Abstract
Objective:
Decision-makers in the US may be interested in the applicability to their populations of cost-effectiveness results generated from clinical trial populations.
Methods:
An economic model estimating the cost-effectiveness of prasugrel plus aspirin relative to clopidogrel plus aspirin for patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI) was developed from a managed care organization (MCO) perspective. The model estimated 15-month cardiovascular events or bleeding-related outcomes, life expectancy, and costs for patients who received thienopyridine treatment during and after a PCI following a diagnosis of ACS. Post-ACS event rates for patients treated with clopidogrel were from an MCO. The relative risks of these events with prasugrel compared with clopidogrel were from a head-to-head clinical trial.
Results:
The results of the base-case analysis indicated that, in an MCO population, use of prasugrel-based therapy rather than clopidogrel-based therapy at current prices resulted in cost-savings and fewer clinical events over the 15 months after an ACS diagnosis followed by PCI. At possible lower prices for generic clopidogrel-based therapy, the cost-effectiveness ratio for prasugrel-based therapy compared with clopidogrel-based therapy was between $6643 and $13,906 per life-year gained. The results were most sensitive to the relative costs of the two treatments and the cost for hospital stays.
Limitations:
Limitations of the study included lack of follow-up of patients disenrolling from the MCO before the end of the 15-month observation period, the assumption of equal relative risks of events in an MCO as in the clinical trial, and the lack of information on the ratio of cost to charges in the MCO database.
Conclusions:
Use of prasugrel-based therapy compared with clopidogrel-based therapy in ACS patients having a PCI resulted in cost-savings at current prices and favorable cost-effective ratios at likely generic prices for clopidogrel-based therapy because of offsetting savings in the costs of rehospitalization.
Introduction
Improved pharmacologic management and increased use of percutaneous coronary revascularization procedures have led to improved short-term outcomes in patients presented with acute coronary syndrome (ACS). However, late adverse outcomes including rehospitalization, recurrent myocardial infarction (MI), and mortality still hamper the recovery process and affect clinical practiceCitation1.
Antiplatelet drugs that are able to prevent excessive aggregation of platelets are valuable in the treatment of an ACS episode. Studies of aspirin alone or in combination with clopidogrel or prasugrel have shown a reduced risk of death and cardiovascular (CV) events following an ACS episode, while having increased bleeding rates associated with more effective therapiesCitation2–6.
Several publications have presented estimates of the cost-effectiveness and/or cost-utility of clopidogrel plus aspirin (clopidogrel-based therapy) compared with aspirin aloneCitation7–11 or of prasugrel plus aspirin (prasugrel-based therapy) compared with clopidogrel-based therapyCitation12 in the US. The results of these studies indicated cost-effectiveness ratios of well under $50,000 per life-year (LY) gained for clopidogrel-based therapy compared with aspirin, with base-case estimates ranging from $935 to $15,696 per LY gained and cost-savings with prasugrel-based therapy compared with clopidogrel-based therapy due to reductions in CV events in the period following an ACS episode.
Decision-makers in the US may be interested in the applicability of cost-effectiveness results generated from clinical trial populations to general practice populations. The goal of this paper was to generate estimates of the cost-effectiveness of prasugrel-based therapy compared with clopidogrel-based therapy in a managed care organization (MCO) population, with its different characteristics, practice patterns, and adherence patterns, compared with a clinical trial population.
Methods
Model overview
Model structure
A disease-progression model was developed from a US payer (e.g., MCO) perspective using Microsoft Excel software. Patient events and costs were estimated for ACS episodes and percutaneous coronary intervention (PCI) treatment during the index hospitalization, as well as at 30 days, at 1 year, and at 15 months. In addition, to estimate the cost-effectiveness of prasugrel-based therapy compared with clopidogrel-based therapy, patients’ remaining life expectancy for those surviving to 15 months was estimated based on the events experienced in the first 15 months. Life expectancy was discounted back to the index ACS episode at 3%.
In the model, once discharged from the hospital, patients could experience CV events or a bleeding event that could result in an emergency room/department (ER) visit or in further hospitalization. The monthly event rates for months 2–15 were estimated by dividing the total number of events observed during months 2–15 by the person-months of enrollment for the index population in the i3 InVision database during this time period.
The model included several assumptions. Estimates were based on the assumption that the relative risks of events for the two treatments are the same in the MCO setting as those observed in the clinical trial. Patients who left the plan, either because of death or disenrollment, were assumed to no longer be at risk for further CV or bleeding events that must be treated and paid for by the health plan. The percentage of patients that left the plan that were due to mortality was assumed to be the same for both treatmentsCitation6. Discontinuations of prasugrel-based therapy before 15 months, either because of leaving the plan or choosing to discontinue its use, were assumed to be the same as those observed for clopidogrel-based therapy in the i3 InVision databaseCitation6. After discontinuation of the initial therapy, all patients were assumed to continue taking aspirin only for the remaining 15 months or for as long as they remained enrolled in the MCO. There were assumed to be no crossovers between the arms. The life-expectancy gain from the MCO perspective was assumed to include only the gain from those patients who stayed enrolled for the full 15-month time period.
Only health utilization (in or out of network) billed through the MCO would be part of the database records for the clopidogrel cohort.
Model outcomes
Costs and health outcomes were reported for each treatment arm and time horizon and included the following: costs disaggregated into thienopyridine drug, ER visits, and inpatient stays, including hospital costs and physician fees; number of CV and bleeding events; number of rehospitalizations; and number of LYs gained (for lifetime horizon only).
Model input values
Clopidogrel-based therapy CV-related and bleeding-related event rates
The CV event rates for those patients taking a clopidogrel-based therapy regimen after an ACS episode with PCI were estimated from an analysis of the i3 InVision database and are presented in .
Table 1. Monthly CV-related and bleeding-related event rates after an ACS episode with PCI, per 100 patientsCitation13,Citation14.
The follow-up period for the database sample was 15 months, which for each patient began on the discharge date of the index hospital stay. ER visits that did not result in admission to the hospital were recorded separately. CV-related readmissions were classified according to their associated Diagnosis-Related Group code. Revascularization procedures were considered to be any hospitalization with a Diagnosis-Related Group code for a PCI, stent, and/or coronary artery bypass graft (codes 106, 124, 125, 518, 550, 526, 527, 549, and/or 555–558). A more detailed description of the database and the patient sample included in the analysis is provided in the supplemental appendix.
Because of difficulty in accurately identifying treatment-related bleeding events in the i3 InVision database, the rates for bleeding events requiring hospitalization for base-case estimates were taken from the TRITON-TIMI 38 eight-country economic cohort data and are presented in . Alternative bleeding rates were tested in the sensitivity analyses. For an upper bound, the model used the rates observed in a study of the risk of bleeding with clopidogrel therapy after implantation of a drug-eluting stent in an MCO populationCitation15. For a lower bound, the model used a rate based on the i3 InVision database rate of hospitalization for gastrointestinal hemorrhages, which was lower than that in the trial population. Gastrointestinal hemorrhages are serious bleeding events sometimes seen with ACS episodes, and could plausibly be attributable to thienopyridines.
The risk of bleeding with each treatment regimen was adjusted downward to the published aspirin-only level, 0.78 (95% confidence interval: 0.60, 1.02) of the clopidogrel-based therapy bleed rateCitation16, for the time that patients were not taking a thienopyridine but remained enrolled in the MCO.
Prasugrel-based therapy CV-related and bleeding-related event rates
The relative risk for prasugrel-based therapy compared with clopidogrel-based therapy for each type of CV- or bleeding-related event observed in the enrollees in the TRITON-TIMI 38 trial 8-country economic cohort was used to calculate the monthly probability of having an event for patients in the database who were taking prasugrel-based therapy within each time period (index hospital to 30 days and 31 days to 15 months). presents the relative risks for events with prasugrel-based therapy. The same relative risk for prasugrel-based therapy compared with clopidogrel-based therapy was applied to all population sub-groups.
Disenrollment or mortality rates and life expectancy
In the i3 InVision database, 35% of the clopidogrel-based therapy sample disenrolled before 15 months. The database did not present the reasons for disenrollment, which might have been death, change of employment, or change of health plan. In any of these cases, disenrollees ceased to pay premiums and incur healthcare expenses. The mortality rates were assumed to be the same for the clopidogrel-based therapy and prasugrel-based therapy treatment groupsCitation6.
Life-expectancy gains associated with prasugrel-based therapy compared with clopidogrel-based therapy were estimated only for patients who remained enrolled for the full 15 months after the index episode, thus under-estimating the likely impact on life expectancy of the index population. The estimated gains were based on the rate of non-fatal MIs and strokes experienced in the first 15 months after the index ACS episode, and on published life-expectancy estimates from the Framingham Heart StudyCitation17,Citation18 for different age ranges. These are presented in Appendix in the supplemental appendix.
Thienopyridine drug costs
Drug costs were calculated from the loading doses ($24.32 for clopidogrel and $36.42 for prasugrel) and daily maintenance dose ($6.08 for 75-mg clopidogrel and $6.07 for 10-mg prasugrel) and from the person-months of treatment observed in the i3 InVision database. Costs for thienopyridine drugs were obtained from the electronic Drug Topics Red BookCitation19. Because the costs of clopidogrel are likely to change after generic formulations enter the market, alternative costs for clopidogrel ($3 and $4 per day less than prasugrel) were tested in sensitivity analyses. The mean duration of treatment with a thienopyridine drug was assumed to be the same for both clopidogrel-based therapy-treated and prasugrel-based therapy-treated patients and was derived from actual observed use seen in the i3 InVision database from three input parameters: (1) the mean duration in the MCO for those patients who disenrolled before 15 months (6.7 months); (2) the proportion of MCO patients who disenrolled before 15 months (35%); and (3) the proportion of enrolled days for which drug was dispensed based on prescription refills (82.03% for the full, starting cohort and between 73.99–84.01% for the different sub-populations analyzed). The costs to the MCO were adjusted for out-of-pocket prescription copayments.
CV- and bleeding-event costs
Direct medical costs were estimated in the model by applying a discount off-charge to hospital and physician charge data. All charges were adjusted to 2009 US dollars, using the medical care component of the Consumer Price IndexCitation20, where necessary, and are shown in Appendix in the supplemental appendix.
Table 2. Base-case scenario for the total MCO population for the 15-month time horizon, per 100 patients.
The CV- and bleeding-related medical costs in the model included ER visits for CV- or bleeding-related events and inpatient hospital and physician costs due to rehospitalization for a CV event or a bleeding event.
The mean charges for ER visits and facility charges for hospital stays for CV events were taken from the i3 InVision database. Physician charges for the hospital stay were estimated using the 75% fees listed in the Physician Fee ReferenceCitation21 for all inpatient procedures and in-hospital patient encounters. Inpatient costs for hospitalized bleeding events were based on the mean costs paid from the MarketScan databaseCitation12.
In the base case, a 50% discount off-charge was assumed for the MCO for ER visits and hospital and physician chargesCitation22. Alternative discounts, ranging from 10–57%, were tested in the sensitivity analyses. The upper bound was based on the average hospital cost-to-charge ratio of 43% that is used by the US Medicare program to convert hospital charges into costsCitation23.
In the lifetime cost-effectiveness analysis, an annual cost of $6836 each year after the 15-month study periodCitation12 was assumed for patients remaining enrolled in the MCO, irrespective of their thienopyridine treatment.
Alternative scenarios
Cost-effectiveness estimates were generated for all ACS-PCI patients who were treated with thienopyridines and who had no history of stroke or transient ischemic attack, to compare patients who were eligible for both thienopyridines. Estimates were generated for sub-groups, including different age ranges, diabetes status, and sub-type of ACS episode. Additionally, a scenario was analyzed that included other CV events deemed unlikely to be associated with the effectiveness or side-effects of antiplatelet therapy but that were observed during the TRITON-TIMI 38 trial. The model was also run for alternative time horizons.
Sensitivity analyses
One-way sensitivity analyses were performed for key parameters, using a credible range of estimates derived from data or expert opinion and presented in a tornado diagram.
A probabilistic sensitivity analysis, in which all parameters estimated with uncertainty were varied at the same time, was run for 10,000 iterations and the results presented as a cost-effectiveness acceptability curve. In the probabilistic sensitivity analysis, a normal distribution was assumed for the clopidogrel event rates taken from the i3 database, and a log-normal distribution was assumed for the relative risks from the TRITON-TIMI 38 trial. Mean duration of thienopyridine treatment, time to disenrollment, and event costs were all assumed to have a gamma distribution.
Results
presents the results for the full MCO cohort, using the base-case assumptions. At branded prices for clopidogrel, the total cost was lower for 100 patients on prasugrel-based therapy than for 100 patients on clopidogrel-based therapy. The difference was –$97,090 and was attributable to a reduced hospitalization rate for patients taking prasugrel-based therapy compared with clopidogrel-based therapy.
In , the total cost and event rates are presented for different sub-populations, different time horizons, and for the alternative scenario that also included other CV events unrelated to ischemic or bleeding events. The cost-saving with prasugrel-based therapy was greatest among patients who had a bare-metal stent implanted or were treated without stenting: –$126,223. The event rates and numbers of rehospitalizations remained lower for prasugrel-based therapy compared with clopidogrel-based therapy for each of the sub-groups and time horizons evaluated, although the cost-saving with prasugrel-based therapy was less for the shorter time horizons of 30 days and 1 year than for 15 months. When other CV-related events were included in the analysis, the cost-savings and number of events avoided with prasugrel-based therapy compared with clopidogrel-based therapy were lower than in the base case. Even when clopidogrel cost was adjusted to $3 per day less than prasugrel, cost-savings were still reduced to –$175 per 100 patients. Only when clopidogrel cost was estimated at $4 per day less than prasugrel, the costs per 100 patients was higher for prasugrel-based therapy by $32,022. However, in the lifetime analyses based on lower clopidogrel prices, the cost per LY gained with prasugrel was $6643 and $13,906, respectively.
Table 3. Scenarios for alternative MCO sub-populations and time horizons, per 100 patients.
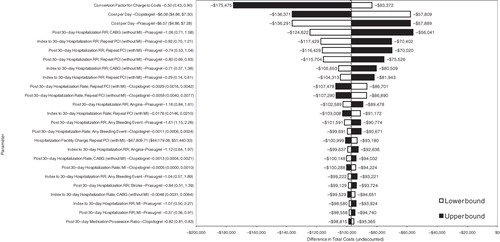
presents the results of the one-way sensitivity analysis for total cost as a tornado diagram. These results indicated that the cost-savings with the prasugrel-based therapy regimen were most sensitive to the price of prasugrel and clopidogrel and to the relative risk of repeat PCI with MI for prasugrel-based therapy compared with clopidogrel-based therapy for both the 30-day and post-30-day time period. The total cost results also were sensitive to the discount off-charges negotiated by the MCO for the hospital facility charges. The results were less sensitive to the relative risk of bleeding with prasugrel-based therapy compared with clopidogrel-based therapy.
Figure 1. One-way sensitivity analysis results per 100 patients: Tornado diagram. CABG, coronary artery bypass graft; MI, myocardial infarction; PCI, percutaneous coronary intervention; RR, relative risk.
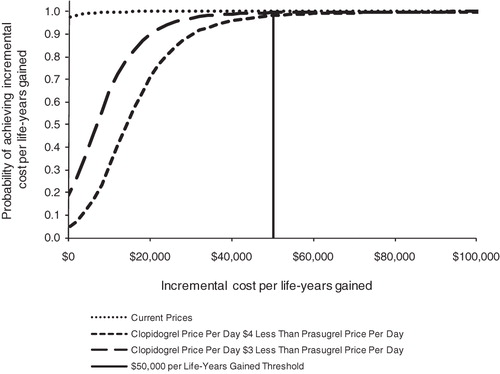
presents the results of probabilistic sensitivity analyses of current prices as well as clopidogrel prices that are $4 and $3 less than the current prasugrel price (hypothetical generic prices). The results indicated that, at current prices, prasugrel-based therapy had both lower total costs and fewer events than clopidogrel-based therapy (i.e., prasugrel dominant) in 97.6% of the 10,000 simulations. With clopidogrel prices of $3 and $4 less per day than prasugrel, the probability that prasugrel is cost-effective using a threshold value of $50,000 per LY gained was 99.5% and 98.2%, respectively.
Discussion
The results of the analysis indicated that prasugrel-based therapy compared with clopidogrel-based therapy has a favorable economic profile for patients with ACS treated with a PCI in an MCO setting. In the base-case analysis, use of prasugrel-based therapy rather than clopidogrel-based therapy at a branded price for clopidogrel resulted in cost-savings and a lower total number of CV and bleeding events over the 15 months among ACS patients treated with PCI. The one-way sensitivity analyses showed that the results are most sensitive to the relative cost of clopidogrel compared with prasugrel, the costs to the MCO for the rehospitalizations, and the relative risks of repeat PCIs with MI from the index period through 15 months. At hypothetical generic prices for clopidogrel, the use of prasugrel-based therapy compared with clopidogrel-based therapy is projected to have a high probability of having a cost-effectiveness ratio.
When other CV events unrelated to MI or coronary thrombosis were included in a scenario analysis, the decrease in cost-savings and events avoided for prasugrel-based therapy compared with clopidogrel-based therapy can be attributed to the higher number of other CV events in the i3 InVision database than in the clinical trial during the post-30-day time period. Because the risk of other CV events was higher with prasugrel-based therapy than with clopidogrel-based therapy in the clinical trial population, the estimated increase in these events in patients taking prasugrel-based therapy partially offsets the estimated decrease in costs associated with repeat PCIs and MIs. The higher incidence of other CV events in the MCO population as compared to clinical trial subjects may be the result of exclusion criteria.
The strengths of the presented analysis include the use of data from a large MCO database for estimating event rates and drug, ER, and hospital facility charges for real-world patients experiencing ACS followed by PCI and treated with clopidogrel-based therapy. The clinical event rates observed in the MCO population were similar to those observed for the clopidogrel-based therapy treated population in the clinical trial. Therefore, the results using the MCO population are similar to those presented for the clinical trial population by Mahoney et al.Citation12, who estimated cost-savings of $221 per patient and fewer events with prasugrel-based therapy than with clopidogrel-based therapy at branded clopidogrel prices.
There are several limitations in the analyses. First, the i3 InVision database did not allow a determination of whether the reason for patient’s disenrollment from the MCO was due to death or transfer out of the plan. Nor did the database include event rates for patients who disenrolled from the plan before the end of the 15-month observation period. Other important data limitations include the lack of long-term outcomes data, lack of actual differentiation (if any) on persistence with clopidogrel vs prasugrel, and the assumption that the treatment efficacy relative risk data from a randomized controlled trial applies to a real-world population that might differ from the clinical trial population in its age distribution, body weight, and comorbidity.
Another limitation was that the i3 InVision database did not include actual MCO payments; rather, it included the hospital facility-billed charges. Thus, it was necessary to estimate the discount off-charge that would be negotiated between the MCO and the hospital from interviews with people familiar with hospital contracts with MCOsCitation22.
In summary, the analyses demonstrate, using MCO data, that treatment with prasugrel-based therapy compared with clopidogrel-based therapy in ACS patients treated with PCI may reduce costs as well as clinical events in the first 30 days and 15 months. Even with discounted prices for clopidogrel, treatment with prasugrel was projected to have very favorable cost-effectiveness ratios.
Conclusions
Use of prasugrel-based therapy compared with clopidogrel-based therapy in ACS patients having a PCI resulted in cost-savings at current prices and favorable cost-effective ratios at likely generic prices for clopidogrel-based therapy because of offsetting savings in the costs of rehospitalization. In addition, the results in the managed care population were similar to estimated results based on the clinical trial population.
Transparency
Declaration of funding
This study was funded by Eli Lilly & Company.
Declaration of financial/other relationships
Dr Mauskopf and Mr Graham are employees of RTI Health Solutions, an independent research organization that received consulting fees from Eli Lilly & Company for the development of this paper. Dr Meadows, Dr Bae, and Mr Zagar are employees of Eli Lilly & Company, the manufacturer of prasugrel. Dr Ramaswamy is an employee of Daiichi Sankyo Inc., a partner with Eli Lilly & Company in marketing prasugrel. Dr Magnuson and Dr Cohen are employees of St. Luke’s Hospital; they served as consultants for this study. Dr Cohen has received speaking honoraria from Eli Lilly & Company and Daiichi-Sankyo, Inc., manufacturers of prasugrel.
Supplementary Material
Download PDF (65.8 KB)Acknowledgments
None.
References
- Abbott JD, Ahmed HN, Vlachos HA, et al. Comparison of outcome in patients with ST-elevation versus non-ST-elevation acute myocardial infarction treated with percutaneous coronary intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2007;100:190-5
- Antiplatelet Trialists’ Collaboration. Collaborative overview of randomised trials of antiplatelet therapy prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ 1994;308:81
- Mehta SR, Yusuf S, and the Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) Study Investigators, The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J 2000;21:2033-41
- Sabatine MS, Cannon CP, Gibson CM, et al; CLARITY-TIMI 28 Investigators. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med 2005;352:1179-89
- Chen ZM, Jiang LX, Chen YP, et al. Addition of clopidogrel to aspirin in 45,852 patients with acute myocardial infarction: randomized placebo-controlled trial. Lancet 2005;366:1607-21
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15
- Schleinitz MD, Heidenreich PA. A cost-effectiveness analysis of combination antiplatelet therapy for high-risk acute coronary syndromes: clopidogrel plus aspirin versus aspirin alone. Ann Intern Med 2005;142:251-9
- Weintraub WS, Mahoney EM, Lamy A, et al. Long-term cost-effectiveness of clopidogrel given for up to one year in patients with acute coronary syndromes without ST-segment elevation. J Am Coll Cardiol 2005;45:838-45
- Mahoney EM, Mehta S, Yuan Y, et al. Long-term cost-effectiveness of early and sustained clopidogrel therapy for up to 1 year in patients undergoing percutaneous coronary intervention after presenting with acute coronary syndromes without ST-segment elevation. Am Heart J 2006;151:219-27
- Beinart SC, Kolm P, Veledar E, et al. Long-term cost effectiveness of early and sustained dual oral antiplatelet therapy with clopidogrel given for up to one year after percutaneous coronary intervention: results from the Clopidogrel for the Reduction of Events During Observation (CREDO) trial. J Am Coll Cardiol 2005;46:761-9
- Cowper PA, Udayakumar K, Sketch MH Jr, et al. Economic effects of prolonged clopidogrel therapy after percutaneous coronary intervention. J Am Coll Cardiol 2005;45:369-76
- Mahoney EM, Wang K, Arnold SV, et al. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel thrombolysis in myocardial infarction (TRITON-TIMI 38). Circulation 2010;121:71-9
- Eli Lilly data on file. Eli Lilly’s analysis of i3 InVision Data Mart data from 2006 to mid-2008. October 2009. Available at: http://www.i3global.com
- Daiichi-Sankyo, Incorporated/Lilly Research Laboratories data on file. TRITON-TIMI 38 eight-country economic cohort data, Indianopolis, Indiana
- Tsai TT, Ho PM, Xu S, et al. Increased risk of bleeding in patients on clopidogrel therapy after drug-eluting stents implantation: insights from the HMO Research Network-Stent Registry (HMORN-stent). Circ Cardiovasc Interv 2010;3:230-5
- Mehta SR, Yusuf S, Peters RJ, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet 2001;358:527-33
- Peeters A, Mamun AA, Willekens F, et al. for NEDCOM. A cardiovascular life history: a life course analysis of the original Framingham Heart Study cohort. Eur Heart J 2002;23:458-66
- Mahoney EM, Jurkovitz CT, Chu H, et al. Cost and cost-effectiveness of an early invasive versus conservative strategy for the treatment of unstable angina and non-ST-segment-elevation myocardial infarction. JAMA 2002;288:1851-8
- Thomson PDR. Drug topics. Red Book for Windows. Version 61127, 58. Montvale, NJ: Thomson PDR, 2010
- US Department of Labor, Bureau of Labor Statistics. Consumer Price Index—all urban consumers (current series), 2010. http://data.bls.gov/PDQ/outside.jsp?survey=cu. Accessed March 29, 2011
- Yale Wasserman. Physicians’ fee reference, 28th edn. Milwaukee, WI: Yale Wasserman, DMD Medical Publishers Ltd, 2011
- Faulkner E, Mauskopf J, Bae J. Hospital contracting trends and implications for adoption and use of new health technologies. J Manag Care Med 2009;12:6-22
- Centers for Medicare and Medicaid Services. 42 CFR, parts 412, 413, 415, 485, and 489. Medicare program: changes to the hospital inpatient prospective payment systems for acute care hospitals and fiscal year 2010 rates; and changes to the long-term care hospital prospective payment system and rate years 2010 and 2009 rates. Fed Regist 2009;74. http://www.cms.gov/AcuteInpatientPPS/10FR/itemdetail.asp?filterType=none&filterByDID=-99&sortByDID=1&sortOrder=ascending&itemID=CMS1227461&intNumPerPage=10/. Accessed August 1, 2010