Abstract
Objective:
To conduct a cost-effectiveness analysis comparing roflumilast/tiotropium therapy vs tiotropium monotherapy in patients with severe-to-very severe COPD.
Methods:
The economic evaluation applied a disease-based Markov cohort model with five health states: (1) severe COPD, (2) severe COPD with a history of severe exacerbation, (3) very severe COPD, (4) very severe COPD with a history of severe exacerbation, and (5) death. Within a given health state, a patient may have a mild/moderate or severe exacerbation or die. Data from roflumilast clinical trials and published literature were used to populate model parameters. The model calculated health outcomes and costs for roflumilast/tiotropium therapy vs tiotropium monotherapy over a 5-year horizon. Incremental cost and benefits were then calculated as cost-effectiveness ratios, including cost per exacerbation avoided and cost per quality adjusted life year ($/QALY).
Results:
Over a 5-year horizon, the estimated incremental costs per exacerbation and per severe exacerbation avoided were $589 and $5869, respectively, and the incremental cost per QALY was $15,815. One-way sensitivity analyses varying key parameters produced an incremental cost per QALY ranging from $1963–$32,773.
Limitations:
A number of key parameters used in the model were obtained from studies in the literature that were conducted under different contexts. Specifically, the relative risk estimate for severe COPD patients originates from a small trial not designed to demonstrate the impact of roflumilast on frequency of exacerbations. In addition, the model extrapolates the relative risk estimates over periods of 5–30 years, even though the estimates were only observed in trials that spanned less than a year.
Conclusions:
The addition of roflumilast to tiotropium is cost-effective for the treatment of severe to very severe COPD patients.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive disease characterized by airflow limitation due to inflammation, obstruction or structural changes to the airways, which causes difficulty in breathingCitation1. While ∼12 million patients in the US have been diagnosed with COPD, it remains largely under-diagnosed, with total prevalence estimated at ∼24 millionCitation1. Patients with COPD experience periods of worsening symptoms including breathlessness, cough and sputum during exacerbations and exacerbations often lead to decreased lung functionCitation2. As the disease progresses, the severity and frequency of exacerbations rise, leading to hospitalization, significant patient disability and reduction in health-related quality-of-lifeCitation2. COPD ranked as the 3rd leading cause of death in the US as of 2009Citation3 and poses a major economic burden to the payers.
Typical treatment recommended by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines to patients with moderate or more severe COPD involves inhaled long-acting bronchodilatorsCitation4, which relaxes muscles around the bronchi to allow easier breathing. These bronchodilators relieve symptoms but fail to address underlying causes of airflow limitation in COPD patientsCitation5.Roflumilast is a once-daily oral phosphodiesterase-4 (PDE4) selective inhibitor that reduces the risk of exacerbations and is indicated for patients with severe-to-very severe COPDCitation6. Roflumilast is the first drug in its class approved in the US as maintenance treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of exacerbations.
In phase III clinical trials, roflumilast has demonstrated efficacy in improving lung function and reducing moderate-to-severe exacerbations. The placebo-controlled, double-blind, multi-center trials M2-124 and M2-125 found that COPD patients randomly assigned to roflumilast for 52 weeks had significantly reduced rates of moderate and severe exacerbationsCitation6. The double-blind, multi-center trials M2-127 and M2-128 studied COPD patients concomitantly being treated with long-acting inhaled bronchodilatorsCitation7. These trials found that patients who were randomized to take roflumilast for 24 weeks showed significantly improved lung function compared to patients taking long-acting inhaled bronchodilator monotherapy.
Despite these efficacy findings from clinical trials, no evaluation of the cost-effectiveness of roflumilast exists in the US in the published literature. It is thus important to assess the real-world value associated with this novel treatment of COPD. This study seeks to evaluate the cost-effectiveness of roflumilast/tiotropium therapy vs tiotropium monotherapy in patients with severe or very severe COPD. Tiotropium is a commonly-used long-acting bronchodilator for the maintenance treatment of COPD in the US. Therefore, US payers would be interested in understanding the cost-effectiveness of adding roflumilast to tiotropium relative to tiotropium alone. Using data from the phase III trials and administrative claims analyses of COPD treatment costs as well as scientific literature on COPD, we estimate incremental cost-effectiveness ratios (ICERs) per exacerbation avoided and per quality-adjusted life year (QALY) over time horizons of 5 and 30 years.
Methods
Model structure and assumptions
The disease-based cost-effectiveness model calculates exacerbations, QALYs and treatment costs. Incremental costs vs benefits associated with roflumilast/tiotropium therapy compared with tiotropium monotherapy are expressed as ICERs.
The modelling technique for the cost-effectiveness analysis is a disease-based Markov cohort model that consists of five health states representing the clinical progression of COPD which were defined using the lung function thresholds from the GOLD guidelinesCitation4. These were adapted to include the impact that severe exacerbations have on lung function (see )Citation2.
Figure 1. Markov mdel of five health states related to chronic obstructive pulmonary disease and the probability of transitioning between them. a Transition probabilities for each treatment cohort are from start state to end state, in direction of arrow. b Transition to the absorbing state Death is possible from all other states. R/T, roflumilast/tiotropium; T, tiotropium. One of three events is possible in each state: (1) non-severe exacerbation (mild and moderate exacerbations); (2) severe exacerbation; and (3) death.
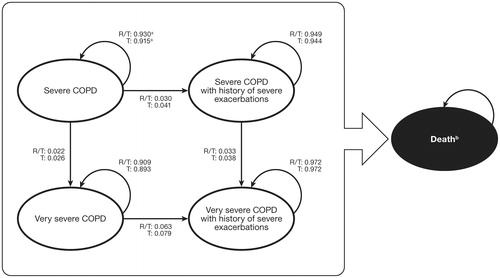
The health states include:
Severe COPD (30% ≤post-bronchodilator FEV1 <50% predicted),
Severe COPD with history of severe exacerbation,
Very severe COPD (post-bronchodilator FEV1 <30% predicted),
Very severe COPD with history of severe exacerbation, and
Death.
The model tracks events and associated health utilities (values assigned to health states, from 0 [death] to 1 [perfect health]) and economic outcomes for roflumilast/tiotropium therapy and tiotropium monotherapy over 3-month cycles for each treated cohort of patients. Model inputs for the clinical and economic outcomes associated with two alternative treatments are based on clinical trial results and published literature. Model outputs include clinical and economic outcomes that are aggregated over each 3-month cycle for the model time horizon.
This model was developed from a US payer perspective; therefore, it includes direct medical and drug treatment costs associated with COPD in the US. Given that COPD is a chronic and slowly-progressing disease with a long-term disease management requirement, a 5-year horizon was chosen for the base case analysis. This timeframe is a reasonable horizon for evaluating the impact of alternative treatments with different costs and outcomesCitation8. A 30-year timeframe was chosen as a lifetime horizon to analyze how model results potentially vary over time. Examining model results over a lifetime horizon is a standard analysis for a slowly-progressing disease in the literatureCitation9,Citation10. Benefits of treatment and costs in the future are discounted to the present at a rate of 3%.
At initiation, patients are in one of two states—severe or very severe COPD. The percentage of very severe patients was assumed to be 21% based on average rates reported in the literatureCitation8,Citation11,Citation12.
Transitions between health states can occur for any one of the following three reasons:
Natural decline in lung function associated with underlying COPD severity, which is faster than the rate of decline in the general population.
Patients experience their first severe exacerbation. This event transitions patients to a health state defined as having a history of severe exacerbation; these patients may then only transition to other states defined as having a history of severe exacerbationCitation2.
Patient dies. This event transitions patients to the absorbing state, death.
These model assumptions, along with other assumptions concerning key parameter inputs, are summarized in .
Table 1. Model assumptions.
Parameters
Transition probabilities
The probability of transitioning between health states, shown in , was estimated based on a published approach, assuming that all patients in the same health state had the same average initial level of FEV1 and average rate of declineCitation9. Transition probabilities were based on relative FEV1 decline for COPD patients in the M2-128 trial compared to individuals in the general population of the same sex and age, where the rate of decline for average healthy men and women was calculated based on predicted FEV1 for men and women aged 60–69 as a function of initial FEV1, age and heightCitation13,Citation14. A weighted average was then calculated to match the proportion of men vs women in the M2-128 trialCitation7. Although roflumilast/tiotropium patients have the same rate of decline of their lung function as tiotropium patients after the first 6 months of treatment, they do experience an initial increase in FEV1 levels upon receiving roflumilastCitation7. This initial increase results in the equivalent of a slower average rate of FEV1 decline. The relative decline in FEV1 was used to calculate the implied 3-month probability of transitioning to a more severe COPD stateCitation9. The initial post-bronchodilator FEV1 for severe patients was assumed to be 1.25 L (equivalent to 40% predicted). Based on published literature, a decrease in lung function of 0.05 L was assumed to occur after the first severe exacerbationCitation2,Citation15. The main inputs associated with this calculation and the resulting transition probabilities are presented in .
Table 2. Estimation of transition probability from severe to very severe COPD (tiotropium monotherapy vs roflumilast/tiotropium therapy).
In any cycle, a patient who is in any one of the four COPD health states may or may not experience an exacerbation or dieCitation9,Citation16. At the end of a cycle, some of these events may lead to a transition from the current health state to another health state. Because COPD is assumed to be non-reversible in the long termCitation10,Citation17, transitions from the very severe to the severe state are not allowed in the model.
For tiotropium patients, the probability of experiencing an exacerbation was based on the published literatureCitation10. Monthly exacerbation rates were converted to 3-month probabilities using the formula:
where P3 is 3-month probability and r1 is 1-month rate.
The probability of all-cause death in a 3-month cycle for severe and very severe patients in both treatment cohorts was also based on published literatureCitation16.
Relative risks
In the pooled analysis of key pivotal safety and efficacy trials M2-124 and M2-125, the overall rate of moderate or severe exacerbations per patient per year was lower in the roflumilast group than in the placebo group. This pattern was apparent regardless of whether patients had received an ICS before randomization or had concomitantly used a LABA or a SAMACitation18. The M2-128 study comparing roflumilast/tiotropium therapy vs tiotropium monotherapy included COPD exacerbations as a secondary endpoint. This study included patients with moderate-to-severe COPD and patients with severe COPD represented ∼33% of the overall study population. In this study, the proportion of patients who experienced moderate or severe exacerbations was 27% lower in the roflumilast group than in the tiotropium group, although the relative risk reduction did not reach statistical significance (p = 0.0867)Citation7.This 27% reduction in the risk of exacerbation corresponds to a relative risk of 0.73.
The assumed relative risk reduction of COPD exacerbation used in this model comes from both the M2-128 and M2-124/125 trials. The relative risk estimates for patients with severe COPD were based on M2-128 and the relative risk estimates for patients with very severe COPD were based on a sub-group analysis of pooled data from the M2-124/125 studies. The proportion of patients with very severe COPD who experienced moderate or severe exacerbations was 20% lower in the roflumilast group than in the placebo groupCitation19, which corresponds to a relative risk of 0.80. The relative risks for each health state were multiplied by the exacerbation probabilities for tiotropium patients to estimate the probability of an exacerbation for patients treated with roflumilast/tiotropium. presents the resulting 3-month probability of an exacerbation, as well as the probability of death, for each health state for each treated cohort.
Table 3. Three-month probability of exacerbation or death.
Costs
Treatment costs included in this model were based on an analysis of COPD costs using administrative claims dataCitation20. The analysis estimated three types of costs for COPD patients independent of disease severity:
COPD maintenance cost without exacerbation,
Cost of mild/moderate exacerbations and
Cost of severe exacerbations.
All-cause total cost estimates for the study quarters with no exacerbation were considered the maintenance cost for an average moderate COPD patient in the USCitation20. The literature on COPD suggests that these costs vary by disease severity and most published economic models of COPD allow at least some of these costs to depend on the disease stateCitation9,Citation21. This model assumes that maintenance costs for patients in the severe and very severe states are multiples of maintenance costs of the average moderate COPD patient in the US. No US study presenting maintenance costs of COPD by disease severity was identified; consequently, the ratios of maintenance costs for severe COPD to moderate COPD (1.365) and for very severe COPD to moderate COPD (1.902), reported in a recent study from the perspective of the Spanish National Health System, were used as multipliersCitation8. These multipliers are the lowest among a set of reviewed studies, making this a conservative assumptionCitation9,Citation12,Citation22,Citation23.
The cost of mild/moderate exacerbations used in the model was calculated as the difference in medical costs for 3-month periods during which such an exacerbation occurred vs 3-month periods in which no exacerbation occurredCitation20. A similar approach was used to calculate the medical cost of treating severe exacerbations. Costs associated with treating patients experiencing an exacerbation are considered an incremental cost in addition to the maintenance costs associated with medical management of a COPD patient.
Medical costs associated with end-of-life care, where a patient transitions from one health state to death, are added to the maintenance cost for medical management in each disease state because state transitions take place at the end of each cycle and it is assumed that patients can only experience one event per cycle. Without this assumption, patients who died in any 3-month period would incur lower medical costs than patients who did not experience an exacerbation. The medical cost associated with transitioning from any health state to death is set such that it is well within the range of end-of-life costs estimated by the Dartmouth Atlas of Health CareCitation24. Both exacerbation costs and medical costs associated with end-of-life care were conservatively assumed to be independent of COPD severity.
The 3-month cost of roflumilast, estimated based on a Wholesale Acquisition Cost (WAC) of $5.75 per 500 mcg, was added to total medical costs for patients in the roflumilast/tiotropium treated cohort. Perfect adherence to the roflumilast/tiotropium regimen was assumed during all 3-month cycles when patients were alive. The dosage and, therefore, the cost of roflumilast were independent of disease severity.
Utilities
COPD leads to lower health utilities (values assigned to health states, from 0 [death] to 1 [perfect health]). Published literature reported lower health state utility associated with patients with more severe COPD or patients with severe exacerbationsCitation9,Citation21. Health state utility estimates included in the model are based on those from a study in which the EQ-5D™ questionnaire was completed by 283 COPD patients in 1996Citation9. The EQ-5D, developed by the EuroQol Group, is a standardized questionnaire from which a single index value for health status can be generatedCitation25. The utility of death is set to zero, a standard approach. Utility for any given state is assumed to be the same across treatment arms. Utilities in states with history of severe exacerbation are assumed to be the same as in states without history of severe exacerbation.
presents all parameters used in the model.
Table 4. Model inputs.
Sensitivity analyses
One-way sensitivity analyses were conducted to assess the robustness of the model results to changes in the model parameters. Parameters were varied one at a time away from the base case value to determine upper and lower bounds on the 5-year ICER ($/QALY and $/exacerbation avoided). For succinctness, similar parameters were grouped into categories and the largest deviations from the base case ICER caused by changes to a parameter in the category were reported. The categories included relative risks, costs of exacerbation, number of years over which average FEV1 decline is calculated, percentage decline in FEV1, health state utilities, monthly exacerbation rates, cost multipliers, quarterly mortality and discount rate. Sensitivities of ICERs in terms of $/severe exacerbation avoided were conducted but are not shown because the relative importance of the parameters in those results resemble that of the $/exacerbation avoided results.
In addition to one-way sensitivity analyses, probabilistic sensitivity analysis (PSA) was conducted to evaluate the impact of simultaneously varying multiple model parameters. For every iteration of the PSA, each parameter was drawn from an appropriate distribution to determine the 5-year ICER ($/QALY). Parameters included in this analysis were those found in the one-way sensitivities to have the largest impact on the model. The distribution from which each parameter was drawn was based on conventionCitation26; the mean of each distribution equaled the value of the parameter in the base case. The parameters included in the PSA along with their distributions were listed in . This simulation procedure was repeated 10,000 times and the results were used to construct a cost-effectiveness acceptability curve (CEAC), which presented the probability of the treatment being cost-effectiveness for different values of the cost-effectiveness ratio threshold.
Table 5. Parameters included in probabilistic sensitivity analysis.
Results
Base case: Five year time horizon
Base case results are presented in . Results include the total number of exacerbations, stratified by severity, life years and quality adjusted life years, costs and incremental cost-effectiveness. Patients treated with roflumilast/tiotropium experienced fewer exacerbations, i.e., 6.379 vs 8.459. Over the model horizon of 5 years, patients treated with roflumilast/tiotropium lived 3.842 years vs 3.837 years for patients treated with tiotropium. These results translated into 2.438 and 2.361 QALYs for roflumilast/tiotropium treated patients and tiotropium patients, respectively.
Table 6. Base case (5 years) and lifetime horizon (30 years) results.
On average, the model predicted that patients treated with roflumilast/tiotropium would incur $140,217 in total healthcare costs vs $138,991 for those treated with tiotropium. Excluding the $9719 attributable to the cost of roflumilast, roflumilast/tiotropium patients incurred $130,497 in total healthcare costs compared to $138,991 for tiotropium patients.
also presents incremental cost-effectiveness ratios for various outcomes calculated by the model: cost per exacerbation avoided, cost per severe exacerbation avoided, cost per life year and cost per QALY. The incremental cost per exacerbation avoided was $589 and per severe exacerbation avoided was $5869. Over the 5-year model time horizon, the incremental cost per QALY was estimated to be $15,815.
Lifetime horizon
Model results from the 30-year time-horizon are also included in . Over the longer horizon, patients in both treatment groups experienced more exacerbations, accumulated additional LYs and QALYs, and incurred more costs than in the 5-year horizon. Also, like in the 5-year horizon, roflumilast/tiotropium patients had favorable results compared to tiotropium patients in terms of exacerbations, life years and other healthcare costs. The incremental costs per exacerbation avoided, per severe exacerbation avoided and per QALY were calculated to be $962, $8674, and $16,351, respectively, for patients taking roflumilast/tiotropium.
Sensitivity analysis
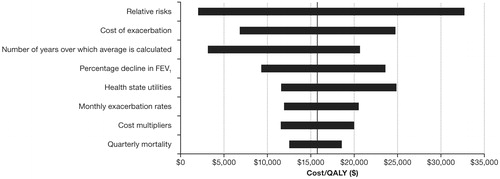
Results of one-way sensitivity analyses, in which each parameter was individually varied to analyze the effect on the model, are presented as tornado diagrams (see and ). shows the ranges within which each parameter was varied. In , the range of ICERs in terms of $/QALY produced by the model when groups of parameters are varied are shown. For example, the top bar shows the range of ICERs in terms of $/QALY when relative risk in the severe state is varied by 10%, holding all else fixed, or when relative risk in the very severe state is varied by 10%, holding all else fixed, to be $1963–$32,773. A 20% increase or decrease in the costs of exacerbation leads to a range of ICERs in terms of $/QALY of $11,871 –$20,580. A 50% increase or decrease in the percentage decline in FEV1 leads to a range of ICERs in terms of $/QALY of $6800–$24,829. Similarly, variations in a number of other parameters produce comparable or smaller ranges of ICERs in terms of $/QALY.
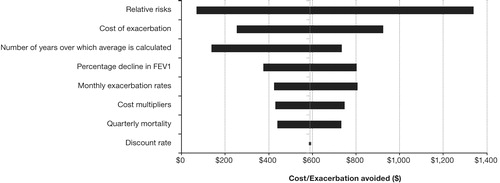
presents similar results for ICERs in terms of $/exacerbation avoided. These results show that ICERs in terms of $/exacerbation avoided showed the most sensitivity to the same parameters that had the most impact on ICERs in terms of $/QALY. A 10% increase or decrease in the relative risk results in ICERs in terms of $/exacerbation avoided to range from $68–$1340. A 20% increase or decrease in the cost of exacerbations results in ICER range in terms of $/exacerbation avoided of $253–$925. A 50% increase or decrease in the number of years over which average FEV1 is calculated leads to the ICER range in terms of $/exacerbation avoided of $137–$733. Variations in other parameters lead to comparable or smaller ranges of ICERs in terms of $/exacerbation avoided.
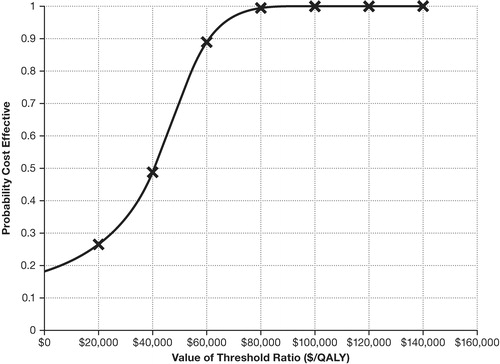
After 10,000 iterations in the PSA, the majority of resulting ICERs fall below common cost-effectiveness thresholds. The CEAC () shows that roflumilast/tiotropium dominated tiotropium in ∼20% of the trials. Moreover, for a threshold of $50,000/QALY, ∼70% of the simulated ICERs are cost-effective. At a threshold of $100,000/QALY, nearly 100% are cost-effective.
Discussion
Treatment of COPD places a significant economic burden on payers in the USCitation27. Roflumilast represents a new class of treatment for patients with severe COPD that reduces the risk of exacerbationsCitation6. In this study, the cost-effectiveness of roflumilast/tiotropium therapy vs tiotropium monotherapy was examined using a disease-based Markov cohort model. Over a 5-year horizon, the model estimated that the incremental cost avoided by adding roflumilast amounted to $589 per exacerbation and $5869 per severe exacerbation. The model estimated the incremental cost per QALY gained by adding roflumilast to be $15,815. The presence of uncertainty in the choice of model parameters emphasizes the need to evaluate the sensitivity of the results to changes in parameters. One-way sensitivity analyses indicated that the incremental cost per QALY may range from $1963–$32,773. Varying multiple parameters simultaneously in the probabilistic sensitivity analysis also showed that the majority of simulated trials were cost-effective. Together, these results indicate that the findings of roflumilast/tiotropium therapy’s cost-effectiveness remain robust to other reasonable choices for the model parameters.
A few parameters in the model displayed the most influence on the final ICERs. For example, relative risk drives differences in patient costs between roflumilast/tiotropium and tiotropium patients because it controls the rate at which patients in each treatment group experience exacerbations of any severity. As exacerbations are costly events for payers, differences in the exacerbation rates between treatment groups result in substantial differences in cost. Moreover, relative risk also indirectly influences the rate at which patients in each treatment group transition to develop very severe COPD. Patients who experience a severe exacerbation escalate in disease severity to very severe COPD faster than patients without a history of severe exacerbation. Thus, by controlling the rate of severe exacerbations, the relative risk also impacts costs through indirectly influencing the rate at which patients transition to the more costly health state.
The relative risk was estimated using data from clinical trials where most patients remained highly adherent to treatments. Patients in real world settings, on the other hand, often exhibit non-adherence. Consequently, the relative risk between tiotropium vs roflumilast/tiotropium therapy among patients in the real world may be higher than what is assumed in the model because non-adherence to roflumilast would reduce its effect on the risk of exacerbations. However, the oral once-daily formulation of roflumilast may help to lessen non-adherence in the real world. Under the non-adherence scenario, patients may incur higher COPD-related costs, but this effect on costs would also be mitigated by lower drug costs from non-adherence. Nonetheless, the relative risk used in the model may be under-estimated because the 0.8 estimate used for very severe COPD patients was based on published data from the M2-124/125 trials rather than the M2-128 trial, where the relative risk was 0.73 among moderate-to-severe COPD patients. If the model used a relative risk closer to the estimate from M2-128 for very severe COPD patients, then the model would show even more favorable ICERs for roflumilast/tiotropium therapy.
The difference in the rate of FEV1 decline among roflumilast/tiotropium and tiotropium patients acted as another key driver of ICERs because it also impacted the transition rate at which patients in either treatment group developed very severe COPD. Although roflumilast is not approved as a bronchodilator, based on data from the M2-128 trial, roflumilast/tiotropium patients experienced an increase in FEV1 and thus declined to very severe COPD at a slower rate. This effect is consistent with findings from other trials involving PDE4 inhibitors in COPD patientsCitation28. By decreasing the rate at which roflumilast/tiotropium patients advance to very severe COPD, this effect allows patients to avoid the high costs associated with very severe COPD for a longer time, thereby reducing total costs.
Results of the model also exhibited sensitivity to particular cost and utility parameters. As explained above, roflumilast impacts cost-effectiveness through two means: reducing the number of exacerbations and slowing the rate at which patients with severe COPD develop very severe COPD by increasing patients’ initial levels of FEV1 during the first 6 months of treatment. Thus, adjusting the utility and cost associated with exacerbations and with the maintenance cost of being in the very severe COPD state ultimately influences the total cost and QALY gains from treating patients with roflumilast/tiotropium relative to tiotropium monotherapy. For example, lowering the cost of exacerbations would reduce the impact of roflumilast because roflumilast reduces medical costs by decreasing the chance of exacerbations. These key cost and utility parameters include the cost and utility decrement associated with any exacerbation, the utility decrement associated with very severe COPD and the cost multipliers used to obtain very severe COPD maintenance costs.
Besides analyzing the sensitivity of the model results to changes in model parameters, this study also evaluated how model results changed when using a lifetime horizon. Over such a horizon, the model results in slightly increased ICERs per QALY and per exacerbation relative to the 5-year horizon. In the medium term (5 years), roflumilast offsets costs for patients by delaying the progression of the disease to very severe COPD, which is costly to treat. However, over the span of 30 years, almost all patients have progressed to very severe COPD. Once patients are in this state, the principal impact of roflumilast on relative costs and QALYs comes from reducing exacerbations, as patients’ disease cannot progress further, and mortality rates are assumed to be identical between the two cohorts. The relative effect of roflumilast is decreased; however, the cost of roflumilast is still incurred by treated patients. This leads to a relative rise in ICERs over the lifetime horizon.
The analyses in this study are subject to common problems relating to generalizability. A number of key parameters used in the model were obtained from studies in the literature that were conducted under different contexts. For example, exacerbation frequency by health state, mortality rate by health state, treatment costs and health state utilities were obtained from other studies where populations may display significant differencesCitation9,Citation10,Citation16,Citation20. In addition, relative risk parameters were obtained from the M2-128 and M2-124/125 trials, where patients enrolled in clinical trials are likely to differ from patients in actual clinical practice due to stringent inclusion and exclusion criteria. In particular, M2-128 was a small study not designed to demonstrate the impact of roflumilast on exacerbation. Thus, the projected reduction in exacerbations based on data from M2-128 needs to be confirmed by further clinical trials. Lastly, the model extrapolates estimates from the M2-124/125 and M2-128 trials over periods of 5–30 years. This extrapolation of effects has inherent limitations because these trials, respectively, spanned only 52 and 24 weeks. Interpretation of the final results from the model ought to give due consideration to these limitations of the data.
Current literature includes few cost-effectiveness studies for roflumilast and no studies using US cost data. This study captures the dynamics of costs and benefits associated with roflumilast treatment for this slowly-progressing disease over a time horizon of up to 30 years. Additionally, by using estimates from a recent administrative claims study, this study offers a current perspective on the cost-effectiveness of this novel treatment. Ultimately, this study demonstrates the cost-effectiveness of roflumilast/tiotropium therapy for US patients over time-horizons of 5 and 30 years. The addition of roflumilast to bronchodilator treatment reduces direct medical costs associated with the management of COPD by decreasing the number of exacerbations patients experience and slowing the rate at which patients’ COPD severity increases. Roflumilast/tiotropium’s potential ability to slow the rate of COPD severity progression also increases QALYs for treated patients in the short-term relative to tiotropium patients. However, this relative benefit is decreased after all patients have advanced to very severe COPD states in the long-term.
Conclusion
This economic evaluation suggests that, compared to treatment with tiotropium monotherapy, treatment with roflumilast/tiotropium therapy reduces direct medical costs, decreases exacerbations and increases QALYs for severe-to-very severe COPD patients in the US. These findings indicate that roflumilast is a cost-effective option for severe and very severe COPD patients.
Transparency
Declaration of funding
This research was sponsored by Forest Research Institute.
Declaration of financial/other relationships
S.S. and M. Mocarski are employees of Forest Research Institute. D.Y. was an employee of Forest Research Institute when this study was conducted. M. Marynchenko, R.B., D.C., A.Y. and E.W. are employees of Analysis Group, Inc., which has received consulting fees from Forest Research Institute for research related to this manuscript.
Acknowledgments
Prescott Medical Communications Group (Chicago, IL) provided editorial assistance with funding supported by Forest Research Institute, Inc., a wholly owned subsidiary of Forest Laboratories, Inc.
References
- National Heart, Lung and Blood Institute. Chronic obstructive pulmonary disease data fact sheet. Bethesda, MD: U.S. Department of Health and Human Services, NIH, NHLBI, 2003. Available at: www.uptakemedical.com/pdfs/copd_fact.pdf. Accessed February 28, 2011
- Anzueto A, Sethi S, Martinez FJ. Exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007;4:554-64
- Kochanek KD, Xu J, Murphy SL, et al. Deaths: preliminary data for 2009. National Vital Statistics Reports 2011;59:1-51
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (Update 2009). Available at: http://www.goldcopd.org/. Accessed March 3, 2010
- Rabe KF, Bateman ED, O’Donnell D, et al. Roflumilast—an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomized controlled trial. Lancet 2005;366:563-71
- Calverly PMA, Rabe KF, Goehring U-M, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet 2009;374:685-94
- Fabbri LM, Calverley PMA, Izquierdo-Alonso JL, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374:695-703
- Rutten-van Mölken MPMH, Oostenbrink JB, et al. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ 2007;8:123-35
- Spencer M, Briggs AH, Grossman RF, et al. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics 2005;23:619-37
- Borg S, Ericcson A, Wedzicha J, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health 2004;7:153-67
- Hoogendoorn M, Rutten-van Mölken MP, Hoogenveen RT, et al. A dynamic population model of disease progression in COPD. Eur Respir J 2005;26:223-33
- Dal NR, Eandi M, Pradelli L, et al. Cost-effectiveness and healthcare budget impact in Italy of inhaled corticosteroids and bronchodilators for severe and very severe COPD patients. Int J Chron Obstruct Pulmon Dis 2007;2:169-76
- Crapo RO, Morris AH, Gardner RM. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981;123:659-64
- McDowell MA, Fryar CD, Ogden CL, et al. Anthropometric reference data for children and adults: United States, 2003–2006 in National Health Statistics Reports, October 10, 2008
- Donaldson GC, Seemungal TAR, Bhowmik A, et al. Relationship Between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57:847-52
- Sin DD, Golmohammadi K, Jacobs P. Cost-effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. Am J Med 2004;116:325-31
- Mannino D. Chronic obstructive pulmonary disease: epidemiology and evaluation. Hospital Physician 2001;37:22-31
- Bateman ED, Rabe KF, Calverly PM, et al. Roflumilast with long-acting {beta}2 agonists for COPD: influence of exacerbation history. Eur Respir J 2011;38:553-60
- Hanania NA, Bateman ED, Calverley PM, et al. Benefits of Roflumilast Treatment on exacerbation outcomes in patients with COPD: pooled results from two pivotal 12-month studies. Presented at CHEST Annual Meeting Oct-Nov 2010, Vancouver, Canada
- Yu AP, Yang H, Wu EQ, et al. Incremental third-party costs associated with COPD exacerbations: a retrospective claims analysis. J Med Econ 2011;14:315-23
- Oostenbrink JB, Rutten-van Mölken MP, Monz BU, et al. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health 2005;8:32-46
- Nielsen R, Johannessen A, Askildsen JE, et al. The excess treatment-related costs of COPD by severity. A twelve-month follow-up study. Am J Respir Crit Care Med 2010;181:A4121
- Jansson SA, Andersson F, Borg S, et al. Costs of COPD in Sweden according to disease severity. Chest 2002;122:1994-2002
- Wennberg JE, Fisher ES, Sharp SM, et al. The care of patients with severe chronic illness: an online report on the Medicare Program by the Dartmouth Atlas Project; The Dartmouth Atlas of Health Care 2006. Available at http://www.dartmouthatlas.org/atlases/2006_Chronic_Care_Atlas.pdf. Accessed March 22, 2010
- EQ-5D: a standardised instrument for use as a measure if health outcome. Available at http://www.euroqol.org/home.html. [Last accessed 13 March, 2011]
- Briggs A, Claxton K, Sculpher M. Decision modelling for health economic evaluation. New York, NY: Oxford University Press, 2006
- Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest 2000;117(2 Suppl):5S-9S
- Brown WM. Treating COPD with PDE 4 inhibitors. Int J Chron Obstruct Pulmon Dis 2007;2:517-33