Abstract
Objective:
Duloxetine is indicated for treatment of major depressive disorders in the UK. While clinical trials have documented its clinical effectiveness, little is known regarding the relationship between duloxetine use and healthcare utilization in community practice. This study quantifies the impact of treatment with duloxetine on healthcare utilization among patients with depression and those with depression and co-existing pain.
Methods
Depressed adults initiating duloxetine during 1/1/2006–9/30/2007 were identified from the General Practice Research Database (GPRD). All-cause hospitalization, accident/emergency visits, specialist referrals, and analgesic use in the 12 months before (pre-period) and after (post-period) duloxetine initiation were compared. Generalized Estimating Equation models evaluated the pre–post change in the odds of hospitalization.
Results:
Nine hundred and nine patients were identified, 413 had pre-period unexplained pain (UPain). Rates of hospitalization declined from the pre- to the post-period. Fewer UPain patients received analgesics post-duloxetine initiation. Multivariate analyses confirmed that the odds of hospitalization were lower after duloxetine initiation. UPain patients with pre-period anticonvulsant use had lower odds of hospitalization in the post-period and the reduction in odds was significantly larger than that of patients without pre-period anticonvulsants. While patients with pre-period anxiolytic use, alcohol/drug dependence, or sleep disorders did not show statistically significant pre–post change in the odds of hospitalization, these changes were significantly different from patients without these conditions.
Limitations:
The study did not include a comparison group of patients who were non-users of duloxetine. Prevalence of chronic conditions might be under-estimated due to coding in the GPRD. Medications were assumed to be taken as prescribed. Study results are not generalizable beyond the population covered by the UK’s primary care system.
Conclusions:
All-cause hospitalization rates lowered among depressed patients and fewer UPain patients received analgesics post-duloxetine initiation. The reduction in the odds of hospitalization was most pronounced among UPain patients receiving pre-period anticonvulsants.
Introduction
Depression is estimated to affect 1.24 million people in England in 2007Citation1. In addition to the substantial impairment in the patient’s cognitive, physical, and social abilities, depression is associated with significant economic burden across a range of costs, including direct medical costs, social service costs, and costs due to productivity lossCitation1–3. Recommended by the National Institute for Health and Clinical Excellence (NICE), selective serotonin reuptake inhibitors (SSRIs) are first-line therapy in the UK. For those patients who have no or minimal response within 3–4 weeks following treatment with first-line drugs, alternative antidepressants, such as selective serotonin and noradrenaline reuptake inhibitors (SNRIs), are recommendedCitation4.
Duloxetine is a SNRI with an indication for treatment of major depressive disorders (MDD) in the UK and US. Clinical trials have documented the clinical effectiveness of duloxetine in relieving depression symptomsCitation5 and in preventing or delaying relapse and recurrence of depressionCitation6–9. However, results of studies evaluating duloxetine from an economic perspective have been inconsistent. In a US claims database analysis, Sheehan et al.Citation10 compared medication adherence, healthcare utilization, and costs of three generations of antidepressants, i.e., TCAs and MAOIs as the first generation antidepressants, SSRIs available before 2002 as the second generation, and SSRIs and SNRIs launched after 2002 as the third-generation. Patients receiving first- and second-generation antidepressants had 44% and 12% higher medical costs than those receiving third-generation antidepressants. However, duloxetine had the highest 6-month unadjusted healthcare expenditure among third-generation antidepressants. In a comparative study of escitalopram and duloxetine based on cost data collected in a double-blind, randomized study, duloxetine reduced hospitalization, and sick leave, but to a lesser extent than escitalopramCitation11. Benedict et al.Citation12 used a decision analytic cost model to estimate the health and economic impacts of duloxetine, venlafaxine XR, SSRIs, and mirtazapine in the treatment of patients with MDD. The study found duloxetine to be less costly and slightly more effective than venlafaxine XR in both MDD patients and in a more severe sub-group. The analysis suggested that duloxetine had the highest probability to be the cost-effective treatment option comparing to SNRIs, SSRIs, and mirtazapine in moderately severe MDD patients in the primary care setting. In UK clinical practice, however, the impact of duloxetine on healthcare utilization in the treatment of depression has not been well studied.
In addition to MDD, duloxetine is indicated in the European Union and the US for treatment of general anxiety disorder, and diabetic peripheral neuropathy (DPN), and, in the US, for fibromyalgia and chronic musculoskeletal pain due to osteoarthritis and chronic low back pain. In a recent claims-based study, Shi et al.Citation13 suggested that opioid use and moderate-to-severe pain were significant predictors of initiating duloxetine in the US. Pain commonly co-exists with depression. Double-blind clinical trialsCitation14,Citation15 and a prospective clinical studyCitation16 have shown that painful physical symptoms have a direct impact on depression remission rates and treatment outcomes. However, little has been studied regarding the relationship between duloxetine use and healthcare utilization among depression patients with comorbid pain conditions.
We conducted a retrospective analysis using the General Practice Research Database (GPRD) in order to address the inconsistent evidence published in the current literature regarding duloxetine use and healthcare utilization, as well as to provide real-world data on the impact of duloxetine use on healthcare utilization in the UK primary care system. The impact of treatment of duloxetine on healthcare utilization among patients with depression were quantified by comparing healthcare utilization before and after the initiation of duloxetine. Rates of all-cause hospitalization, accident/emergency (ER) visits, specialist referrals, and analgesic prescriptions during 12 months before (pre-index period) and 12 months after duloxetine initiation (post-index period) were evaluated and compared. Multivariate models were used to determine demographic and clinical factors that had a significant impact on the change in the odds of all-cause hospitalization from the pre-period to the post-period.
Given duloxetine’s dual indications for pain management and depression, a sub-set of patients with depression and co-existing pain was also identified and analysed for the same outcomes.
Patients and methods
Data source
Data for this study were drawn from the 2003–2008 GPRD. The GPRD is one of the largest computerized databases of medical records in the world and includes data for ∼8.9 million patients (over 3 million active enrollees annually) from over 350 primary care practices throughout the UK. De-identified data are collected directly from general practitioners on enrollees in the primary care system and provide detailed longitudinal medical information relating to symptoms, diagnoses, examinations, tests, and treatment.
Study sample
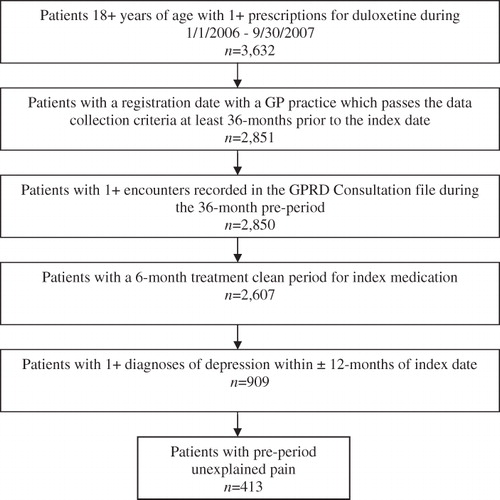
Patients who were 18 years of age or older and had at least one prescription order for duloxetine between 1 January 2006 and 30 September 2007 were identified. The date of the first observed duloxetine prescription in this time window was set as the index date. As a proxy for treatment initiation with duloxetine, patients were excluded if they had a duloxetine prescription within the 6-months prior to the index date. All eligible patients were required to have at least one diagnosis for depression in the 12 months before or after the index prescription. A broad definition of depression was used and included acute or chronic major depressive disorders of various severity levels, as well as depression with or without anxiety disorder and psychoses. To ensure that study patients were active in the GPRD system during the entire study period, the general practices associated with these patients were required to participate in data submission to the GPRD during the entire study time window. Study patients were also required to have registered with their general practitioners on or before the start of the pre-period. As a proxy of continuous enrolment eligibility, all study patients were required to have at least one encounter, face-to-face or non-face-to-face, recorded in the GPRD within the 36-month period prior to the index date.
Within the main depressed patient sample, a sub-set of patients with pre-existing unexplained pain during the 36-month pre-index period (UPain) was identified and analysed separately.
Study outcomes
The main study outcomes were the changes in all-cause hospitalization, accident/emergency (ER) visits, specialist referrals, and analgesic prescriptions during the 12 months before and after duloxetine initiation. These measures reflect either resource intensive medical services, such as inpatient admissions and ER visits, or services commonly sought by depression patients, like visits to mental health specialists and other specialists. Prescription of analgesics was examined as pain was a major comorbid condition of depression. We chose to evaluate these all-cause utilization measures because depression can affect patients’ mental and physical health and all-cause utilization can best reflect healthcare resource use among this patient population. Rates of all-cause hospitalization, ER visits, specialist referrals, and analgesic use were measured as the proportion of patients with utilization of each of these services within each of the pre- and post-index periods. Hospitalization, ER visits, and specialist referrals were identified through codes from the Read coding system and the Oxford Medical Information System (OXMIS) (Read/OXMIS codes), provider types, or consultation types recorded in the GPRD. Analgesics were identified using the British National Formulary codes indicating analgesics. Study outcomes were assessed for both the main depression sample as well as the UPain sub-group.
All-cause hospitalization having a significant impact on economic outcomes in depression, we further evaluated the change in the odds of all-cause hospitalization from the pre- to post-index period using Generalized Estimating Equation models (GEE). Clinical factors with potential impact on the pre–post change of the odds of hospitalization were examined. GEE models were employed on both the main sample of depression patients and the UPain sub-group.
Variables
Patient characteristics were summarized and presented through a series of demographic and clinical variables. Age and gender were measured on the index date. Patient level binary variables were created to indicate whether patients filled prescriptions during the 12-month pre-index period for medications used to treat a variety of mental health conditions: anxiolytics, antipsychotics, stimulants, antimanics, anticonvulsants, and antidepressants. Antidepressants included tricyclics (TCAs), monoamine oxidase inhibitors (MAOIs), SSRIs, non-duloxetine SNRIs, and other antidepressants. Prescription orders during the 12-month pre-index period for cardiovascular medications, thyroid hormones, anti-diabetic medications, gastrointestinal medications, respiratory medications, and analgesics (opioids, non-opioids, and migraine medications) were also captured.
The presence of comorbid conditions was measured during a 36-month pre-index period. A 36-month evaluation period was used since diagnosis codes, especially those for chronic conditions, may not be recorded in the GPRD each time the patient interacts with the provider. A longer evaluation period increases the chances of capturing diagnoses of interest with the potential to impact utilization. We also examined psychiatric conditions including anxiety disorders, attention deficit hyperactivity disorder, schizophrenia, bipolar disorder, and alcohol/drug dependence. Pre-existing medical conditions, such as circulatory, respiratory, and digestive system diseases, sleep disorders, and diabetes were evaluated. The occurrence of various pain conditions were noted, including DPN, fibromyalgia, osteoarthritis, chronic low back pain, and unexplained pain. Medical conditions were identified by Read/OXMIS codes, determined jointly by researchers and medical coding experts after extensive text string searching based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis descriptions and literature reviews. The code sets for unexplained pain were based on text string searching of the key word ‘pain’. Pain conditions and symptoms at various sites such as the joint, musculoskeletal, muscle, non-cardiac chest, abdominal, and others were included. Codes listed under chronic low back pain were removed from the unexplained pain list.
In addition to the demographic and clinical variables, a variable indicating the observation time period either before (=0) or after (=1) the initiation of duloxetine was created for each patient in the study cohort. This observation period indicator was used in the GEE models assessing the changes in the odds of all-cause hospitalization.
Statistical analysis
Frequency distributions and descriptive statistics (i.e., mean and standard deviation) were used to describe the demographic and the pre-index clinical characteristics of the study cohort. Pre-index and post-index period healthcare utilization rates were compared and the differences were evaluated using paired t-tests. Differences with p < 0.05 were considered statistically significant. The statistical analysis was implemented using STATA MP 11.
Multivariate GEE models were used to identify the impact of duloxetine initiation and patient demographic/clinical factors on the pre–post changes in the odds of all-cause hospitalization. GEE models were based on two-period panel data, in which each patient had two observations: one in the pre-duloxetine initiation period and the other in the post-initiation period. Two GEE models were implemented. The first model (Model I) assessed the overall effect of the initiation of duloxetine on the odds of all-cause hospitalization, assuming that the pre–post change did not vary across patients with different covariates. The dependent variable was all-cause hospitalization during the observation period. The independent variables included the demographic characteristics and pre-index clinical conditions and medication use variables detailed in the ‘Variables’ section. The observation period indicator was also included as the key independent variable. As the pre-index health conditions and medication use variables may be correlated with each other and lead to multicollinearity in the regressions, the variance inflation factors (VIF) were examined after implementing an OLS regression with the same dependent and independent variables. A VIF value higher than 10 indicates presence of high multicollinearityCitation16. The VIF values for the independent variables ranged from 1.00–2.04, which indicates that the possibility of multicollinearity was low. The odds ratio (OR) for the observation period indicator captured the impact of duloxetine initiation on the change in hospitalization, as it was expressed as the ratio between the odds of hospitalization in the post-index period and the odds of hospitalization in the pre-index period.
While using the same dependent variables, the second model (Model II) incorporated the interaction terms between the observation period indicator and patient demographic/clinical factors, in addition to evaluating the main effect of these covariates on the odds of hospitalization. The interaction terms between the observation period indicator and patient characteristics captured the relative magnitude of the pre–post change in the odds of hospitalization between patients with and without conditions or medications.
The ORs for observation period indicator, demographics/clinical factors, as well as the interaction terms, were computed as the exponent of the GEE regression coefficients, where logit link and binomial variance functions were used. P-values for all ORs were considered statistically significant when p < 0.05.
Results
Sample size and characteristics
A total of 909 patients met the study criteria (). Of these, 413 patients (45%) had pre-existing unexplained pain during the 36-months pre-index period and qualified for the UPain sub-group. The average age of the overall study sample was 49.6 (SD = ±16.5) years and females accounted for 67.7% of the sample. The UPain sub-group had a mean age of 50.7 (SD = ±16.6) and 69.7% were females.
The prevalence of medical, psychiatric, and pain conditions as well as medication use prior to initiating duloxetine is displayed in . Patients with depression were commonly diagnosed with a disease in the respiratory (51.7%), the digestive (34.9%), or the circulatory (22.8%) system within 36-months prior to initiation of duloxetine. The most prevalent psychiatric condition was anxiety disorders. Unexplained pain was the most common pain type reported.
Table 1. Clinical characteristics.
During the 12-month period prior to duloxetine initiation, 75% of all patients were treated with a SSRI for depression while 11% of patients did not receive an antidepressant of any type during this period. Other frequently prescribed psychiatric medications included anxiolytics and antipsychotics. Pain medications, opioids, and non-opioids were commonly used among patients with depression who initiated duloxetine.
Unadjusted utilization results
Healthcare utilization during the pre- and post-index period is shown in . For all depressed patients, the unadjusted rate of all-cause hospitalization was 4.2 percentage points higher (p = 0.006) in the pre-index period than in the post-index period. There were no significant pre–post differences in the unadjusted rates of ER visits, specialist referrals, or analgesic use. Similarly for the sub-set of patients with UPain, pre–post differences in healthcare utilization were statistically significant, with lower rates of hospitalization (26.2% vs 19.1%, p = 0.003) and analgesic use (77.0% vs 71.7%, p = 0.015) in the post-index period.
Table 2. Comparison of healthcare utilization before and after initiating duloxetine, all depressed patients and patients with pre-period unexplained pain.
Odds ratios of all-cause hospitalization
GEE Model I indicated that the odds of all-cause hospitalization were lower after duloxetine initiation than before initiation among all depressed patients (OR = 0.75, 95% CI: 0.65–0.87, p < 0.001), as well as depressed patients with UPain (OR = 0.70, 95% CI: 0.57–0.87, p = 0.001).
GEE Model II identified four clinical factors whose interaction terms with the observation period indicator were statistically significant, meaning the impact of these clinical factors on the odds of hospitalization was significantly associated with duloxetine initiation (). These factors included pre-index presence of alcohol/drug dependence, sleep disorders, pre-index use of anxiolytics among all depressed patients, and pre-index use of anticonvulsants among patients of the UPain sub-group. UPain patients who received anticonvulsants in the pre-index period experienced a significant decrease (73% reduction, p = 0.031) in the odds of hospitalization in the post-index period. The decrease was also significantly larger than the 38% reduction for patients without any conditions/medication use examined (). In the main depression sample, patients with pre-index period alcohol/drug dependence or sleep disorder had a 75% (p = 0.183) and 57% (p = 0.244) increase in the odds of hospitalization from pre- to post-index period, respectively; while patients with pre-index anxiolytic use experienced a 33% (p = 0.265) decrease in the odds of hospitalization. Although these pre–post changes in the hospitalization rates within each of the mentioned sub-groups did not reach statistical significance, they were significantly different from the change observed for patients without any conditions/medication use examined (4% decrease).
Figure 2. Odds ratios of clinical factors interacted with observation period indicator in the regressions of all-cause hospitalization, all depressed patients and patients with pre-index unexplained pain, GEE Model II.
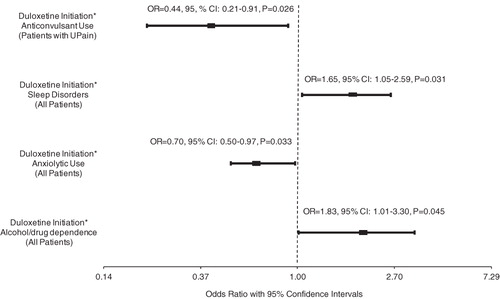
Table 3. Odds ratios for post-duloxetine initiation period in the regressions of all-cause hospitalization for patients with and without select clinical conditions/medications.
Discussion
Using a large administrative database which contains a 5.5% sub-sample of patients in the UK primary care health system, this study examined healthcare utilization in the 12-month period before and after treatment initiation with duloxetine among adult patients with depression as well as a sub-set of depressed patients with pre-existing unexplained pain. Results from both descriptive and multivariate analyses showed lower rates of all-cause hospitalization following duloxetine initiation compared to the pre-initiation period. Additionally, descriptive results indicated that patients with pre-existing unexplained pain were less likely to receive analgesics in the post-index period than in the pre-index period.
Currently there is a paucity of research investigating the relationship between duloxetine use and healthcare utilization in community practice. Using medical claims in the US, Sheehan et al.Citation10 found that 15.9% of patients who received duloxetine were hospitalized during the 6-month follow-up period, compared to 21% of patients on the first generation antidepressants and 13% of patients on the second generation. However, study patients could have depression only (63%), anxiety only (31%), or both conditions (6%), and findings were not reported for the depression sub-population separately.
To the best of our knowledge, the present study is the first examination in community practice of the relationship between duloxetine and healthcare utilization among patients with depression in the UK. In addition to the main finding of reduced odds of all-cause hospitalization after duloxetine initiation, we explored the interactions between patient demographic and clinical characteristics and duloxetine initiation. Because of duloxetine’s analgesic properties, we were interested in the effects of duloxetine initiation on the rate of all-cause hospitalization among patients with pain conditions or who were receiving analgesics. Previous studies suggest that presence of painful physical symptoms predicts substantially poorer treatment outcomesCitation17,Citation18. Our findings indicated that there were fewer patients in the UPain sub-group being prescribed analgesics after initiating duloxetine. Furthermore, GEE Model II showed that the hospitalization outcome was similar irrespective of the presence of an (unexplained) pain diagnosis at baseline or not, as the odds ratio for the interaction term between unexplained pain and the observation period indicator was 1.02 (95% CI, 0.73–1.41, p = 0.925). Similarly, pre-index presence of fibromyalgia, osteoarthritis, and analgesic opioids and non-opioids use were not significant factors based on GEE Model II in both the main depression patient cohort and the UPain sub-group.
In the analysis of the UPain sub-group, we found that the most pronounced reduction in the odds of hospitalization (73% reduction) was among patients who were prescribed with anticonvulsants in the pre-index period. Anticonvulsants are used to treat a variety of conditions including epilepsy, bipolar disorder, and pain. Although we were unable to capture the underlying conditions for which medications were prescribed using the GPRD, a review of the anticonvulsant prescriptions within our sample showed gabapentin, pregabalin, lamotrigine, and carbamazepine were the most frequently prescribed. In addition to epilepsy treatment, these medications are used in managing pain associated with conditions such as neuropathy and fibromyalgiaCitation19–22, for which duloxetine could also be used. In our study, of the 86 patients using anticonvulsants during the pre-index period, 75.6% (n = 65) continued on anticonvulsants after duloxetine initiation. We did not know, however, if both anticonvulsants and duloxetine were prescribed concomitantly in the 12 months post-index period as these are binary variables indicating if the patients filled any prescriptions of interests. Future research is warranted to elucidate the association between concomitant use of anticonvulsants and duloxetine among depressed patients with pain symptoms.
The study is subject to some limitations. The present study adopted a pre–post comparison design and did not include a comparison group of patients who were non-users of duloxetine. Thus, we were not able to address the effects of disease progression on the study outcome. We also did not require adherence to duloxetine once treatment was initiated, which can affect the healthcare utilization outcomes examined. Of the 909 patients in the study sample, 170 (18.7%), 294 (32.3%), and 109 (12.0%) patients had at least one prescription for a tricyclic, SSRI, or other antidepressant medication in the post-period, respectively. As a result, the post-period utilization may have been influenced by antidepressants other than duloxetine. Future studies employing a cohort comparison design that takes treatment adherence into consideration are needed to validate the findings from this study. Our study was also subject to the limitations of the data source used. There was a potential for under-estimating the prevalence of chronic conditions using the GPRD because GPs may not record patients’ comorbid conditions for each encounter. In order to increase the opportunities for capture of medical conditions, we extended the evaluation period for pre-period medical condition to 36 months. We also examined medication use reflective of underlying medical conditions. Despite extensive review by medical coding experts and researchers, the Read/OXMIS codes used in identifying medical conditions and healthcare utilization events may not be comprehensive and this can lead to under-reporting of conditions/medication use. By using pharmacy data in the GPRD, we could only assume patients took the medications as instructed, but we did not necessarily know if patients actually took the medications. Over-the-counter medications and prescriptions provided in a hospital or secondary care setting were not captured. Finally, our study results are not generalizable beyond the population covered by the UK’s primary care system.
Conclusions
This study was the first attempt to examine the relationship between treatment with duloxetine and healthcare utilization. Lower rates of analgesic use in the post-initiation period were observed among patients with pre-existing pain. The odds of all-cause hospitalization were lower in the post-initiation period than the pre-initiation period, and the reduction in the odds of hospitalization was most pronounced among patients with pre-existing pain and on treatment with anticonvulsants before starting duloxetine. This study also found no significant differences in the unadjusted rates of ER visits and specialist referrals between the pre- and post-initiation period for both the entire study sample and the sub-sample of patients with pre-existing pain.
Transparency
Declaration of funding
This study was fully funded by Eli Lilly and Company.
Declaration of financial/other relationships
NS, ZC, and ED are employees of Thomson Reuters and provided consulting services to Eli Lilly. AT was an employee of Thomson Reuters and provided consulting services to Eli Lilly. MH and AS receive salary and hold stocks from Eli Lilly that commercializes duloxetine and has financed this publication.
Acknowledgements
The authors acknowledge the contribution of Suellen Curkendall, PhD, Director of Outcomes Research at Thomson Reuters, who provided valuable feedback on the manuscript.
CMRO peer reviewers may have received honoraria for their review work. The peer reviewers on this manuscript have disclosed any relevant financial relationships.
References
- McCrone P, Dhanasiri S, Patel A, et al. Paying the price: the cost of mental health care in England to 2026. London: King’s Fund, 2008
- Ustun TB, Ayuso-Mateos JL, Chatterji S, et al. Global burden of depressive disorders in the year 2000. Br J Psychiatry 2004;184:386-92
- Thomas CM, Morris S. Cost of depression among adults in England in 2000. Br J Psychiatry 2003;183:514-9
- Anderson I, Pilling S, Barnes A, et al. Depression: the treatment and management of depression in adults. National Collaborating Centre for Mental Health Commissioned by the National Institute for Health and Clinical Excellence, 2009. United Kingdom
- Cowen PJ, Ogilvie AD, Gama J. Efficacy, safety and tolerability of duloxetine 60 mg once daily in major depression. Curr Med Res Opin 2005;21:345-56
- Perahia DG, Gilaberte I, Wang F, et al. Duloxetine in the prevention of relapse of major depressive disorder: double-blind placebo-controlled study. Br J Psychiatry 2006;188:346-53
- Kelin K, Berk M, Spann M, et al. Duloxetine 60 mg/day for the prevention of depressive recurrences: post hoc analyses from a recurrence prevention study. Int J Clin Pract 2010;64(6):719-26
- Perahia DG, Maina G, Thase ME, et al. Duloxetine in the prevention of depressive recurrences: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2009;70:706-16
- Hudson JI, Perahia DG, Gilaberte I, et al. Duloxetine in the treatment of major depressive disorder: an open-label study. BMC Psychiatry 2007;7:43
- Sheehan DV, Keene MS, Eaddy M, et al. Differences in medication adherence and healthcare resource utilization patterns: older versus newer antidepressant agents in patients with depression and/or anxiety disorders. CNS Drugs 2008;22:963-73
- Wade AG, Fernandez JL, Francois C, et al. Escitalopram and duloxetine in major depressive disorder: a pharmacoeconomic comparison using UK cost data. Pharmacoeconomics 2008;26:969-81
- Benedict A, Arellano J, De Cock E, et al. Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. J Affect Disord 2010;120:94-104
- Shi L, Liu J, Campbell C, et al. Factors associated with duloxetine treatment among patients with major depressive disorder in Veterans Health Administration: a retrospective study. Curr Med Res Opin 2010;26(12):2715-21
- Fava M, Mallinckrodt CH, Detke MJ, et al. The effect of duloxetine on painful physical symptoms in depressed patients: do improvements in these symptoms result in higher remission rates? J Clin Psychiatry 2004;65:521-30
- Arnold LM, Meyers AL, Sunderajan P, et al. The effect of pain on outcomes in a trial of duloxetine treatment of major depressive disorder. Ann Clin Psychiatry 2008;20:187-93
- Kutner M, Nachtsheim C, Neter J. Applied Linear Regression Models. 4th edn. McGraw–Hill Irwin, 2004. United States
- Leuchter AF, Husain MM, Cook IA, et al. Painful physical symptoms and treatment outcome in major depressive disorder: a STAR*D (Sequenced Treatment Alternatives to Relieve Depression) report. Psychol Med 2010;40:239-51
- Bair MJ, Robinson RL, Eckert GJ, et al. Impact of pain on depression treatment response in primary care. Psychosom Med 2004;66:17-22
- Tesfaye S. Advances in the management of diabetic peripheral neuropathy. Curr Opin Support Palliat Care 2009;3:136-43
- Zakrzewska JM. Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother 2010;11:1239-54
- Rao SG. Current progress in the pharmacological therapy of fibromyalgia. Expert Opin Investig Drugs 2009;18:1479-93
- Marcus DA. Fibromyalgia: diagnosis and treatment options. Gend Med 2009;6(2 Suppl):139-51