Abstract
Objective:
With the availability of several bowel cleansing agents, physicians and hospitals performing colonoscopies will often base their choice of cleansing agent purely on acquisition cost. Therefore, an easy to use budget impact model has been developed and established as a tool to compare total colon preparation costs between different established bowel cleansing agents.
Methods:
The model was programmed in Excel and designed as a questionnaire evaluating information on treatment costs for a range of established bowel cleansing products. The sum of costs is based on National Health Service reference costs for bowel cleansing products. Estimations are made for savings achievable when using a 2-litre polyethylene glycol with ascorbate components solution (PEG + ASC) in place of other bowel cleansing solutions. Test data were entered into the model to confirm validity and sensitivity. The model was then applied to a set of audit cost data from a major hospital colonoscopy unit in the UK.
Results:
Descriptive analysis of the test data showed that the main cost drivers in the colonoscopy process are the procedure costs and costs for bed days rather than drug acquisition costs, irrespective of the cleansing agent. Audit data from a colonoscopy unit in the UK confirmed the finding with a saving of £107,000 per year in favour of PEG + ASC when compared to sodium picosulphate with magnesium citrate solution (NaPic + MgCit). For every patient group the model calculated overall cost savings. This was irrespective of the higher drug expenditure associated with the use of PEG + ASC for bowel preparation. Savings were mainly realized through reduced costs for repeat colonoscopy procedures and associated costs, such as inpatient length of stay.
Conclusions:
The budget impact model demonstrated that the primary cost driver was the procedure cost for colonoscopy. Savings can be realized through the use of PEG + ASC despite higher drug acquisition costs relative to the comparator products. From a global hospital funding perspective, the acquisition costs of bowel preparations should not be used as the primary reason to select the preferred treatment agent, but should be part of the consideration, with an emphasis on the clinical outcome.
Introduction
With a prevalence of ∼5% in western countriesCitation1, colorectal cancer (CRC) management and treatment is a growing issue in patient care. In the UK alone, more than 30,000 patients are diagnosed with and treated for CRC each yearCitation2. Options for treatment are highly dependent on the stage of the disease. If detected at an early stage, 5-year survival rates can reach 90% or moreCitation3,Citation4. As malignancies in the colon and rectum usually originate from benign adenomas, prevention is possible if polyps are removed before becoming malignant.
The most reliable test currently available to screen for CRC and polyp removal is colonoscopyCitation5,Citation6. Over the past years, there has been an increasing demand for colonoscopies in the UK due to the advent of bowel cancer screeningCitation7. It has been demonstrated that in population screening programmes an increase in completed colonoscopies is linked to a reduction in mortality from CRCCitation8. An accurate colonoscopy is dependent on thorough cleansing of the colon and rectumCitation9. Typically the oral laxatives routinely used in the UK are either the hyperosmotic sodium phosphates and NaPic + MgCit or the PEG3350-based solutions, including the 2-litre PEG + ASC.
If total cleansing of the gut allowing full mucosal visualization is not achieved, a repeat procedure associated with additional cost should be considered to ensure pathological changes are not missed which may result in mis-diagnosisCitation8,Citation10. Colonoscopists cannot repeat the procedure immediately when confronted with poor bowel cleansing and also rarely acknowledge repeating colonoscopies. This has been highlighted in a publication that surveyed gastroenterologists and found that, when confronted with an intermediate-quality preparation which prevented full mucosal visualization, most gastroenterologists recommend a significantly shorter follow-up/repeat intervalCitation11.
In Europe, prior to the introduction of a low volume (2 litre) PEG + ASC bowel preparation, patients had the choice of consuming either a high volume (4 L) PEG + electrolyte solution or low volume hyperosmotic preparations. The 4 L PEG + electrolyte solutions were preferred in terms of efficacy and safety, however they suffered from reduced patient compliance. In a large comparative randomized controlled trial (RCT) 2 L PEG + ASC bowel preparation was demonstrated to improve patient acceptability without compromising the high degree of efficacy and safety associated with 4 L PEG + electrolyte preparationsCitation12.
Comparative RCTs have demonstrated the 2 L PEG + ASC solution to be as effective as older established hyperosmotic regimens (sodium phosphate and NaPic + MgCit oral solutions) with the additional benefit of cleansing superiority in the ascending colon and caecumCitation12–14. As a result, the 2-litre PEG + ASC solution has become a commonly adopted standard for colon cleansing in Europe.
Faced with a variety of cleansing agents, the choice of bowel preparation to be used is often determined by the acquisition cost of the bowel preparation in isolation. However, acquisition cost should not be the primary decision-making factor, as cleansing efficacy of the bowel is a vital pre-requisite for accurate diagnosis together with any appropriate interventions.
In light of recent safety alerts by bodies such as the UK National Patient Safety Agency (NPSA) and the US Food and Drug Administration (FDA), regarding the administration and supply of various bowel cleansing preparations clinicians need to be mindful of the continuing need to ensure patients safety as part of a routine colonoscopy assessment.
Clinical safety and efficacy should be the primary determinants of bowel preparation choice, but costs are clearly important in the current environment of cost containment. When calculating the costs for colonoscopy, not only the costs for the cleansing agent itself and the single procedure have to be taken into account, but also the repeat procedure costs due to poor and/or incomplete visualization of all areas of the colon mucosa, either as a result of poor patient compliance or due to a lack of efficacy.
Assessment of the efficacy of a bowel preparation during colonoscopy is frequently subjective and intra-operator consistency is rarely achieved without the use of empirical assessment tools. So far, gastroenterologists and hospital administrators are not able to directly compare the overall long-term budget impact of high quality colonoscopy, including outputs from appropriate bowel cleansing agents.
This paper introduces a budget impact model which compares total treatment costs for high quality colonoscopy between 2-litre PEG + ASC and two other commonly used hyperosmotic bowel cleansing agents in the UK: sodium picosulphate with magnesium citrate, and sodium bisphosphate/sodium phosphate. The primary aim of the model is to verify the budget impact, given that data indicates that PEG + ASC is highly effective in the cleansing of the colon (full visualization of 100% of mucosa in all areas of the rectum and colon) without reduced patient acceptability and/or safety findings seen for selected other bowel cleansing products. The model calculates the budget impact of bowel cleansing agents on total costs for colonoscopies and provides the hospital with an opportunity to maximize cost efficiencies in relation to the conduct of colonoscopies.
Methods
Perspective
The model addresses the major cost drivers relevant to colonoscopy from the perspective of the UK NHS. Within the payer structure, there are two competing cost structures: drug acquisition costs and procedural costs. Purchasers within the NHS or third party payers are understandably motivated by the drug acquisition costs that are incurred for medical treatment. Costs considered in the model include colonoscopy procedures, hospital bed days and bowel cleansing agents. To ensure a conservative analysis, indirect costs such as lost working days are not included. Additionally, treatment-related adverse events are not considered due to there being published RCT evidence showing lower rates of adverse events with 2 L PEG + ASC vs the comparatorsCitation15.
Structure of the budget-impact model
The model is programmed in Microsoft Excel. It is constructed as a questionnaire and is intended for use by gastroenterologists, pharmacists and payors.
The model collects a number of different data variables (see for details). Based on the questionnaire responses, the information is displayed on a report sheet. The model generates outputs related to workload and costs using the respective cleansing products relative to each other.
Table 1. Variable inputs from audit data collected from St. Georges Hospital, London into the budget impact model questionnaire plus drug acquisition costs without fixed costs.
Total treatment costs for both the low-volume PEG + ASC solution and the alternative cleansing product were calculated as follows:
total treatment costs for low-volume PEG + ASC = total treatment costs for frail outpatients (defined by the consulting physician to be at risk of clinically significant comorbidities requiring hospital admission for supervised bowel preparation) using PEG + ASC, + total treatment costs for inpatients using PEG + ASC + total treatment costs for out-patients using PEG + ASC; and
total treatment costs for the alternative product = total treatment costs for frail out-patients using the alternative product + total treatment costs for in-patients using the alternative product + total treatment costs for out-patients using the alternative product.
In the absence of direct comparative data on the rate of repeat procedures, a surrogate measure for the need to repeat a colonscopy was required for this model. Repeat procedures are subject to variability in terms of both patients and the performing clinicians. Therefore, the model assumes that a failed or incomplete colonoscopy based on published comparative cleansing efficacy data would indicate a failure and require a repeat procedure. gives the published comparative efficacy dataCitation13,Citation14. The model allows for up to two rounds of repeat procedures. The budget impact model assumes that a 10% higher efficacy will result in 10% fewer repeat procedures in line with the published data.
Table 2. Published efficacy results obtained in controlled clinical trials.
The model calculates the total treatment costs for frail outpatients, inpatients and outpatients, respectively, by adding (if available) the respective first acquisition drug costs, costs of procedure, costs of bed days, second acquisition drug costs, repeat drug costs, and costs of procedure repetitions. Successful colon preparation was regarded as a high quality colonoscopy with 100% mucosal visualization as defined, for example, by the Harefield Cleansing Scale.
It needs to be noted that frail out-patients often require hospitalization prior to colonoscopy to ensure appropriate safety monitoring during consumption of the bowel preparation before the colonoscopy. The model calculates the total amount of money (excluding lost revenue from Bowel Cancer Screening Programme) expended by the hospital, and what benefit or deficit results from using the respective bowel cleansing agent.
Total costs are compared using either the 2-litre PEG + ASC or the selected alternative bowel cleansing agents. For hospitals that participate in the UK bowel cancer screening programme, the model estimates the loss of revenue as a result of repeated procedures for these patients as payment is based on a single colonoscopic investigational fee.
Data inputs
This model was created and validated to estimate the budget impact of a colonoscopy service in a UK hospital. Comparative efficacy data for different colon cleansing preparations were derived from published studiesCitation13,Citation14. It is important to note that the two comparative studies used in the model evaluation were based on an outpatient cohort only. Outpatients are acknowledged to be associated with higher efficacy results due to their enhanced mobility. As a result, the budget impact model provides a conservative result for inpatients.
Costs for colonoscopy procedures and hospital bed days were derived from the NHS Reference Costs 2010/2011. Drug costs were taken from the Monthly Index of Medical Specialities (MIMS), version Jan 2012.
The model was tested using collected audit data using the questionnaire of a major UK colonoscopy unit (St. Georges, London, UK).
Sensitivity analysis
In order to test the sensitivity of the model to various data inputs, five sets of test data were applied to the model. Mean values were calculated for the five test data sets, as well as for each group of patients. For test data, the fixed costs were £897.00 for one inpatient colonoscopy procedure, £504.00 for one outpatient colonoscopy procedure, and £227.00 for one bed day (all numbers according to 2010/2011 NHS reference costs). The comparative bowel cleansing agents were sodium picosulphate with magnesium citrate and sodium biphosphate/sodium phosphate. The reference in all data sets was the 2-litre PEG + ASC solution based on comparative clinical study resultsCitation13,Citation14.
Validity of the model
In order to adapt this model to clinical practice in the UK, the validity of the model was tested from a budget impact point of view. The model validity was assessed through a combination of face-to-face and group meetings with gastroenterologists and pharmacists across the UK. Following confirmation of the face validity a structural validity assessment was undertaken by an independent third party (Pierrel Research Europe, Essen, Germany).
To demonstrate how the budget impact model works, audit data from St. George’s Hospital, London, UK, was applied to the model.
Limitations of the model
For the applied model, the assumption is that all colon cleansing products differ in frequency of needed repetitions of colonoscopy procedures and of needed admissions to the hospital.
In a complete budget-impact model, a wider period of time should be considered, in particular the colonoscopy preparation phase.
The following points are not considered in the model:
additional costs resulting from differences concerning compliance/non-compliance, e.g., withdrawal from treatment; and
additional costs resulting from differences concerning adverse reactions and the management thereof.
Results
In , the summarized cost differences between the 2-litre PEG + ASC solution and the alternative hyperosmotic agent sodium picosulphate with magnesium citrate are listed for each cost factor by patient group.
Table 3. Overall cost differences (£) NaPic + MgCit (comparator) – PEG + ASC using the test data (500 colonoscopies per year).
shows the mean cost differences over all five test data sets for each cost factor included in the model used in the sensitivity analysis. In all three patient groups (inpatients, outpatients, frail out-patients), the drug costs for first and repeated procedures form the lowest proportion of the overall cost differences generated between 2-litre PEG + ASC and the alternative products. Although two different products were compared to 2-litre PEG + ASC, it is improbable that the use of one and the same comparator product would lead to a different result. Instead, the costs for the colonoscopy procedures emerged as the main cost drivers. For frail outpatients, the costs for first and repeated bed days have a considerable impact as well.
Figure 1. Cost difference (in £) between comparator products and PEG + ASC in colonoscopy costs using test data.
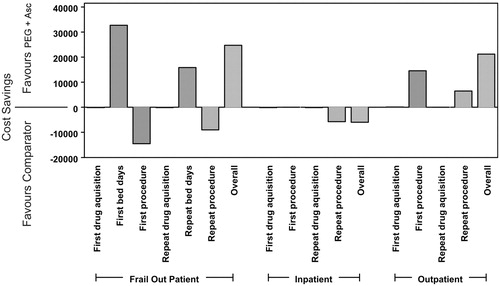
The drug acquisition costs are not the driving factor in the overall cost structure for an efficient colonoscopy service when cleansing outcomes are considered and the fact that the bowel preparation acquisition cost accounts for less than 3% of the total cost.
In order to verify the findings derived from the test data sets, a set of audit data from a large gastroenterology unit in St. George’s Hospital, London, was applied to the model. The results of the total resource cost calculations and mean costs per patient are summarized in . The drug acquisition costs were £25,184.88 for PEG + ASC and £8695.21 for NaPic + MgCit; procedure costs were £1,410,649.77 and £1,495,564.99, respectively. The mean per patient total costs were £603.91 and £636.60 for PEG + ASC and NaPic + MgCit, respectively, indicating a 5.4% lower budget impact despite corresponding mean drug acquisition costs of £10.49 and £3.62.
Table 4. Total and per patient cost calculations (£) using either NaPic + MgCit or PEG + ASC for the audit data collected from St. Georges Hospital, London.
In all three patient groups, cost savings were realized when PEG + ASC was used for bowel preparation (details are summarized in ). Differences in drug acquisition costs were minimal in favour of NaPic + MgCit. However, the significant overall driver of savings was the reduced need for repeat procedures in all patient groups.
Table 5. Detailed total annual colonoscopy costs (£) from St. George’s Hospital, London.
These data confirm the finding of the test data analysis that the drug acquisition costs have the least impact on the overall colonoscopy cost structure, and that higher drug costs for more effective colon cleansing are compensated by savings through reduction of procedure repetitions.
In the example presented above, the total costs for colonoscopy were £1,527,207.17 with NaPic + MgCit as cleansing agent, including all three patient groups; the estimated costs with PEG + ASC were £1,419,978.08. Based on the data entered into the questionnaire of the model, usage of 2-litre PEG + ASC preparation would save the unit at least £107,229 (∼7.5% of total costs) per year. This sum does not include extra costs associated with Bowel Cancer Screening colonoscopies being repeated. Repeat procedures associated with bowel cancer screening need to be covered ‘out of pocket’. It should be noted that the budget impact model uses the 2010/11 NHS reference cost for an outpatient colonoscopy rather than the higher screening colonoscopy fee, making the model conservative in relationship to revenue generated from bowel cancer screening. Overall, total estimated revenue from colon cancer screening would increase, when using the 2-litre PEG + ASC as bowel preparation, in comparison, for example, to NaPic + MgCit based on the units repeat rate.
The complete entries in the questionnaire of the model are displayed in . Results of the model calculations are shown in , and revenues from the Bowel Cancer Screening Programme are listed in .
Table 6. Bowel cancer screening costs and revenues (UK).
Discussion
In a recent study, colonoscopy has been shown to be inversely associated with colorectal cancer mortalityCitation8. Every increase in colonoscopy rates by 1% is associated with a reduction of death hazard by 3%. Therefore, the increased use of colonoscopy is associated with a reduction in the CRC mortality rate based on a population analysisCitation8. Furthermore, another study showed that the costs caused by a detected lesion increases rapidly with progression of the lesion. According to the results of Bini et al.Citation16, an adenoma will cost ∼$2000, whereas an adenocarcinoma will cost ∼$7600–7800. Therefore, early detection of lesions is of high importance to keep overall healthcare budgets as low as possible. Therefore, a cleansing preparation which enables 100% mucosal visualization to be routinely achieved is of vital importance for cancer prevention. The goal of any colorectal cancer screening programme is to allow the clinical detection and removal of small polyps and flat adenomas as early as possible to improve patient outcomes and long-term treatment costs.
Previous studies comparing the effectiveness of several colon cleansing products have shown comparable rates of mild-to-moderate gastrointestinal adverse reactions such as abdominal pain, nausea and vomiting. Complications arising directly from the colonoscopy procedure are, in general, very rare and are irrespective of the bowel cleansing agent usedCitation17–19. PEG-based solutions are preferentially recommended in at-risk patients due to their established safety profilesCitation20. However, sodium phosphate can cause electrolyte abnormalities, such as hypokalaemia and hypophosphataemia and also other complications, including renal impairment and pathological ulcerations on the colon mucosaCitation21,Citation22.
As the budget impact model has not included costs for adverse events it remains a conservative tool in its assessment of total budget impact.
A recent meta-analysis has summarized in detail relative bowel cleansing efficacies for published study results using different agentsCitation23. The meta-analysis provides the conclusion that PEG-based regimens offer the optimum choice for bowel preparation.
One of the key influencing factors in obtaining high efficacy with bowel preparations is high patient acceptability, which has been a commonly experienced problem with the older, high volume (4 L) PEG-based products. The use of the bowel cleansing agents remains a necessary, but unpleasant experience for patients undergoing colonoscopy and, as a result, investigators should ensure that patients do not have to undergo the process more often than necessary. Additionally, the presented Excel-based budget impact model confirms that the colonoscopy process itself is the major cost driver. Total costs can be reduced if the cleansing agent used for bowel preparation allows reliable cleansing (100% mucosal visualization), negating unnecessary repeat procedures, which will also allow an optimized use of resources within a colonoscopy unit. Using the presented budget impact model, both hospitals and payors are given a conservative tool to assess when a change in bowel cleansing agent, for example, to 2-litre PEG + ASC, is economically reasonable based on improved colon cleansing.
The validation and sensitivity analysis of the budget impact model using five test data sets clearly shows that the main cost driver in the process of colonoscopy is not the drug acquisition costs, but the colonoscopy process itself. Therefore, to minimize the budget impact of a colonoscopy service and maximize the use of available resources the aim should be to reduce the number of repeat or shortened intervals between colonoscopies by using the most clinically effective bowel cleansing agent. As mentioned above, 2-litre PEG + ASC has been shown in comparative RCTs to be highly effective and well accepted by patientsCitation12–14.
The data set derived from the UK-based colonoscopy unit confirms the finding demonstrated by the sensitivity analysis that the cost offset associated with less procedure repetitions outweighs the expenditures for the cleansing agent. In the given example, the savings amount to ∼£107,000 (7.5% of the modelled costs). Whilst this cannot be generalized across the UK, it demonstrates that the utilization of 2-litre PEG + ASC can provide a cost saving for colonoscopy services.
As a future step in the development of the model, it could be applied to other European healthcare environments. This includes adaptation of fixed treatment costs according to national standards, i.e., costs for bed-days, medications, and procedures for inpatients and out-patients. If applicable, national screening programmes or other national specifics in cancer prevention would have to be incorporated.
Conclusions
We have demonstrated that the primary cost driver associated with colonoscopy is the procedure itself and not the drug acquisition cost. In this example the use of a PEG + ASC bowel preparation is shown to contribute to a reduction in overall costs and more efficient resource utilization. This is despite the initial up-front higher drug acquisition cost. This is of importance, today, as many decisions are driven by drug acquisition costs alone without full consideration of the long- and short-term clinical and economic outcomes. Budget impact models are an effective tool in informing healthcare providers and payors as to the most cost-effective use of resources.
Transparency
Declaration of funding
The Excel-based budget impact model was validated by Pierrel Research Europe GmbH, Essen, Germany on behalf of Norgine Ltd, Norgine House, Widewater Place, Moorhall Road, Harefield, Uxbridge, UK. Publication was prepared by Michael Köhler, Pierrel Research Europe GmbH on behalf of Norgine Ltd, including any calculation of data.
Declaration of financial/other relationships
HJG has worked for Norgine. AC is an employee of Norgine. RL has acted as a consultant to Norgine, but has not received financial benefits for the enclosed contribution. HJG and AC developed the concept for the budget impact model and were responsible for design, validation, and implementation of data. AC also developed the Excel-based budget impact model. RL contributed clinical data from the gastroenterology unit of St Georges Hospital, London. All authors read and approved the final manuscript.
Supplementary Material
Download PDF (1.9 MB)Acknowledgements
The peer reviewers on this manuscript have disclosed any relevant financial relationships.
References
- Palmer KR, Morris AI. A snapshot of colonoscopy practice in England: stimulus for improvement. Gut 2004;53:163‐5
- Office for National Statistics. Cancer statistics-registration England, 1999. London: The Stationery Office 2002; series MB1 No. 30
- Endoscopy Section Committee of the British Society of Gastroenterology. Future requirements for colonoscopy in Britain. Occasional report. Gut 1987;28:772‐5
- Corbett M, Chambers SL, Shadbolt B, et al. Colonoscopy screening for colorectal cancer: the outcomes of two recruitment methods. MJA 2004;181:423‐7
- Ball JE, Osbourne J, Jowett S, et al. Quality improvement programme to achieve acceptable colonoscopy completion rates: prospective before and after study. BMJ 2004;329:665‐7
- Lindsay DC, Freeman JG, Cobden I, et al. Should colonoscopy be the first investigation for colonic disease? BMJ 1988;296:167‐9
- Bowles CJA, Leicester R, Romaya C, et al. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow? Gut 2004;53:277‐83
- Rabeneck L, Paszat LF, Saskin R, et al. Association between colonoscopy rates and colorectal cancer mortality. Am J Gastroenterol 2010;105:1627‐32
- Taylor SA, Halligan S, Goh V, et al. Optimizing bowel preparation for multidetector row CT colonography: effect of Citramag and Picolax. Clin Radiol 2003;58:723‐32
- Rex DK, Imperiale TF, Latinovich DR, et al. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol 2002;97:1696‐700
- Ben-Horin S, Bar-Meir S, Avidan B. The impact of colon cleanliness assessment on endoscopists’ recommendations for follow-up colonoscopy. Am J Gastroenterol 2007;102:2680‐5
- Ell C, Fischbach W, Bronish HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008;103:883‐93
- Bitoun A, Ponchon T, Barthet M, et al. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther 2006;24:1631‐42
- Worthington J, Thyssen M, Chapman G, et al. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin 2008;24:481‐8
- Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparations for colonoscopy. Aliment Pharmacol Ther 2007;25:373‐84
- Bini AJ, Rajapaksa RC, Weinshel EH. The findings and impact of nonrehydrated guaiac examination of the rectum (FINGER) study. A comparison of 2 methods of screening for colorectal cancer in asymptomatic average-risk patients. Arch Intern Med 1999;159:2022‐6
- Pérez Roldán F, González Carro M, Legaz Huidobro LM, et al. Endoscopic resection of large colorectal polyps. Rev Esp Enferm Dig 2004;96:36‐47
- Lüning TH, Keemers-Gels ME, Barendregt WB, et al. Colonoscopic perforations: a review of 30,366 patients. Surg Endosc 2007;21:994-7
- Levin TR, Zhao W, Conell, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med 2006;145:880‐6
- Conner A, Tolan D, Hughes S, et al. Consensus guidelines for prescription and administration of oral bowel cleansing agents. 2009; www.rcr.ac.uk/docs/radiology/pdf/oral_bowel_cleansing_guidelines.pdf
- Belsey J, Epstein O, Heresbach D. Systematic review: adverse event reports for oral sodium phosphate and polyethylene glycol. Aliment Pharmacol Ther 2009;29:15‐28
- Barkun A, Chiba N, Enns R, et al. Commonly used preparations for colonoscopy: efficacy, tolerability and safety -- a Canadian Association of Gastroenterology position paper. Can J Gastroenterol 2006;20:699‐710
- Belsey J, Crosta C, Epstein O, et al. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985-2010. Aliment Pharmacol Ther 2012;35:222‐37