Abstract
Objective:
The aim of this study was to assess the cost-utility and value of reducing the uncertainty associated with the decision to use first-line biologic treatment (bDMARD) after the failure of one or more traditional drugs (tDMARD) in moderate-to-severe rheumatoid arthritis (msRA) in Finland.
Research design and methods:
The treatment sequences were compared among 3000 hypothetical Finnish msRA patients using a probabilistic microsimulation model in a lifetime scenario. Adalimumab + methotrexate, etanercept + methotrexate, or tocilizumab + methotrexate were used as first biologics followed by rituximab + methotrexate and infliximab + methotrexate. Best supportive care (BSC), including tDMARDs, was assumed to be used after the exhaustion of the biologics. Methotrexate alone was added as a further comparator. Efficacy was based on ACR responses that were obtained from a mixed treatment comparison. The resources were valued with Finnish unit costs (year 2010) from the healthcare payer perspective. Additional analyses were carried out, including productivity losses. The Health Assessment Questionnaire (HAQ) values were mapped to the EQ-5D values using the tocilizumab trials; 3% annual discounting for costs and quality-adjusted life years (QALY) and extensive sensitivity analyses were completed.
Main outcome measures:
Incremental cost per QALY gained and multinomial expected value of perfect information (mEVPI).
Results:
bDMARDs significantly increase the QALYs gained when compared to methotrexate alone. Tocilizumab + methotrexate was more cost-effective than adalimumab + methotrexate or etanercept + methotrexate in comparison with methotrexate alone, and adalimumab + methotrexate was dominated by etanercept + methotraxate. A QALY gained with retail-priced (wholesale-priced) tocilizumab + methotrexate costs €18,957 (€17,057) compared to methotrexate alone. According to the cost-effectiveness efficiency frontier and cost-effectiveness acceptability frontier (CEAF), tocilizumab + methotrexate should be considered before rituximab + methotrexate, infliximab + methotrexate, and BSC. Based on the CEAF, tocilizumab + methotrexate had a 60–93% probability of being cost-effective with €20,000 per QALY gained (mEVPI €230–2182).
Conclusions:
Tocilizumab + methotrexate is a potentially cost-effective bDMARD treatment for msRA, indicating a low value of additional research information with the international threshold values.
Limitations:
Efficacy based on an indirect comparison (certolizumab pegol, golimumab excluded), fixed treatment sequence after the exhaustion of first bDMARD, Swedish resource use data according to HAQ scores, and inpatient costs assumed to include surgery.
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease that causes a significant economic burden on societyCitation1,Citation2 and reduces the quality-of-life (QoL) of those affectedCitation3–7. Clinically significant RA has been diagnosed in 0.8% of the Finnish populationCitation8 and the incidence is 44.5/100,000 in adultsCitation9.
When ineffectively treated, RA can result in permanent disabilityCitation10. The goal of RA treatment is to achieve remission or low disease activity, with normal functioning and QoLCitation11. At the onset of RA, aggressive treatment with traditional disease modifying anti-rheumatic drugs (tDMARD) and low-dose corticosteroidsCitation11,Citation12 is recommended. Among tDMARDs, methotrexate (MTX) is the ‘anchor drug’Citation12,Citation13. TNF inhibitors are usually started if the response to tDMARDs is not satisfactoryCitation14. TNF inhibitors are followed by rituximab (RTX), abatacept (ABAT), or tDMARDs (e.g., cyclosporine, leflunomide, or MTX).
This study assessed (1) the cost-effectiveness (CE) and (2) the value of additional research information in reducing the uncertainty related to the decision to use the first-line biologic disease modifying anti-rheumatic drug (bDMARD) + MTX or MTX alone in the treatment of moderate-to-severe RA (msRA) after one or more tDMARD failures. The first-line bDMARD comparators included two established and reimbursed TNF inhibitors – the most used (adalimumab, ADAL) and most affordable (etanercept, ETAN) – and a new option (tocilizumab, TOC). The compared sequences consisted of one first-line bDMARD comparator followed by (→) a fixed treatment sequence (RTX → infliximab [INFL] → best supportive care [BSC], including the sequence of leflunomide [LEFL] → cyclosporine [CSA] → MTX). The fixed treatment sequence was adapted based on the clinical expertise and the recent CE evaluation of second-line bDMARD treatments in FinlandCitation15. In line with clinical recommendations, the bDMARDs were given with MTX and an outcome assessment was conducted at 6-month intervals.
Patients and methods
The CE of different treatment sequences was assessed using an Excel-based probabilisticCitation16 individual sampling model. In the primary analysis, costs and outcomes were considered from the public healthcare payer perspective (named payer costs), whereas the societal perspective (including productivity losses) was adopted as the secondary perspective.
Patients and simulations
The CE was assessed in a hypothetical RA population consisting of 3000 patients with active msRA and an inadequate response to the first-line mono or combination therapy treatment with tDMARDs using a simulation model. The patient profiles were taken from the pooled TOC trial (OPTION, TOWARD and LITHECitation17–19) msRA population who had inadequate response (IR) to tDMARDs. The patients were 52.5 years old on average, had an HAQ score of 1.51 at the baseline and weighed 73 kg; 18% of the population were men. The characteristics of the patients in the TOC trials were comparable to the indirect comparison populationCitation20, the Finnish RA populationCitation21–23, and the average weight of FinnsCitation24.
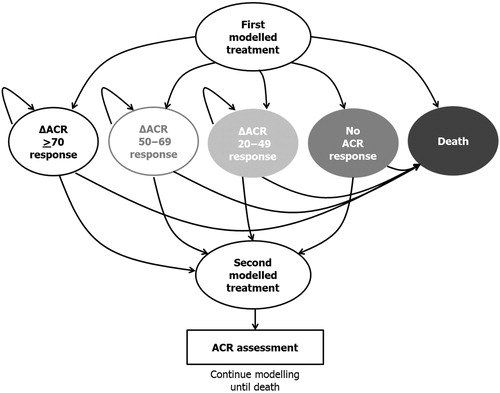
The model () used in the analysis was an individual sampling model with a structure that allows PSA (probabilistic sensitivity analysis, probability distributions for known uncertain parameters). The individual sampling means that the characteristics of the patients are tracked and the patients have individual histories that affect their outcomes. The analysis was performed by recording the outcomes of 3000 patients (seeding was used to force the inclusion of the same population) in 1000 PSA simulations.
Efficacies
In the model, all patients were assumed to undertake the initiated treatment for at least one cycle (6 months). The response status of the patient was assessed after the first cycle to determine whether the treatment would continue or the patient would be switched to the next treatment line. The probability of transition to the next treatment was determined by the probability of response and a constant withdrawal rate () from the initiated treatment. The ACR response ratesCitation25–28 were used as the measure of efficacy. Response to treatment was defined as a response equal to or higher than ACR20. Due to a lack of head-to-head clinical data between bDMARDs, the results of a recent mixed treatment comparison (MTCCitation20) were used to reflect the differences across treatments (, please see Bergman et al.Citation20 for further information regarding the indirect comparison methods used).
Table 1. Adjusted ACR responses, constant withdrawal rates, HAQ drops, and HAQ progressions together with their probabilistic distributions.
Response to treatment was assumed to have an impact on the disease severity reflected by the patients’ HAQ scores. Data from the phase III TOC clinical trialsCitation17–19 was analyzed to estimate the relationship between the ACR response and the individual HAQ score: the higher the observed response, the greater the drop in HAQ score (). For every response to a new treatment, the corresponding HAQ score reduction was applied in the first treatment cycle (negative HAQs were not permitted). The reduction was assumed to be the same regardless of treatment. The patients were at risk of withdrawalCitation29,Citation30 while under treatment, and the HAQ score was assumed to change according to the progression ratesCitation27,Citation31 shown in . The estimated rate of HAQ score change based on the ACR response was applied at the beginning of each treatment cycle (responders only) and assigned to each simulated individual regardless of the previous ACR response. Evidence related to the long-term sustained benefit of treatment after withdrawal is scarce. A rebound effect (increase in HAQ score) has been suggested to occur when therapy is withdrawnCitation32. In our analysis, HAQ worsening equal to the initial HAQ improvement was assumed to occur immediately at the point of treatment withdrawal.
Mortality and transition limitations
The probability of death in the model was based on age- and gender-specific mortality in the year 2010 according to the official national statistics for Finland. The patients’ HAQ scores modified the risk of death according to Wolfe et al.Citation33 ().
Some transitions in the model were assumed to occur only once (patients cannot initiate the same treatment again and no transitions are allowed once an individual dies). Unlike the conventional Markov-type models, more than one transition could occur during a 6-month cycle (i.e., treatment response, withdrawal, and/or death may occur during a cycle) in the individualistic discrete event simulation model.
Quality-of-life values
The quality-of-life (QoL) effects of the treatments were estimated based on the generic EQ-5D. The EQ-5D values were assumed to change following the changes in the patients’ HAQ scores. The association between HAQ scores and EQ-5D was estimated from the OPTIONCitation17 and LITHECitation19 data using a non-linear mixed model: EQ5D =0.82 − 0.11*HAQ − 0.07*(HAQ*HAQ) (p < 0.0001 for all HAQ coefficients)Citation34. The model coefficients were in line with those reported by Boggs et al.Citation35. In comparison to the linear model, the inclusion of the model term for the square of the HAQ score improved the model fit and produced a significant coefficient for the non-linear term.
Resource use and unit costs
All healthcare costs were presented in the most recent 2010 values (drug costs were from the Finnish Medicine Tariff 9/2011; the cheapest available generic prices were used and an assumption of no drug wastage was applied, ). TOC costs were presented using two approaches: (1) the pharmacy retail price without value added tax (VAT 9%) – that is, the drug cost when the intravenous (IV) drug is given in private hospitals or homes – and (2) the wholesale price, which served as a proxy for the drug cost when the intravenous drug is given in publicly-funded hospitals. The national unit costs from the year 2006Citation36 were transformed into 2010 values using a multiplier of 1.12739 based on the official Finnish healthcare price index obtained from Statistics Finland. All unit cost parameters were handled as fixed tariffs.
Table 2. Resource use and costs (healthcare unit costs at 2010 values; drug costs at 9/2011 values).
Five different cost categories were considered for all treatments: drug, administration, monitoring, hospitalizations, and travelling. In Finland, the initiation of the first biologic treatment necessitates screening procedures (i.e., chest X-ray and laboratory tests) related to the evaluation of drug safety at the initiation and an application for reimbursement for bDMARD. In the model, all patients were assumed to follow a routine monitoring protocol: the patients visit a specialist physician and a general practitioner (GP) once every 6 months (), if not otherwise needed due to medication. The laboratory values of the patients during MTX (also in combination with bDMARD), CSA, and LEFL treatment were assumed to be monitored every 2 months on average, based on the Helsinki University Hospital practice [HUS care protocol 7/2009], with telephone contact after the laboratory results were ready.
All patients were assumed to be able to inject ETAN and ADAL. In the Finnish practice, TOC is given as an infusion once a month, and is assumed to take some 1.5 h of nursing time (a clinician visit takes place every three visits); please note that the TOC infusion typically takes 1 h. RTX is given as daily infusions every 9 months (a nurse gives the infusion and a clinician sees the patient every 6 months). INFL is given as infusions seven times per year, each taking some 2 h of nursing time (the clinician visit takes place every 6 months).
Due to scarce Finnish data, the method for estimating hospitalizations followed the Swedish study by Kobelt et al.Citation37 (). In the secondary scenario, productivity losses for the total societal costs were mapped based on Kobelt et al.Citation37 using the average Finnish annual productivity loss of €24,309Citation36 (in order to obtain conservative estimates productivity costs were not indexed to 2010 price level).
Outcomes
The primary outcome measure was the incremental cost per quality-adjusted life-year (QALY) gained, also illustrated as the CE efficiency frontier (CEEF). Overall survivals were reported as secondary outcomes. In addition, the CE acceptability frontier (CEAF) was drawn based on the PSA simulation resultsCitation16. The microsimulation + PSA approach was used to depict the patient (heterogeneity) and parameter (variability) uncertainty associated with the CE results simultaneously. The multinomial expected value of perfect information (mEVPI) was estimated to assess the consequences of a wrong decision and the value of reducing uncertainty related to the model parameters.
Sensitivity analyses
Because there is uncertainty related to the choice of some model parameters, the impact of changing the assumptions was tested in multiple sensitivity analysis scenarios. The impact of response criteria was tested in two scenarios: a stricter scenario including only ACR50 and ACR70 responses, and the strictest scenario consisting of only ACR70 responses. The impact of treatment times on the studied drugs was tested using mean treatment times instead of the constant probabilities of withdrawal: ADAL/ETAN/INFL 2.5 years, ABAT/RTX/TOC 3.75 years, MTX alone 15 years and other tDMARD 2 yearsCitation15. In one scenario we assumed no time-dependent HAQ improvement for the TOC responders.
In a sensitivity analysis scenario the patients were assumed to lose 50% of the benefit obtained from treatment at the point of discontinuation instead of the 100% rebounding. Because the risk of mortality may not be related to HAQ scores, we assumed no additional risk to die in one scenario. The impact of the chosen QoL assessment method was tested using EQ-5D and HAQ score relationships suggested by Bansback et al.Citation30, Hawthorne et al.Citation38, and Hurst et al.Citation39. In addition, the requirement for positive QoL (i.e., QoL could not be worse than death in the base case) was relaxed. In further scenarios, a constant number of hospitalizations (4.11 days/yearCitation40), regardless of HAQ score, ABAT instead of RTX in the fixed sequence, baseline ages of 40 and 60 years, a 10-year modelling time, baseline HAQs of 1.3 and 1.7, as well as 6% and 0% discounting were applied.
Results
The detailed results of the base-case analyses are shown in . When the patients received MTX alone after tDMARD failure, the average expected payer (societal) lifetime costs were €96,753 (€111,927) and the remaining average lifetime’s gain of QALYs was 5.83.
Table 3. Base-case simulation results.
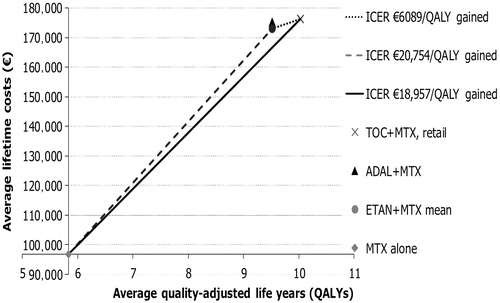
In comparison with MTX alone, the most cost-effective strategy from the payer (societal) viewpoint was to use TOC with the incremental cost-utility ratio (ICUR) of €17,057 (€17,091) and €18,957 (€18,991) per QALY gained based on the wholesale price and retail price of TOC, respectively. In comparison with ETAN, the ICURs of retail-priced TOC were €6089 (€2762), while the wholesale-priced TOC dominated ETAN. Treatment with ETAN was more cost-effective (dominating) than treatment with ADAL. The CEEF, average expected results, and ICURs are presented in from the payer perspective and using the retail price for TOC.
Figure 2. The cost-effectiveness efficiency frontier (CEEF) presents the cost-effective treatment options and their expected lifetime average costs and effectiveness.
Probabilistic analysis
The results of PSA are depicted in , for which the four treatment sequences were considered. MTX alone was the optimal and potentially cost-effective treatment (highest expected net monetary benefit and probability of CE > 50%) with the WTP below €17,276 per QALY gained when TOC had the wholesale price. The corresponding threshold was €19,107 when TOC had the retail price. With the WTP exceeding €17,323 (€19,516) per QALY gained, wholesale-priced (retail-priced) TOC was the optimal treatment.
Figure 3. (a) Wholesale price for TOC, and (b) retail price for TOC. Probabilistic sensitivity analysis (PSA) results: cost-effectiveness acceptability frontier presenting the optimal treatments together with multinomial expected value of perfect information (mEVPI) for the comparison between ADAL, ETAN, and TOC before the fixed sequence of RTX followed by INFL followed by BSC, and MTX alone.
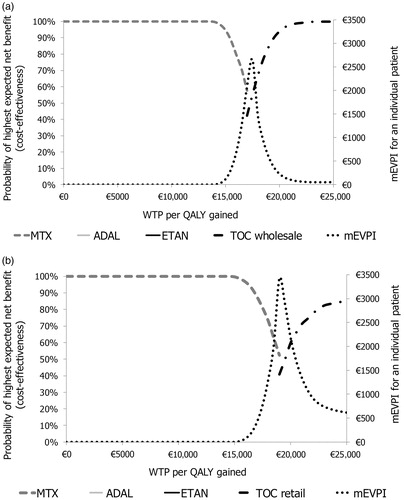
With the WTP of €20,000 and €25,000 per QALY gained, the wholesale-price (retail-priced) TOC had a 93.4% (59.8%) and 98.7% (84.8%) CE probability (). With the WTP of €20,000 and €25,000 per QALY gained, the respective per-patient mEVPIs were €230 (€2182) and €53 (€616) ().
Sensitivity analyses
The results of the one-way sensitivity analysis scenarios are shown for the most cost-effective options (ADAL + MTX excluded) in . The modelling assumptions only had a small impact on the relative results. Exclusion of the ACR20 responses reduced the ICURs by €2822–4456, whereas exclusion of both ACR20 and ACR50 responses (i.e., use of ACR70 responses only) reduced the ICURs by €4483–8755. The use of fixed mean treatment times instead of constant risk of withdrawal increased the ICURs by €4608–6283. When no HAQ improvement for TOC responders over time was assumed, the ICUR for TOC increased by €2937–3631.
The assumed rebound rate had the most significant effect in the lifetime scenario: when a 50% rebound effect was assumed instead of 100%, the ICURs decreased by €9684–12,646. The exclusion of excess HAQ scores associated with the mortality risk reduced the ICURs by €1178–1381.
Table 4. Deterministic sensitivity analyses.
The chosen method for mapping the QoL and HAQ scores had a significant impact on the absolute results. The formulae by Bansback et al.Citation31, Hawthorne et al.Citation38 and Hurst et al.Citation39 resulted in €2236–3518, €7712–10,426 and €103–870 higher ICURs, respectively. The acceptance of negative QoL reduced the ICURs by €558–772.
The highly unlikely constant rate of hospitalizations (i.e., 4.11 hospital days/year were assumed to be independent of the patient’s status) based on a Finnish sourceCitation40 resulted in €6955–7085 higher ICURs. When RTX was replaced with ABAT, the ICURs increased by €4676–5402.
Patients with the average baseline age of 40 years resulted in €1639–1993 lower ICURs and the average baseline age of 60 years resulted in €2547–3575 higher ICURs in comparison with the base-case results with the average age of 52.5 years. Truncating the modelling to the 10-year timeframe increased the ICURs by €25,521–30,775. Varying the baseline HAQ from 1.3 to 1.7 only had a minor effect on the ICURs. Undiscounted ICURs were €3527–4292 lower and 6% discounted ICURs were €4329–5261 higher compared to the base-case results with the 3% annual discounting.
Discussion
According to this study, both retail and wholesale-priced TOC was more cost-effective than ETAN and ADAL in comparison with MTX alone, and both ETAN and wholesale-priced TOC dominated ADAL. When using wholesale prices and the payer perspective, additional QALY was gained with TOC costs of €17,057 compared to MTX alone. Additional QALY was gained with retail-priced TOC costs of €18,957 compared to MTX alone, and €6089 compared to ETAN from the payer perspective. TOC was cost-effective due to its ACR responses and due to HAQ improvement over time when using TOC (and resulting changes in costs, QoL, and mortality): the sensitivity analyses on these, however, indicated only a minor effect on the CE results. Generally, the pricing of ADAL, ETAN, and retail-priced TOC seems to be relatively similar in Finland and, thus, efficacy made the biggest difference.
The maximum per patient mEVPIs were €3421 (TOC retail pricing) and €2657 (TOC wholesale pricing) with the ICUR values. The estimates were rather low, suggesting that both the value of the additional research information and the consequences of a wrong decision were likely to be low in this setting. With the WTP of €20,000 per QALY gained, TOC + MTX had a 60–93% CE probability, depending on the place of use. With WTP of €0–30,000 per QALY gained, only treatments with MTX alone and TOC + MTX were potentially cost-effective.
When comparing the results of this analysis with a recent Finnish analysisCitation15 for second-line treatments, we see that the results of this analysis are less uncertain (i.e., the CE probabilities are higher). We used averages (strictly speaking, average is the expected value of distribution; thus averages should be used in economic evaluations) instead of the medians reported in another recent Finnish study of INFLCitation41. In addition, theirCitation41 setting was not decision analytical and did not include an active comparator drug, even though they reported ICERsCitation42. Thus, our results are not comparable with that study.
Consequently, direct comparisons between the current and previous CE studies in RA must be done with caution due to the differing study designs, comparators, costs, timelines, treatment lines, response definitions, outcome definitions, and patient populations. High variations in the reported ICERs were found in the review by Chen et al.Citation43, but, on average, the ICERs were some £30,000 per QALY. Our results also seem to be in line with more recent studies (not included in the reviewCitation43) assessing the life-time CE of first-line biologic treatment for RACitation44–47. These studies, however, did not include TOC as a comparator and, in comparison with the most previous RA models, our analysis was based on a microsimulation + PSA model with, for example, patient tracks and multiple event risks (not the more common Markov model without patient tracks, mutually exhaustive events and/or PSA), MTC results based on the iteration process (not on the more common simple meta-analysis or mathematically adjusted indirect comparison), and non-linear QoL values from relevant studies (TOC trials, not from independent sources). In addition, we reported mEVPI. A review of existing RA models based on the publications would be an interesting study.
There were some limitations in our analyses that may influence the usability of our results. Because there were no randomized, controlled clinical trials comparing the efficacy of all biologics after tDMARD failure, the results from MTCCitation20 were used. Because certolizumab pegol and golimumab were not included in the MTC, their CE was not assessed. Adherence and persistence were not modelled due to data limitations, comparability of available data (i.e., the assessment of adherence/persistence in patients obtaining infusions is likely to be easier than in patients using subcutaneous injections or tablets) and the ‘cost-efficacy’ type of analysis (i.e., can the treatments be efficient?). Moreover, ADAL and ETAN have had around a 75% market share among all TNFs in Finland; ADAL having the highest share.
To simplify the relatively complex analysis we fixed the treatment sequence after the initial bDMARDs to consist of RTX, INFL, and BSC, as this was observed to be a cost-effective second-line treatment sequence in Hallinen et al.Citation15. The BSC sequence was assumed to include CSA, LEFL, and MTX in that order. In reality, the treatment sequence after the failure of the first bDMARD varies and is individually tailored. However, the choice of a fixed treatment sequence did not have a significant impact on the results because the same sequences were applied in all comparisons (except MTX alone). The choice of RTX as the first biologic treatment after the first bDMARDs was supported by a sensitivity analysis replacing RTX with ABAT (the sequence with RTX is more cost-effective).
In our model we made assumptions regarding the changes in the patients’ condition (HAQ scores) while under treatment. For bDMARDs other than TOC, the condition was assumed to remain at the level of the initial response to treatment as long as they were receiving treatmentCitation28. As long-term TOC studies have demonstrated improvements in HAQ over time, a slight reduction in the HAQ score (−0.01622) was assumed to occur in each cycle while under treatmentCitation31. In a sensitivity analysis we assumed no HAQ improvement for TOC; the resulting ICURs for TOC were comparable with the ICUR for ETAN in comparison with MTX alone. The base case HAQ of 1.3–1.7 only had a minor effect on the ICURs. The probability of discontinuing the treatment after the initial response (withdrawal rate) was assumed to be the same for all bDMARDs. Because the estimate was an average estimate reported in one studyCitation29, we tested the impact of this assumption in a sensitivity analysis with fixed treatment timesCitation15. The method of handling time under treatment had an impact on the absolute but not the relative results.
In the absence of Finnish resource use data in RA according to patients’ HAQ scores, we estimated hospitalizations and productivity losses based on a Swedish studyCitation37. The assumption seems reasonable because the mean HAQ and annual days in hospital were comparable in Sweden and FinlandCitation37,Citation40. In a sensitivity scenario we assessed the impact of hospital days by using constant hospital days irrespective of the patients’ HAQ scores. The change in this assumption did not change the relative results. For simplicity, the average inpatient costs were assumed to include the costs of any possible surgery, and the drug administration/monitoring was assumed to include all outpatient costs.
The method used for linking the EQ-5D measured quality-of-life and HAQ scores had a relatively large impact on the obtained absolute ICURs (€103–10,426 higher ICURs). Although the ICURs in the primary analysis are lower, we believe them to be reasonable as the TOC trial data indicated a superior fit for the non-linear relationship between HAQ and EQ-5D compared to the linear relationshipCitation34 that was used in Hawthorne et al.Citation38 and Bansback et al.Citation30. Also, the results based on Hurst et al.Citation39 were close to those used in this study.
The modelled msRA patients died just before their 80th birthday. In comparison with the gender-weighted expected lifetime of 50-year-old Finns (79 years) based on the official Finnish statistics, the survival of the modelled patients seemed to be credible.
Finally, intensive anti-rheumatic drug therapy can make a difference in a variety of outcomesCitation9,Citation48,Citation49. However, new options are needed: in real life, ∼25% of the patients get hardly any response from the currently used bDMARDsCitation14. TOC is one of the newest RA drugs and the CE of TOC may encourage its use in clinical care.
Conclusion
Tocilizumab + methotrexate is a cost-effective initial biologic treatment for patients with moderate-to-severe rheumatoid arthritis after failure with one or more tDMARDs. The results are relatively robust and indicate a relatively low value for additional research information for the adaptation decision with the corresponding population and parameter definitions.
Transparency
Declaration of funding and author contributions
This work was supported by Roche Oy, Espoo, Finland. EJS contributed to the concept, design, and modelling, acquired data, analysed and interpreted data, and drafted and revised the manuscript. TAH contributed to the design and interpretation of data, and helped to draft and revise the manuscript. VV acquired data, and helped to draft the manuscript. MJK and KP contributed to the concept and interpretation of data, and revised the manuscript. All authors read and approved the final manuscript.
Declaration of financial and other relevant relationships
EJS and TAH have disclosed that they are consultants and shareholders in ESiOR Oy, a company that was commissioned by Roche Oy to perform this study. VV has disclosed that he is an employee of Roche. KP and MJK have disclosed that they received consulting fees from Roche and other companies such as Abbott, Bristol Myers-Squibb, MSD, Pfizer, and UCB. The final manuscript has been read and approved by all the authors, and all authorship decisions were made on the basis of scientific consideration.
Acknowledgments
The authors wish to thank Juhana Heinonen, Kalevi Nikula, Anne Hautala, and Wolfgang Berger (Roche) for contacts and/or beneficial comments during the writing process. Parts of this study were presented during the 74th ACR Annual Meeting (ACR Notable Poster nomination) in Atlanta and the 13th Annual European Congress of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in Prague in November 2010.
References
- Huscher D, Merkesdal S, Thiele K, et al. Cost of illness in rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis and systemic lupus erythematosus in Germany. Ann Rheum Dis 2006;65:1175-83
- Maetzel A, Li LC, Pencharz J, et al. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis 2004;63:395-401
- Loza E, Abásolo L, Jover JA, et al. Burden of disease across chronic diseases: a health survey that measured prevalence, function, and quality of life. J Rheum 2008;35:159-65
- Saarni SI, Härkänen T, Sintonen H, et al. The impact of 29 chronic conditions on health-related quality of life: a general population survey in Finland using 15D and EQ-5D. Qual Life Res 2006;15:1403-14
- West E, Jonsson SW. Health-related quality of life in rheumatoid arthritis in Northern Sweden: a comparison between patients with early RA, patients with medium-term disease and controls, using SF-36. Clin Rheum 2005;24:117-22
- Alonso J, Ferrer M, Gandek B, et al. Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Qual Life Res 2004;13:283-98
- Picavet HS, Hoeymans N. Health related quality of life in multiple musculoskeletal diseases: SF-36 and EQ-5D in the DMC3 study. Ann Rheum Dis 2004;63:723-9
- Aho K, Kaipiainen-Seppänen O, Heliövaara M, et al. Epidemiology of rheumatoid arthritis in Finland. Semin Arthritis Rheum 1998;27:325-34
- Puolakka K, Kautiainen H, Pohjolainen T, et al. Rheumatoid arthritis remains a threat to work productivity: a nationwide register based incidence study from Finland. Scand J Rheumatol 2010;39:436-8
- Turesson C, O'Fallon WM, Crowson CS, et al. Occurrence of extraarticular disease manifestations is associated with excess mortality in a community based cohort of patients with rheumatoid arthritis. J Rheum 2002;29:62-7
- Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis 2010;69:964-75
- Katchamart W, Trudeau J, Phumethum V, et al. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2009;68:1105-12
- Pincus T, Marcum SB, Callahan LF. Longterm drug therapy for rheumatoid arthritis in seven rheumatology private practices: II. Second line drugs and prednisone. J Rheum 1992;19:1885-94
- Leirisalo-Repo M. Tulehduksellisten reumatautien uudet biologiset lääkkeet. Duodecim 2007;123:2459-67
- Hallinen TA, Soini EJ, Eklund K, et al. Cost-utility of different treatment strategies after the failure of tumour necrosis factor inhibitor in rheumatoid arthritis in the Finnish setting. Rheumatology (Oxford) 2010;49:767-77
- Barton GR, Briggs AH, Fenwick EAL. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97
- Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987-97
- Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum 2008;58:2968-80
- Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate. Results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609-21
- Bergman GJ, Hochberg MC, Boers M, et al. Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum 2010;39:425-41
- Laas K, Roine R, Räsänen P, et al. Health-related quality of life in patients with common rheumatic diseases referred to a university clinic. Rheum Int 2009;29:267-73
- Laas K, Peltomaa R, Puolakka K, et al. Early improvement of health-related quality of life during treatment with etanercept and adalimumab in patients with rheumatoid arthritis in routine practice. Clin Exp Rheum 2009;27:315-20
- Laas K, Peltomaa R, Kautiainen H, et al. Pharmacoeconomic study of patients with chronic inflammatory joint disease before and during infliximab treatment. Ann Rheum Dis 2006;65:924-8
- Paturi M, Tapanainen H, Reinivuo H, et al. Finravinto 2007 -tutkimus [The National FINDIET 2007 Survey]. Helsinki: National Public Health Institute, 2008
- Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology (Oxford) 2009;48:436-9
- Suomalaisen Lääkäriseuran Duodecimin ja Suomen Reumatologisen yhdistyksen asettama työryhmä. Nivelreuma. Helsinki: Käypä Hoito suositus, 2009
- National Institute for Health and Clinical Excellence. Adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis. London: NICE TA130, 2007
- Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years). Rheumatology (Oxford) 2006;45:1167-9
- Geborek P, Crnkic M, Petersson IF. Etanercept, infliximab and leflunomide in established rheumatoid arthritis: clinical experience using a structured follow up programme in southern Sweden. Ann Rheum Dis 2002;61:793-8
- Bansback N, Brennan A, Ghatnekar O. Cost effectiveness of adalimumab in the treatment of patients with moderate to severe rheumatoid arthritis in Sweden. Ann Rheum Dis 2005;64:995-1002
- van Vollenhoven RF, Ducournau P, Wintfeld N, et al. Health Assessment Questionnaire-Disability Index Scores in patients with rheumatoid arthritis treated with tocilizumab plus conventional anti-rheumatic drugs. Value Health 2009;12:A434
- Brennan A, Bansback N, Reynolds A, et al. Modelling the cost-effectiveness of etanercept in adults with rheumatoid arthritis in the UK. Rheumatology (Oxford) 2004;43:62-72
- Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994;37:481-94
- Ducournau P, Kielhorn A, Wintfeld NS. Comparison of linear and nonlinear utility mapping between HAQ and EQ-5D using pooled data from the tocilizumab trials OPTION and LITHE. Rheumatology (Oxford) 2009;48(1 Suppl):i107-8
- Boggs R, Sengupta N, Ashraf T. Estimating health utility from a physical function assessment in rheumatoid arthritis (RA) patients treated with adalimumab (D2E7). Value Health 2002;5:452-3
- Hujanen T, Kapiainen S, Tuominen U, et al. Terveydenhuollon yksikkökustannukset Suomessa vuonna 2006. Helsinki: Stakesin Työpapereita, 2008
- Kobelt G, Eberhardt K, Jönsson L, et al. Economic consequences of the progression of rheumatoid arthritis in Sweden. Arthritis Rheum 1999;42:347-56
- Hawthorne G, Buchbinder R, Defina J. Functional status and health-related Quality of Life Assessment in patients with rheumatoid arthritis. West Heidelberg: Monash University, 2000
- Hurst N, Kind P, Ruta D, et al. Measuring health-related quality of life in rheumatoid arthritis validity/responsiveness and reliability of EUROQOL (EQ-5D). Br J Rheumatol 1997;36:551-9
- Laas K, Peltomaa R, Kautiainen H, et al. Clinical impact of switching from infliximab to etanercept in patients with rheumatoid arthritis. Clin Rheumatol 2008;27:927-32
- Virkki LM, Konttinen YT, Peltomaa R et al. Cost-effectiveness of infliximab in the treatment of rheumatoid arthritis in clinical practice. Clin Exp Rheumatol 2008;26:1059-66
- Soini E. Incremental or average cost-utility of routine cataract surgery? Health Qual Life Outcomes 2006;4:74. http://www.hqlo.com/content/4/1/74/comments. Accessed September 30, 2009
- Chen YF, Jobanputra P, Barton P, et al. A systematic review of the effectiveness of adalimumab, etanercept and infliximab for the treatment of rheumatoid arthritis in adults and an economic evaluation of their cost-effectiveness. Health Technol Assess 2006;10:1-229.
- Davies A, Cifaldi MA, Segurado OG, et al. Cost-effectiveness of sequential therapy with tumor necrosis factor antagonists in early rheumatoid arthritis. J Rheumatol 2009;36:16-26
- Brennan A, Bansback N, Nixon R, et al. Modelling the cost effectiveness of TNF-alpha antagonists in the management of rheumatoid arthritis: results from the British Society for Rheumatology Biologics Registry. Rheumatology (Oxford) 2007;46:1345-54
- Tanno M, Nakamura I, Ito K, et al. Modeling and cost-effectiveness analysis of etanercept in adults with rheumatoid arthritis in Japan: a preliminary analysis. Mod Rheumatol 2006;16:77-84
- Spalding JR, Hay J. Cost effectiveness of tumour necrosis factor-alpha inhibitors as first-line agents in rheumatoid arthritis. Pharmacoeconomics 2006;24:1221-32
- Tiippana-Kinnunen T, Laaksonen L, Kautiainen H, et al. Impact of early radiographic remission on the 15-year radiographic outcome in patients with rheumatoid arthritis. Scand J Rheumatol 2011;40:263-8
- Immonen K, Finne P, Grönhagen-Riska C, et al. A marked decline in the incidence of renal replacement therapy for amyloidosis associated with inflammatory rheumatic diseases - data from nationwide registries in Finland. Amyloid 2011;18:25-8