Abstract
Background:
Bone metastases are common in patients with advanced breast cancer, and place patients at risk for skeletal-related events (SREs) including pathologic fracture, spinal cord compression, hypercalcemia of malignancy, and the need for radiotherapy and/or surgery to bone. These SREs are associated with reduced survival and quality-of-life. The nitrogen-containing bisphosphonates Zometa (zoledronic acid, ZOL) and Aredia (pamidronate disodium, PAM) reduce SRE risk in patients with bone metastases from breast cancer. This database analysis compared SRE and mortality rates in a real-life setting in women with breast cancer receiving ZOL and PAM, and assessed long-term ZOL benefit.
Methods:
A retrospective, claims-based analysis was conducted using commercial and Medicare Advantage data from >45 US managed-care plans. Eligible adult patients had diagnoses for breast cancer and bone metastasis between 01/01/01 and 12/31/06, continuous enrollment in the health plan, and no evidence of bone metastasis or intravenous bisphosphonate (IV-BP) use for 6 months before their first ZOL or PAM infusion. Patients were followed until disenrollment (including mortality) or end of the analysis period (12/31/07). Persistency was defined as absence of a >45-day gap between IV-BP treatments.
Results:
Of 8757 patients (mean age, 58.1 [SD 12.4] years), ∼ 30% were treated with ZOL, 15% with PAM, and 55% with no IV-BP. Patients treated with ZOL had a moderately lower incidence of SREs (mean, 36.2 vs 40.0 SREs/100 person-years; p = 0.0707) and significantly lower mortality (mean, 6.5 vs 11.2 deaths/100 person-years; p < 0.001) compared with PAM-treated patients. Longer persistency with ZOL was associated with lower risk of fracture and all SREs (trend-test p = 0.0076 and p = 0.0200, respectively).
Limitations:
Interpretation of this claims-based analysis must be tempered by the inherent limitations of observational data, such as imbalances in patient populations and the potential for bias in treatment selection.
Conclusions:
This analysis suggests that fewer than half of breast cancer patients with bone metastases receive IV-BPs. Longer persistence with ZOL was associated with lower SRE risk, and ZOL-treated patients had longer survival and a non-significant trend toward fewer SREs compared with PAM.
Introduction
An estimated 65–75% of patients with advanced breast cancer develop bone metastasesCitation1. Patients with bone metastases are at increased risk for skeletal-related events (SREs) such as pathologic fractures, spinal cord compression, the requirement for surgery or palliative radiotherapy to bone, and hypercalcemia of malignancyCitation2. Approximately two-thirds of patients with bone metastases secondary to breast cancer develop SREs (i.e., ∼ 50% of all patients with advanced breast cancer), experiencing an average of three or four SREs each yearCitation3. These SREs can limit patients’ functional independence and undermine their quality-of-lifeCitation4 and place a heavy burden on affected patients and on the healthcare system. Pathologic fractures have also been associated with an increased risk of death; a retrospective analysis in patients with bone metastases from breast cancer (n = 1130) showed a significant correlation between pathologic fracture and death (hazard ratio [HR] = 1.32; p < 0.01)Citation5. Treatment of SREs is also associated with substantial increases in healthcare costsCitation6,Citation7.
Malignant bone disease is characterized by increased and imbalanced bone turnover, resulting in accelerated bone resorption and, sometimes, aberrant increases in bone formation. Bisphosphonates (BPs) are inhibitors of osteoclast-mediated bone resorptionCitation1. Numerous clinical studies have established that BPs reduce the risk of SREs, preserve quality-of-life, and may provide additional benefits for patients with malignant bone diseaseCitation1,Citation8. Nitrogen-containing BPs, such as ZOMETA (zoledronic acid, ZOL) and Aredia (pamidronate disodium, PAM), are the current standard of care in the US for the prevention of SREs from bone metastases secondary to breast cancerCitation9–11. (ZOMETA is a registered trademark of Novartis Pharmaceuticals Corporation, USA; Aredia is a registered trademark of Novartis Pharmaceuticals Corporation, USA.)
Although the dosing regimens of ZOL and PAM have been established, the most appropriate time to initiate treatment and the optimal duration of therapy have not been determined. In a head-to-head study, ZOL was demonstrated to be non-inferior to PAM and provided benefits beyond PAM in patients with breast cancer (n = 412), including significantly reducing the ongoing risk of SREs during the 25-month study (p = 0.025) and prolonging time to first radiotherapy to bone (p ≤ 0.05)Citation12.
Although clinical studies yield valuable information regarding the efficacy and safety of BPs, they often fail to consider how real-world use of these agents affects patient outcomes in clinical practice. Using a claims-based analysis of managed-care plan data, this retrospective analysis compared the SRE and mortality rates associated with ZOL and PAM in patients with bone metastases secondary to breast cancer, and assessed the effect of ZOL treatment duration on SRE and mortality rates. Therefore, the objectives of our study were to evaluate the relative efficacy of ZOL and PAM on SRE risk reduction, and to examine the influence of timing and duration of BP therapy on clinical outcomes in patients with bone metastases from breast cancer.
Methods
Data sources
This was a retrospective analysis using medical, pharmacy, and patient enrollment data obtained between July 1, 2000, and December 31, 2007, from two large managed-care databases. Medical claims are collected from healthcare sites (inpatient hospital, outpatient hospital, emergency room, physician’s office, surgery center, etc.) for provided services. Medical claims include diagnoses recorded with the International Classification of Diseases, Ninth Revision (ICD-9-CM) diagnosis codes, procedures recorded with ICD-9-CM procedure codes, Current Procedural Terminology (CPT) or Health Care Financing Agency (HCFA) Common Procedure Coding System (HCPCS) codes, site of service codes, provider specialty codes, revenue codes (for facilities), and paid amounts. Claims for ambulatory services submitted by individual providers use the HCFA-1500 format, and claims for facility services submitted by institutions use the UB-82 or UB-92 format. Typically, facility claims do not include any drugs administered in hospital. An interval of ∼6 months following the delivery of services was required for the medical claims data to be considered complete. Claims for pharmacy services are typically submitted electronically by the pharmacy at the time prescriptions are filled. Pharmacy claims data include drug name, dosage form, drug strength, fill date, days of supply, and de-identified patient and prescriber codes. Pharmacy claims are typically added to the research database within 6 weeks of dispensing.
Patient Selection
This analysis included commercial and Medicare Advantage health plan members with ≥1 medical claim for breast cancer (ICD-9-CM code 174.x) from January 1, 2001, through December 31, 2006. To be included in the analysis, members were also required to have evidence of bone metastasis and ZOL or PAM use during the same period. Bone metastasis was identified based on either the presence of ≥1 claim indicating bone metastasis (ICD-9-CM diagnosis code 198.5x) or the presence of ≥1 claim for breast cancer in any position and a diagnosis code 170.x (malignant neoplasm of bone and articular cartilage) in a secondary position. The date of the first claim for bone metastasis and ZOL or PAM use was set as the index date. To be included in the analysis, members were also required to be ≥18 years of age as of the index date and to have continuous health plan enrollment for ≥6 months before the index date. Members were excluded from the analysis if they had evidence of bone metastasis, PAM use, or monthly ZOL use during the 6 months before the index date. Pamidronate and ZOL were identified by NDC or HCPCS codes (PAM: J2430; ZOL: C9115, J3487). Members were also excluded for evidence of use of oral tiludronate, intravenous (IV) ibandronate, oral or IV etidronate, or yearly ZOL, identified by NDC or HCPCS codes (IV ibandronate: HCPCS C9229, J1740; IV etidronate: J1436; yearly ZOL: J3488, Q4095) at any time during the analysis period (July 1, 2000, to December 31, 2007).
Patients were permitted to have variable follow-up periods, with a maximum follow-up period of 7 years. The duration of follow-up was calculated as the number of days from the index date until the earliest of disenrollment from the health plan (including because of death), switch from ZOL to PAM (or from PAM to ZOL), or the end of the analysis period (December 31, 2007). Patients who died during an inpatient admission were identified from facility claims to distinguish disenrollment because of death vs disenrollment because of other factors that may not have been represented in the data. Patients who initiated treatment with ≥1 medical claim for PAM on or at any time following the index date were assigned to the PAM group, and patients who initiated treatment with ≥1 medical claim for ZOL on or at any time following the index date were assigned to the ZOL group. For patients who initiated both PAM and ZOL following the index date, the earlier claim was used for cohort assignment. Patients with no evidence of ZOL or PAM use on or following the index date were assigned to the group without IV-BP treatment (no IV-BP).
Patient demographic and treatment characteristics
Age was determined as of the index year, and insurance type (commercial or Medicare) and sex were determined from enrollment data. A Charlson-Quan comorbidity score was calculated based on the presence of diagnosis codes on medical claims in the pre-index period. Use of oral BPs (alendronate, ibandronate, and risedronate) during the pre-index period was identified from pharmacy claims. All chemotherapy received during the post-index period was identified by HCPCS and procedure codes.
Treatment discontinuation from ZOL was defined as the first appearance of a >45-day gap between treatments. The discontinuation date was defined as the service date for the last ZOL claim before the gap. Persistence was defined as the number of days from the first date of treatment with ZOL to the earlier of the discontinuation date, disenrollment, switch (to PAM), or end of the analysis period (December 31, 2007). Patients were assigned to a persistency category based on length of ZOL treatment (31–90 days, 91–180 days, 181–365 days, 366–546 days, 547–730 days, ≥731 days).
Analysis outcomes
Patient mortality was assessed during the post-index period. Occurrence of death was identified from facility claims based on patient discharge status for death on facility claims or a diagnosis code for death (ICD-9-CM 798.xx) on a facility or physician claim. Service dates at >60 and >90 days after the death date were analyzed for patients with a death indicator to confirm the mortality algorithm. The time to mortality was calculated as the number of days between the index date and the death date. Because only one of the two health plan databases used for this analysis included mortality data, patient mortality could only be determined for a sub-set of the patient sample. Fractures were identified from medical claims based on the presence of ICD-9-CM diagnosis codes () or CPT procedure codes for surgery to bone (). The occurrence of SREs was also determined in the pre-index and post-index periods. Evaluated SREs included fracture and surgery to bone (as described above), as well as spinal cord compression or radiation to bone. Spinal cord compression was identified from medical claims based on ICD-9-CM diagnosis code 336.9, and radiation to bone was identified from medical claims based on CPT procedure codes 77401–77406, 77418, 77422–77423, 77407–77411, 77412–77416, or 79101.
Table 1. ICD-9-CM codes to identify fractures.
Table 2. CPT procedure codes to identify fractures.
Statistical analysis
Unadjusted bivariate comparisons of baseline characteristics and outcome measures were performed using appropriate tests (e.g., t-test, Mann Whitney-U test, chi-square test) based on the distribution of the measure. As the length of follow-up time will vary, person-time was used because it estimates the actual time at risk (e.g., in years) that all persons contributed to the study. Incidence rates, allowing for variable follow-up time, were calculated using Stata® stptime version 9.2 (StataCorp, College Station, TX). Because of variability in the length of follow-up periods, analysis outcomes were measured as risk per 100 person-years. A Fisher’s exact test was employed to test for differences between treatment groups and differences across persistency categories (e.g., 91–180 days, 181–365 days). Multivariate analysis adjusting for covariates was conducted using the Cox proportional hazards model. Confounding factors for which adjustments were made include age, pre-index Charlson-Quan comorbidity score, pre-index evidence of oral BP use, and pre-index skeletal events. Analyses were conducted using Stata statistical software version 10.0 (StataCorp).
Results
Baseline demographics and patient characteristics
Among 8757 patients included in this analysis, 2639 (30%) had received ZOL (ZOL group), 1303 (14.9%) had received PAM (PAM group), and 4815 (55%) had not been treated with IV-BPs (no IV-BP group) (). Patients in the ZOL group were, on average, older than patients in the PAM (p = 0.0130) or no IV-BP group (p = 0.0006). Additionally, enrollment in a commercial insurance plan was more common and enrollment in Medicare Advantage was less common in the ZOL group vs the PAM group (p = 0.0006) or no IV-BP group (p < 0.0001). The pre-index Charlson comorbidity score was similar among patients who received ZOL and PAM (p = 0.7038), but was significantly higher among ZOL-treated patients vs no IV-BP patients (p < 0.0001). Patients in the ZOL group were more likely than those in the PAM (p = 0.0009) or no IV-BP group (p < 0.0001) to receive oral BPs during the pre-index period, although the incidence of SREs among the three treatment groups was similar during the pre-index period. Patients in the ZOL group had a longer average length of follow-up (575.16 days) than patients in the PAM (522.21 days, p = 0.0004) or no IV-BP group (491.63 days, p < 0.0001). Also, patients treated with ZOL were more likely than patients in the no IV-BP group to receive adjuvant chemotherapy (p < 0.0001).
Table 3. Patient demographics and clinical characteristics.
Clinical outcomes
The SRE and death rates were lower among patients treated with ZOL vs PAM (). In a multivariate Cox proportional hazards model that adjusted for age, pre-index Quan-Charlson comorbidity score, pre-index evidence of oral BPs, and pre-index skeletal complications, ZOL reduced the relative risk of an SRE vs PAM (HR = 0.58; p < 0.001). In unadjusted analyses, there was a non-significant trend toward a lower mean skeletal morbidity rate with ZOL vs PAM (36.2 vs 40.0 per 100 person-years; p = 0.0707). Moreover, the death rate was a statistically significant 42% lower for ZOL vs PAM (6.5 vs 11.2 per 100 person-years; p < 0.001) ().
Figure 1. Kaplan-Meier survival estimates of patients with bone metastases and breast cancer treated with zoledronic acid or pamidronate; p < 0.001. PAM, pamidronate; ZOL, zoledronic acid.
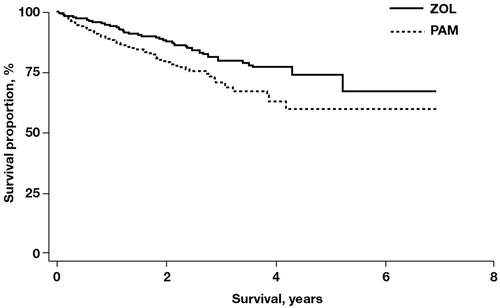
Table 4. Risk of SREs and mortality in patients treated with zoledronic acid or pamidronate.
Relationship between treatment persistency and skeletal-related event risk
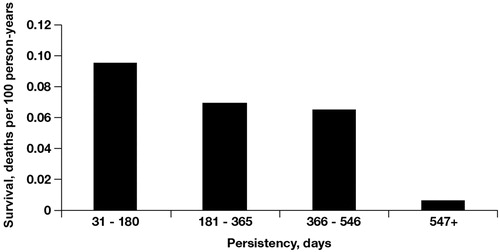
The length of uninterrupted ZOL treatment was used to assign patients to persistency categories. Approximately 87% of ZOL-treated patients received uninterrupted therapy for no more than 1 year, and the remaining 13% received ZOL for longer than 1 year. Patients treated with monthly infusions of ZOL had a significantly lower risk of SREs, including fractures, compared with patients treated less frequently (). Persistent use of monthly ZOL for ≥18 months was associated with a 50% lower risk of a SRE and a 63% lower risk of a fracture compared with persistent monthly use of ZOL for ≤3 months.
Figure 2. Risk of ≥1 event per 100 person-years by zoledronic acid persistency. Skeletal-related event (SRE) trend, p = 0.0216; fracture trend, p = 0.003.
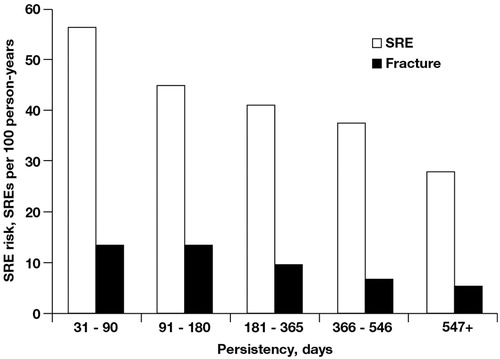
Analysis outcomes were also evaluated using a Cox proportional hazards model adjusted for age, pre-index Quan-Charlson, pre-index evidence of oral BP, and pre-index skeletal complications. The overall adjusted risk of SREs (p = 0.0200) and the overall adjusted risk of fracture (p = 0.0076) were lower among ZOL-treated patients than among no IV-BP patients. Also, the adjusted risk of fracture was lower among patients receiving ZOL for ≤365 days than among no IV-BP patients (p ≤ 0.057). However, statistically significant differences in the adjusted risk of fracture for ≥366 days or in the adjusted risk of SREs were not observed between ZOL-treated and no IV-BP patients ().
Table 5. Relationship between zoledronic acid persistency and skeletal-related events.
Relationship between treatment persistency and mortality
Patients treated with monthly infusions of ZOL for increasingly longer periods of time (persistency) had significant reductions in mortality rates compared with patients treated less frequently (p = 0.003 for trend; ). Persistent use of monthly ZOL for ≥18 months was associated with a 93% lower mortality rate than use for ≤3 months.
Effect of a delay in treatment initiation on clinical outcomes
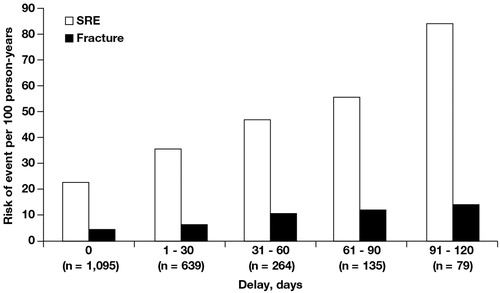
Patients who initiated ZOL treatment shortly after diagnosis of bone metastasis (within 2 weeks) experienced significantly fewer SREs, including fractures, compared with patients in whom initiation of ZOL was delayed for ≥60 days (). The risk of SREs per 100 person-years was 22.7% with early ZOL initiation compared with 47.02% for delayed initiation. Similarly, the risk of a fracture per 100 person-years was 4.3% with early ZOL initiation vs 10.2% with delayed initiation.
Discussion
This retrospective analysis revealed that in a real-world setting the majority of breast cancer patients diagnosed with bone metastases fail to receive IV-BP therapy. Among patients who did receive IV-BPs, SRE rates were numerically lower and mortality rates were significantly lower in ZOL-treated patients than in PAM-treated patients. This is consistent with the superiority of ZOL over PAM demonstrated in secondary endpoints of a prospective, phase 3, head-to-head trial in breast cancerCitation12. During the 25-month course of the study, ZOL reduced the risk of SREs by an additional 20% relative to PAM (p = 0.025; n = 412)Citation12. Among patients with breast cancer and ≥1 osteolytic lesion, ZOL lowered the risk of developing SREs by 30% beyond the effects of PAM (p = 0.010; n = 528)Citation13. These results suggest that ZOL has benefits beyond PAM in patients with breast cancer both in clinical trials and clinical practice.
Although the optimal timing and duration of IV-BP therapy remain to be determined, the current retrospective analysis provides additional insight into the importance of persistence and duration of IV-BP therapy on clinical outcomes. Among ZOL-treated patients, the greatest reductions in SRE and mortality rates were observed among patients receiving early and sustained ZOL therapy. Patients who initiated therapy within 2 weeks of diagnosis and patients who were treated regularly with ZOL for ≥18 months had reduced SRE and mortality rates compared with patients who initiated therapy later or who were persistent with therapy for a shorter duration, respectively. These results are consistent with a previous retrospective analysis of a national medical claims database (n = 4546) in which greater persistency with ZOL was also associated with lower rates of SREs (p < 0.05) and a longer duration of follow-up (possible surrogate endpoint for survival; p < 0.05)Citation14. The results of these claims-based analyses are consistent with expert recommendations that BPs be initiated as soon as bone metastases are diagnosed and continued for as long as they are toleratedCitation15.
Although prevention of potentially life-limiting or debilitating SREs could contribute to improved survival with IV-BPs, previous studies comparing PAM with placebo (n = 382)Citation16 and ZOL with placebo (n = 228)Citation17 have not demonstrated a survival benefit with BP therapy. This may be a result of the limited size and duration of these studies (i.e., 24 and 12 months, respectively) that could have prohibited identification of modest survival benefits. Furthermore, only ∼20% of patients completed 24 months of therapy in the PAM trialCitation16. It is also interesting to note that even in the larger phase 3 trial comparing denosumab with ZOL in patients with bone metastases from breast cancer (n = 2046) there was no relative survival benefit for either of the antiresorptive agents after a median follow-up of 17 monthsCitation18. This suggests that superior SRE risk reduction alone is not sufficient to prolong survival. We hypothesize that the reduction in mortality rates among ZOL-treated vs PAM-treated patients (n = 3942) may be related to the size of the population and the duration of follow-up (up to 7 years) in our study, and may also be related to the potential anti-cancer activity of ZOL observed in pre-clinical studies. Specifically, ZOL directly and indirectly inhibits multiple steps in cancer development and progression, including cancer cell adhesion and invasion, cancer cell proliferation, and angiogenesisCitation19,Citation20. Both ZOL and PAM have been shown to stimulate cancer cell apoptosis and activate anti-cancer immune responses, including expansion of gammadelta (γδ) T cells, which play a key role in immune surveillance against neoplasiaCitation19,Citation20. Pre-clinical studies have also reported that ZOL possesses inherent anti-cancer activity and exhibits synergy with cytotoxic agents in various cancer typesCitation21–23.
Consistent with benefits beyond bone health, ZOL treatment has resulted in improved clinical outcomes in recent studies among patients with early or advanced cancer. Several pilot clinical studies reported that, compared with adjuvant hormonal therapy or chemotherapy alone, ZOL reduced the persistence of disseminated tumor cells in the bone marrow of patients with breast cancerCitation24–26. In the ABCSG-12 study, addition of ZOL to adjuvant endocrine therapy resulted in a relative reduction of 36% in the risk of disease progression compared with endocrine therapy alone in pre-menopausal women with early breast cancer (HR = 0.64; p = 0.01; n = 1803)Citation27, and has now demonstrated improved overall survival (HR = 0.59; p = 0.042) at a median follow-up of 76 monthsCitation28. The ZO-FAST study (n = 1065) demonstrated that the immediate addition of ZOL to adjuvant letrozole produced a relative reduction of 41% in the risk of disease recurrence or death after 36 months in post-menopausal women with early breast cancer (HR = 0.59; p = 0.0314 vs delayed initiation of ZOL [e.g., only for severe bone loss or fracture])Citation29. In the AZURE study (n = 3360), which evaluated the addition of ZOL to a variety of anti-cancer regimens in patients with early breast cancer, women who were >5 years post-menopause at baseline had significantly better disease-free survival (HR = 0.75; p < 0.02) and overall survival (HR = 0.74; p = 0.04) vs women who did not receive ZOLCitation30. An exploratory sub-group analysis of AZURE (n = 205) reported that ZOL plus neoadjuvant chemotherapy reduced residual invasive tumor size compared with chemotherapy alone (p = 0.006). The pathologic complete response rate (breast and axilla) was also higher in the ZOL-plus-chemotherapy arm (11.7%) compared with the chemotherapy-alone arm (6.9%; p = 0.146)Citation31. Several studies have also reported that ZOL can improve overall survival vs no ZOL among patients with other malignancies, including multiple myeloma, bladder cancer, and lung cancerCitation32–34. The largest and most recent of these studies, the Medical Research Council Myeloma IX study, reported that ZOL significantly increased overall survival (p = .012) and progression-free survival (p = .018) compared with the early-generation BP clodronate in patients with newly diagnosed multiple myeloma (n = 1960)Citation33. Survival benefits were independent of SRE prevention, suggesting that ZOL may improve survival via anti-cancer activityCitation33. In addition, BP use for the treatment or prevention of osteoporosis in post-menopausal women has been associated with reduced risk of breast cancerCitation35–37.
The current retrospective analysis provides important insight, but must be interpreted with several limitations in mind. Although claims data are valuable for the examination of healthcare outcomes and treatment patterns, they are collected for the purpose of payment, not research. Therefore, certain limitations are associated with the use of claims data. First, the presence of a claim for a filled prescription does not indicate that the medication was consumed or administered as prescribed. Second, medications filled over-the-counter or provided as samples by the physician are not observed in the claims data. Third, the presence of a diagnosis code on a medical claim may, in some cases, not indicate disease presence, as the diagnosis code could be incorrectly coded or included as rule-out criteria rather than actual disease. Fourth, certain information is not readily available in claims data that could have an effect on study outcomes, such as certain clinical and disease-specific parameters. For example, there is guidance regarding ZOL dosing adjustments for patients with impaired renal function. However, laboratory values were not available for the full study sample and were not used in the current analysis; IV-BP therapy might have been contraindicated because of laboratory values in the no IV-BP group. Moreover, this analysis used an algorithm to identify death in the claims; however, identification of death in the claims without the use of corroborating data may under-estimate mortality, and length of follow-up time is only a surrogate estimate of survival. Finally, this analysis did not include prospective, randomized patient cohorts. Results presented are based on analysis of observational data wherein patients were not randomized to treatment, time of treatment initiation, or length of persistency. Clear imbalances existed between the patient populations, suggesting the potential for bias in treatment selection. Nevertheless, despite these limitations, claims-based data provide healthcare professionals with crucial information regarding how real-world BP use affects clinical outcomes in patients with malignant bone disease. Although BPs have been the cornerstone in the management of SREs in this patient population for many years, denosumab, a monoclonal antibody that inhibits the receptor activator of nuclear factor kappa-B ligand, has recently emerged as another potential treatment option. Denosumab has been approved for prevention of SREs in patients with bone metastases from solid tumors, including breast cancer. However, limited experience exists with denosumab in advanced cancers outside of the clinical trial setting. Therefore, additional follow-up is needed to determine the potential effects of treatment discontinuations and if the efficacy demonstrated in clinical trials will translate to clinical practice.
In conclusion, IV-BPs are under-utilized in general, and ZOL-treated patients have lower SRE and mortality rates compared with PAM-treated patients with breast cancer and bone metastases in a real-world setting. Early and sustained ZOL treatment may improve clinical outcomes among patients with malignant bone disease. Further evaluations of optimal usage of IV-BPs are needed in the clinical practice setting.
Transparency
Funding
Novartis Pharmaceuticals Corporation provided funding for this research and financial support for medical editorial assistance.
Financial/other relationships
HJH is an employee of OptumInsight. OptumInsight received funding from Novartis to conduct the research. SK was employed by Novartis Pharmaceuticals Corporation during the time that this manuscript was developed.
Acknowledgments
We thank Ann Marie Fitzmaurice, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript. Presented in part at the 7th European Breast Cancer Conference (EBCC-7) Barcelona, Spain, March 24–27, 2010.
References
- Coleman RE. Bisphosphonates: clinical experience. Oncologist 2004;9:14-27
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94
- Lipton A, Theriault RL, Hortobagyi GN, et al. Pamidronate prevents skeletal complications and is effective palliative treatment in women with breast carcinoma and osteolytic bone metastases: long term follow-up of two randomized, placebo-controlled trials. Cancer 2000;88:1082-90
- Weinfurt KP, Castel LD, Li Y, et al. Health-related quality of life among patients with breast cancer receiving zoledronic acid or pamidronate disodium for metastatic bone lesions. Med Care 2004;42:164-75
- Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer 2007;110:1860-7
- Botteman M, Barghout V, Stephens J, et al. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006;17:1072-82
- Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol 2006;4:341-7
- Lipton A. Bisphosphonate therapy in the oncology setting. Expert Opin Emerg Drugs 2003;8:469-88
- Zometa® (zoledronic acid) Injection [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation. 2011
- Aredia® (pamidronate disodium) for injection [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2011
- Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des 2010;16:1262-71
- Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003;98:1735-44
- Rosen LS, Gordon DH, Dugan W, Jr. et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer 2004;100:36-43
- Hatoum HT, Lin SJ, Smith MR, et al. Zoledronic acid and skeletal complications in patients with solid tumors and bone metastases: analysis of a national medical claims database. Cancer 2008;113:1438-45
- Aapro M, Abrahamsson PA, Body JJ, et al. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol 2008;19:420-32
- Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocol 19 Aredia Breast Cancer Study Group. J Clin Oncol 1998;16:2038-44
- Kohno N, Aogi K, Minami H, et al. Zoledronic acid significantly reduces skeletal complications compared with placebo in Japanese women with bone metastases from breast cancer: a randomized, placebo-controlled trial. J Clin Oncol 2005;23:3314-21
- Stopeck AT, Lipton A, Body JJ, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol 2010;28:5132-9
- Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev 2008;34:453-75
- Lipton A. Emerging role of bisphosphonates in the clinic–antitumor activity and prevention of metastasis to bone. Cancer Treat Rev 2008;34:S25-S30
- Senaratne SG, Pirianov G, Mansi JL, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer 2000;82:1459-68
- Ottewell PD, Monkkonen H, Jones M, et al. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst 2008;100:1167-78
- Neville-Webbe HL, Rostami-Hodjegan A, Evans CA, et al. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer 2005;113:364-71
- Aft R, Naughton M, Trinkaus K, et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol 2010;11:421-8
- Rack B, Jückstock J, Genss E, et al. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res 2010;30:1807-13
- Lin AY, Park JW, Scott J, et al. Zoledronic acid as adjuvant therapy for women with early-stage breast cancer and disseminated tumor cells in bone marrow. Presented at 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, 30 May – 3 June 2008. Abstract 559
- Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med 2009;360:679-91
- Gnant M, Mlineritsch B, Luschin-Ebengreuth G, et al. Overall survival with adjuvant zoledronic acid in premenopausal breast cancer patients with complete endocrine blockade—long-term results from ABCSG-12. Poster presented at 2011 ASCO Annual Meeting, Chicago, IL, 3–7 June 2011. Abstract 520
- Eidtmann H, de Boer R, Bundred NJ, et al. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol 2010;21:2188-94
- Coleman RE, Marshall H, Cameron D, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011;365:1396-1405
- Coleman RE, Winter MC, Cameron D, et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer 2010;102:1099-105
- Zaghloul MS, Boutrus R, El-Hosieny H, et al. A prospective, randomized, placebo-controlled trial of zoledronic acid in bony metastatic bladder cancer. Int J Clin Oncol 2010;15:382-9
- Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 2010;376:1989-99
- Zarogoulidis K, Boutsikou E, Zarogoulidis P, et al. The impact of zoledronic acid therapy in survival of lung cancer patients with bone metastasis. Int J Cancer 2009;125:1705-9
- Chlebowski RT, Chen Z, Cauley JA, et al. Oral bisphosphonate use and breast cancer incidence in postmenopausal women. J Clin Oncol 2010;28:3582-90
- Newcomb PA, Trentham-Dietz A, Hampton JM. Bisphosphonates for osteoporosis treatment are associated with reduced breast cancer risk. Br J Cancer 2010;102:799-802
- Rennert G, Pinchev M, Rennert HS. Use of bisphosphonates and risk of postmenopausal breast cancer. J Clin Oncol 2010;28:3577-81