study, a series of one-way sensitivity analyses was conducted on the model using the base-case results to determine the impact of different plausible variations in the outcomes analysed.
For the THR population, one-way sensitivity analyses were conducted for RECORD1Citation5 and RECORD2Citation6. RECORD1 and RECORD2 were also combined as a sensitivity analysis.
For the TKR population, a one-way sensitivity analysis was conducted for the RECORD3Citation7 data. RECORD4 resultsCitation8 were also used as a sensitivity analysis.
Results
Calculated resource use and healthcare costs
compares drug costs between rivaroxaban and enoxaparin according to need for administration and monitoring, major bleeding events, and number of physician visitsCitation19,Citation20,Citation25–27. shows resource use and per-patient costs associated with a diagnosis of VTE for inpatients and outpatients according to number of physician visits and diagnostic modality usedCitation19,Citation26. presents resource use and per-patient costs associated with treatment of VTE for inpatients and outpatients according to type of treatment and tests requiredCitation19,Citation21,Citation26,Citation28–30.
Table 2. Resource use and costs associated with prophylaxis.
Table 3. Resource use and costs associated with VTE diagnosis.
Table 4. Resource use and costs associated with VTE treatment.
Base-case deterministic analysis for RECORD1, 2, and 3
THR population
When equal durations of prophylaxis (35 days) were compared in the RECORD1 trial, rivaroxaban, though equally as effective as enoxaparin, was found to be a cost-saving option in patients undergoing THR. Specifically, prophylaxis with rivaroxaban was associated with total cost savings of $695/patient when compared to prophylaxis with enoxaparin ()Citation5–7. When 35 days of rivaroxaban prophylaxis were compared with 10–14 days of enoxaparin in the RECORD2 trial, rivaroxaban was associated with lower costs and greater health benefits vs enoxaparin. In this trial, rivaroxaban was associated with 0.0099 fewer symptomatic VTE events per patient (i.e. 9.9 fewer events/1000 patients), resulting in an estimated cost savings of $244/patient in our analysis.
Table 5. Base-case model predicted cost savings and symptomatic VTE events reduction.
TKR population
In the RECORD3 trialCitation7, the base-case model predicted average cost savings for rivaroxaban of $411/patient; in addition, rivaroxaban was associated with 13.1 fewer symptomatic VTE events/1000 patients.
Sensitivity analyses of robustness of base-case cost-offset estimates
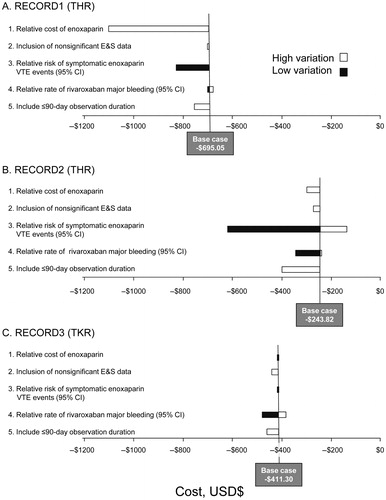
RECORD1 ()
Sensitivity analysis of rivaroxiban using RECORD1Citation5 data found that the variables that had the greatest impact on the benefit associated with rivaroxaban predicted by the base case ($695 savings vs enoxaparin) were a high relative cost of enoxaparin, which resulted in a savings of $1101 with rivaroxaban use, and a high relative risk of symptomatic VTE events with enoxaparin, which resulted in a savings of $827 with rivaroxaban use. In contrast, assuming a lower relative risk of symptomatic VTE events with enoxaparin had little effect upon the incremental costs. Finally, increasing the duration of the observation period included in the analysis to ≤90 days was associated with a greater cost offset with the use of rivaroxaban (a savings of $755), owing to the lower level of symptomatic VTE events occurring in the post-prophylaxis period in the rivaroxaban vs the enoxaparin arm.
RECORD2 (Figure 1b)
Sensitivity analysis of predicted benefit using the RECORD2Citation6 trial data found that the variables that had the greatest impact on the benefit associated with rivaroxaban predicted by the base case were the rate of symptomatic VTE events in the enoxaparin arm compared with the rivaroxaban arm, the time horizon considered, and the prices of the two agents. Assuming a higher rate of symptomatic VTE events in the enoxaparin arm resulted in cost savings of >$600/patient with rivaroxaban use. When a lower rate of VTE events was applied in the enoxaparin arm, rivaroxaban was predicted to be associated with cost savings of ∼$135/patient. Finally, increasing the duration of the observation period included in the analysis to 90 days was associated with a greater cost offset with the use of rivaroxaban ($393/patient), owing to the lower level of symptomatic VTE events occurring in the post-prophylaxis period in the rivaroxaban vs the enoxaparin arm (23 fewer symptomatic VTE events/1000 patients).
When the results of the RECORD1Citation5 and RECORD2Citation6 trials were pooled, the model predicted average cost savings for rivaroxaban of $555/patient. In addition, rivaroxaban was associated with five fewer symptomatic events/1000 patients.
RECORD3 (Figure 1c)
Sensitivity analysis of predicted benefit of rivaroxaban using RECORD3 data found that the variables that had the greatest impact on the benefit associated with rivaroxaban predicted by the base case were relative rates of VTE events and drug costs. Rivaroxaban was predicted to be cost saving in all scenarios compared with enoxaparin. Assuming a higher rate of symptomatic VTE events in the enoxaparin arm also led to cost savings, while simultaneously increasing the clinical benefit of rivaroxaban in terms of the number of symptomatic VTE events avoided. Assuming a lower price for rivaroxaban increased the cost savings for this arm; the converse was also true. Using observed event rates for which differences did not reach significance caused a small improvement in the cost savings.
When the results of the RECORD4Citation8 trial were used, the model predicted average cost savings for rivaroxaban of $408/patient when compared to 10–14 days of enoxaparin 30 mg bid prophylaxis.
Sensitivity analyses of robustness of base-case reduction of symptomatic VTE estimate
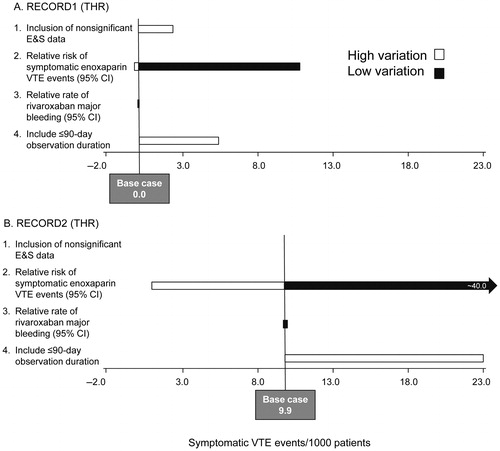
RECORD1 ()5
When the sensitivity analysis was undertaken using the RECORD1 trial dataCitation5, the parameters whose uncertainty had the largest effect on the results were the relative risk (RR) of symptomatic VTE events in the enoxaparin arm compared with the rivaroxaban arm and the time horizon considered. Assuming a higher RR of symptomatic VTE events in the enoxaparin arm, the estimated reduction of symptomatic VTE events with rivaroxaban was an average of 10.7/1000 patients compared with the enoxaparin arm. When a lower RR of VTE events was applied in the enoxaparin arm, rivaroxaban was predicted to be associated with 0.2/1000 patients fewer symptomatic VTE events. Using a 90-day time horizon to capture subsequent symptomatic VTE events, the benefit of rivaroxaban was predicted to be greater and, as such, was associated with cost savings relative to enoxaparin.
RECORD2 (Figure 2b)6
When the sensitivity analysis was undertaken using the RECORD2 trial dataCitation6, the parameters whose uncertainty had the largest effect on the results were again the rate of symptomatic VTE events in the enoxaparin arm compared with the rivaroxaban arm and the time horizon considered. Assuming a higher rate of symptomatic VTE events in the enoxaparin arm, the estimated reduction of symptomatic VTE events with rivaroxaban was an average of 40.3/1000 patients compared with the enoxaparin arm. When a lower rate of VTE events was applied in the enoxaparin arm, rivaroxaban was predicted to be associated with 1.1/1000 patients fewer symptomatic VTE events. Using a 90-day time horizon to capture subsequent symptomatic VTE events, the benefit of rivaroxaban was predicted to be greater and, as such, was associated with cost savings relative to enoxaparin.
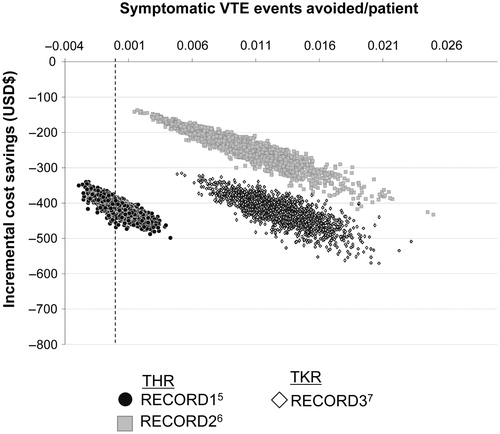
Cost-effectiveness by probabilistic sensitivity analysis
presents the expected number of symptomatic VTE events avoided per patient and the incremental cost saving per patient for the three populations considered (RECORD1, RECORD2, and RECORD3)Citation5–7. Each point in the scatter plot represents one iteration of the probabilistic sensitivity analysis. The plots show a positive correlation between improved effectiveness and greater cost savings.
Discussion
Most surgical patients who require hospitalization should be considered at high risk for VTE and should be given appropriate prophylaxisCitation1. A fundamental consideration in determining the degree of VTE prophylaxis that a surgical patient may need is the thromboembolic risk of the procedure itselfCitation1. Surgical orthopedic procedures, particularly THR and TKR, are associated with an increased risk of VTE, whereas general surgery or open gynecologic or urologic procedures are considered to present a moderate risk of VTECitation1. The incidence of DVT in hospital following orthopedic surgery is ∼50%, and PE, a common complication of DVT, is responsible for ∼10% of all in-hospital deathsCitation1,Citation2. Elective THR and TKR procedures in the US in 2008 were reported to be 276,528 and 615,716, respectivelyCitation3. Therefore, the impact of VTE after orthopedic surgery represents an important clinical reality for healthcare in the US.
The base-case analysis considered a short-term time horizon, taking into account only costs and events occurring during the prophylaxis period (≤35 days for THR patients and 10–14 days for TKR patients) in order to allow for an economic comparison between rivaroxaban and enoxaparin over that time period. Clinical trial data indicate that 35 days of prophylaxis with rivaroxaban are associated with fewer symptomatic VTE events than 10–14 days’ prophylaxis with enoxaparin in patients undergoing THRCitation6. The results of this cost-effectiveness analysis were consistent with those findings and also predicted that rivaroxaban would be associated with lower costs, because the three additional weeks of drug costs incurred were offset by the reduction in the total number of events treated. In both the THR and TKR populations, rivaroxaban was associated with lower costs than enoxaparin when equal durations of prophylaxis were compared. Given that only statistically significant differences in trial endpoints between the two arms were included in the base-case analysis of the model, there were no differences in terms of the number of symptomatic VTE events avoided in the prophylaxis period between the interventions when equal durations of prophylaxis were compared in THRCitation5. However, sensitivity analysis showed results consistently in favor of rivaroxaban when non-statistically significant results were used to populate the model—a result of the lower event rates observed in the rivaroxaban arm of the trials.
Sensitivity analyses were undertaken to explore the effect of changes to the input parameters on the model results. Extending the time horizon to 90 days, thus extrapolating the trial outcomes to the acute phase of the condition (i.e., the post-prophylaxis module in the model), allowed for the assessment of asymptomatic VTE developing into symptomatic VTE. This extended analysis was based on previously published studies concerning disease outcomes in the post-treatment periodCitation16,Citation17.
Results suggested that using a longer modeling duration (90 days) produces cost savings for rivaroxaban, along with an improvement in the incremental number of symptomatic VTE events avoided. The other key parameter identified in this analysis was the RR of symptomatic VTE in the enoxaparin arm compared with the rivaroxaban arm; when the difference between the arms was greater for this outcome, the results were more favorable to rivaroxaban.
Overall, the evaluation has several strengths. In particular, the model structure was based on published recommendations and previously conducted economic evaluationsCitation10,Citation31, and therefore represents current best practice economic modeling in this disease area. Furthermore, the economic evaluation replicated the clinical outcomes observed in the trials, providing credibility to the results of the cost-effectiveness analysis. Published resource-use data were used as a basis for estimating the costs of treatment administration, monitoring, and management of prophylaxis-related bleeding and VTE events.
In addition to the study’s strengths, there were also limitations. While the model discussed here is based on clinical trial data, the small number of events observed in the clinical trials means that, although the probability of some events occurring was different among prophylactic agents and regimens, the differences were not statistically significant. Following a conservative approach, only statistically significant differences were captured in the base-case economic analysis; therefore, differences in PE and bleeding events were not captured. The true relationship between asymptomatic and symptomatic DVT is uncertain and has thus been approximated using previous studies of longer-term outcomesCitation23,Citation32. Despite being routinely used in clinical trials, tests for asymptomatic VTE are not normally part of routine practice. Thus, asymptomatic VTE events detected in a clinical trial context would be treated, which may not reflect what happens in routine practice, where asymptomatic VTE is normally undetected and, therefore, untreated.
The model is based on evidence from large clinical trials and includes the most-robust available evidence for assessing the cost-effectiveness of rivaroxaban compared with enoxaparin in the trial period. There is evidence that a significant proportion of patients develops DVT or PE up to 3 months after general or orthopedic surgeryCitation23,Citation32. Therefore, prolonged prophylaxis during the post-discharge period may provide clinical and cost benefits in high-risk patients. Further research into the long-term outcomes for patients treated with rivaroxaban and enoxaparin after major orthopedic surgery may therefore be beneficial in establishing a longer-term assessment of cost-effectiveness. Key challenges for the future include identifying the patients who require prolonged prophylaxis and determining the duration of treatmentCitation33.
Conclusion
This analysis found that rivaroxaban, when compared with enoxaparin, reduced both the costs and the number of symptomatic VTE events in patients undergoing major orthopedic surgery. The key cost savings predicted by the model were those associated with the prevention and management of VTE events. On this basis, rivaroxaban may be considered to provide value for money to health payers in the US.
Transparency
Declaration of funding
This study was supported by Bayer HealthCare Pharmaceuticals and Johnson & Johnson Pharmaceutical Research & Development, LLC.
Declaration of financial/other relationships
Nishan Sengupta is an employee of Janssen Global Services, LLC.
Acknowledgment
The authors would like to acknowledge Michael Craig, who provided editorial support with funding from Janssen Scientific Affairs, LLC.
References
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines. 8th edn. Chest 2008;133(6 Suppl):381S‐453S
- Sullivan SD, Kahn SR, Davidson BL, et al. Measuring the outcomes and pharmacoeconomic consequences of venous thromboembolism prophylaxis in major orthopaedic surgery. PharmacoEconomics 2003;21:477‐96
- Agency for Healthcare Research and Quality. Healthcare Cost & Utilization Project (HCUP). United States Department of Health & Human Services 2009. Available at: http://www.ahrq.gov/data/hcup. Accessed September 2, 2011
- XARELTO full prescribing information. Titusville, NJ: Janssen Pharmaceuticals, Inc, 2011
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765‐75
- Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31‐9
- Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776‐86
- Turpie AG, Lassen MR, Davidson BL, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet 2009;373:1673‐80
- Baser O, Supina D, Sengupta N, et al. Impact of postoperative venous thromboembolism on Medicare recipients undergoing total hip replacement or total knee replacement surgery. Am J Health Syst Pharm 2010;67:1438‐45
- Sullivan SD, Davidson BL, Kahn SR, et al. A cost-effectiveness analysis of fondaparinux sodium compared with enoxaparin sodium as prophylaxis against venous thromboembolism: use in patients undergoing major orthopaedic surgery. PharmacoEconomics 2004;2:605‐20
- Sullivan SD, Kwong L, Nutescu E. Cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against venous thromboembolism in patients undergoing hip fracture surgery. Value Health 2009;9:68‐76
- Bishop B, Wilson AG, Post D, et al. A pilot study of home treatment of deep vein thrombosis with subcutaneous once-daily enoxaparin plus warfarin. J Manag Care Pharm 2006;12:70‐5
- Botteman MF, Caprini J, Stephens JM, et al. Results of an economic model to assess the cost-effectiveness of enoxaparin, a low-molecular-weight heparin, versus warfarin for the prophylaxis of deep vein thrombosis and associated long-term complications in total hip replacement surgery in the United States. Clin Ther 2002;24:1960‐86
- Spruill WJ, Wade WE, Leslie RB. Cost analysis of fondaparinux versus enoxaparin as venous thromboembolism prophylaxis in elective hip replacement surgery. Blood Coagul Fibrinolysis 2004;5:539‐43
- Friedman RJ, Gallus AS, Cushner FD, et al. Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin 2008;24:87‐97
- Quinlan DJ, Eikelboom JW, Dahl OE, et al. Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. J Thromb Haemost 2007;5:1438‐43
- White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998;158:1525‐31
- Bureau of Labor Statistics UDoL. Consumer Price Index. All urban consumers. Available at: http://www.bls.gov/news.release/cpi.t01.htm. Accessed September 2, 2011
- McGarry LJ, Thompson D, Weinstein MC, et al. Cost effectiveness of thromboprophylaxis with a low-molecular-weight heparin versus unfractionated heparin in acutely ill medical inpatients. Am J Manag Care 2004;10:632‐42
- DRG Expert: A Comprehensive Guidebook to the DRG Classification System. 24th edition. Salt Lake City, UT: Ingenix; 2008
- Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: an administrative claims analysis from 30 managed care organizations. J Manag Care Pharm 2007;13:475‐86
- Arias E. United States Life tables, 2006. Available at http://www.cdc.gov/nchs/data/nvsr/nvsr58/nvsr58_21.pdf. Accessed September 2, 2011
- Goldhaber SZ, Tapson VF. A prospective registry of 5,451 patients with ultrasound-confirmed deep vein thrombosis. Am J Cardiol 2004;93:259‐62
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. Oxford: Oxford University Press, 2006, pp. 256
- AnalySource Online. Available at: http://www.analysource.com [Last accessed 2 September, 2011]
- Centers for Medicare and Medicaid Services. Medicare DRG Payments. Available at: https://www.cms.gov/AcuteInpatientPPS/FFD/list.asp. [Last accessed 2 September, 2011]
- Beebe M, Dalton J, Espronceda M. Current Procedural Terminology (CPT): 2008 Professional edition. Chicago: American Medical Association, 2008
- Thompson Healthcare. Red book: pharmacy’s fundamental reference. Montvale, NJ: Thompson Healthcare, 2008
- Centers for Medicare and Medicaid Services. 2011. Clinical lab fee schedule, Health and Human Services. Available at: https://www.cms.gov/ClinicalLabFeeSched. Accessed September 2, 2011
- Gould MK, Dembitzer AD, Sanders GD, et al. Low molecular weight heparins compared with unfractionated heparin for treatment of acute deep vein thrombosis. Ann Intern Med 1999;130:789‐99
- Oster G, Tuden RL, Colditz GA. A cost-effectiveness analysis of prophylaxis against deep-vein thrombosis in major orthopedic surgery. JAMA 1987;257:203‐8
- Douketis JD, Eikelboom JW, Quinlan DJ, et al. Short-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of prospective studies investigating symptomatic outcomes. Arch Intern Med 2002;162:1465‐71
- Carter CJ. New developments in acute anticoagulation therapy: what improvements over traditional heparin are on the horizon? Postgrad Med 1996;99:129‐36