Abstract
Objective:
Medicaid infants are at high risk of severe respiratory syncytial virus (RSV) disease. The study objective was to estimate the cost-effectiveness of palivizumab in a Medicaid population.
Methods:
A societal cost-utility analysis was conducted of prophylaxis with palivizumab vs no prophylaxis among four groups of premature infants: (1) <32 weeks gestational age (wGA) and ≤6 months chronologic age (CA); (2) 32–34 wGA, ≤3 months CA with 2009 American Academy of Pediatrics (AAP) risk factors (RF); (3) 32–35 wGA, ≤6 months CA with 2006 AAP RF; and (4) 32–35 wGA, ≤6 months CA with ≤1 RF. Full dosing of palivizumab was assumed throughout the RSV season (consistent with the FDA-approved label). All costs were in 2010 US dollars. The societal public payer spend for palivizumab was estimated using Medicaid reimbursement methodologies for the top 10 palivizumab-using states in 2010 minus mandatory manufacturer rebates. This study reports the incremental cost-effectiveness ratios (ICERs) in cost per quality-adjusted life-year (QALY) gained. Sensitivity and probabilistic analyses were also conducted.
Results:
Palivizumab saved costs and improved QALYs among infants <32 wGA. Palivizumab was cost-effective in infants 32–34 wGA with 2009 AAP RF ($16,037 per QALY) and in infants 32–35 wGA with 2006 AAP RF ($38,244 per QALY). The ICER for infants 32–35 wGA with ≤1 RF was $281,892 per QALY. Influential variables in the sensitivity analysis included the background rate of RSV hospitalization, the cost of palivizumab, and the efficacy of palivizumab.
Key limitations:
These results are not generalizable to commercially insured infants or infants outside of the US.
Conclusions:
This is the first cost-utility analysis of palivizumab in a Medicaid population. Palivizumab, when dosed consistent with the FDA-approved labeling, was either cost-saving or cost-effective among current guideline-eligible infants in the Medicaid population. Palivizumab did not demonstrate cost-effectiveness in 32–35 wGA infants with ≤1 RF.
Introduction
Respiratory syncytial virus (RSV) is the leading cause of infant hospitalization in the USCitation1. Infants at highest risk for an RSV-related hospitalization are infants with chronic lung disease of prematurity (CLDP), children with hemodynamically significant congenital heart disease (CHD), or premature birth at less than 36 weeks gestational age (wGA)Citation2,Citation3. Several studies have suggested that the risk of RSV hospitalization is higher among ethnic minorities, including African Americans, Hispanics, and Native AmericansCitation1,Citation4–10. Several studies have reported disproportionate rates of prematurity and low birth weight among ethnic/minority populationsCitation11–13, thereby increasing the risk of severe RSV disease for this population. Furthermore, the rate of ethnic/racial groups in Medicaid in several states is higher compared with the overall ethnic/racial rate in the US populationCitation14. Observational studies also have indicated high rates of severe RSV disease among infants insured by MedicaidCitation1,Citation15,Citation16.
Palivizumab was approved by the Food and Drug Administration (FDA) in 1998. Palivizumab is indicated for the prevention of serious lower respiratory tract disease caused by RSV in pediatric patients at high risk for RSV diseaseCitation17. However, immunoprophylaxis for all infants born 32–35 wGA is not recommended by the American Academy of Pediatrics (AAP) due to ‘cost considerations’. According to the 2009 AAP guidelines, ‘economic analyses fail to demonstrate overall savings in healthcare dollars because of the high cost if all at risk infants receive prophylaxis’Citation18. Therefore, the 2009 AAP guidelines recommend prophylaxis with palivizumab in a sub-population of infants 32–34 wGA with risk factor criteria (i.e., being of young chronologic age CA [≤3 months] and the presence of ≥1 of the following two risk factors: day care attendance and/or having a sibling younger than 5 years). Approximately 1.6 million Medicaid births were financed in the US between 2002 and 2003, representing ∼40.8% of total births annually in the USCitation19. We estimate that ∼95,000 Medicaid births a year are premature infants of 32–35 wGA and that only 15% of these infants will meet current 2009 AAP eligibility criteria for RSV prophylaxis based on gestational age and risk factor criteria.Citation20
Few economic analyses examined RSV prophylaxis in Medicaid populationsCitation21–23. An earlier study by Shireman et al.Citation22 examined the cost-benefit ratio of RSV immune globulin and palivizumab in premature infants or infants with CLDP (10 months or younger) covered by the Kansas Medicaid program during the 1999–2000 RSV season, whereas Wegner et al.Citation23 examined the direct costs of palivizumab for infants born at 32–35 wGA in North Carolina Medicaid’s program during the 2002–2003 RSV season. Recently, Hampp et al.Citation21 examined the cost to avoid an RSV hospitalization among various high-risk cohorts using Florida Medicaid claims data during 2004–2005. Although these studies concluded that the costs of palivizumab exceeded the benefits, these studies did not measure quality-adjusted life-years (QALY), the unit of the effectiveness of a drug or therapy that incorporates the quality and quantity of life gained compared with no therapy in a cost-utility analysis. Furthermore, these studies did not factor in all direct medical costs after the initial RSV episodeCitation24 or the significant federally-mandated rebates public payers receive from the manufacturerCitation25–Citation27. The US Panel of Cost-Effectiveness in Health and Medicine and the 2000 Institute of Medicine report Vaccines for the Twenty-First Century recommended dollars per QALY using the societal perspective as the standard for US cost-effectiveness evaluationsCitation28,Citation29. A cost-effectiveness analysis based on cost per QALY gained, instead of cost per event avoided as previously reported, would allow comparisons of the value of drug therapies across multiple disease areas.
The previous economic studies of palivizumab across various Medicaid programs did not account for the effect of risk factors on severe RSV disease in their economic evaluations. Although prematurity alone can significantly increase the risk for serious RSV disease, several studies have shown that the presence of other risk factors increases the risk of RSV hospitalization. Some risk factors include infant exposure to tobacco smoke, day care attendance, and crowded residential living conditionsCitation30–33. Multivariate regression techniques and risk-scoring tools from Canada and Spain have recently been developed to identify high-risk populations for serious RSV diseaseCitation31,Citation32,Citation34–39. These studies have shown an independent effect of various risk factors on RSV hospitalization. In addition, the prospective 2-cohort study in infants of 32–35 wGA by Figueras-Aloy et al.Citation32 demonstrated that the presence of ≥2 risk factors increases the risk of RSV hospitalization in an additive to multiplicative fashion depending on the risk factors. Infants with school-aged siblings, a mother smoking any number of cigarettes daily during pregnancy, and young CA (i.e., ≤10 weeks at the start of the RSV season) had an overall odds ratio for RSV hospitalization of 9.89 (3.96–24.67) compared with an infant without any risk factors.
The incorporation of risk factors in cost-effectiveness analyses (CEAs) could significantly affect the results. A recent CEA conducted in Canada included risk factor data from a validated risk-scoring tool using the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC) datasetCitation35,Citation37. Infants classified as ‘moderate risk’ and ‘high risk’ from the risk-scoring tool had RSV hospitalization rates of 7.1% and 18.7%, respectivelyCitation37 compared with low-risk infants who had a hospitalization rate of 1.7%. The results of the Canadian cost-effectiveness model indicated that the incremental cost-effectiveness ratios (ICERs) for RSV prophylaxis in infants of 33–35 wGA classified as moderate or high risk were acceptable under their particular healthcare system (moderate-risk group: C$34,215 per quality adjusted life year [QALY]; high-risk group: C$5,765 per QALY)Citation35. Lanctot et al.Citation35 also demonstrated that the ICERs became more compelling as the number of risk factors increased. Similarly, other studies have demonstrated that palivizumab is cost-effective across several high-risk populations outside the USCitation40–43.
In light of these recent findings, the objective of this analysis was to assess the cost-effectiveness of palivizumab prophylaxis in premature infants in Medicaid programs in the US. To our knowledge, this is the first cost-utility analysis of palivizumab conducted in a Medicaid population using Medicaid-specific data. Our economic analysis includes healthcare costs associated with severe RSV disease in Medicaid infants and children, utilities associated with the RSV-related hospitalization, and an estimate of the societal spend for palivizumab that incorporates manufacturer rebates. A Medicaid-specific cost-effectiveness analysis would help decision-makers in setting RSV prophylaxis policies that target patients who would benefit the most from prophylaxis against severe RSV diseaseCitation44.
Methods
Study population
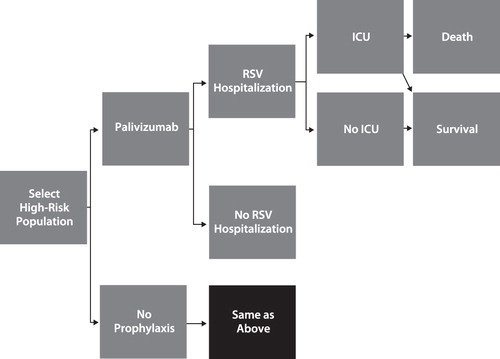
We conducted a cost-effectiveness analysis of prophylaxis with palivizumab vs no prophylaxis. The cost-utility model used the societal perspective and, therefore, includes direct and indirect costs. A decision tree model () was constructed to compare the cost and outcomes of RSV prophylaxis among four Medicaid premature infant populations: (1) <32 wGA and ≤6 months CA; (2) 32–34 wGA, ≤3 months CA with 2009 AAP risk factors (i.e., having siblings <5 years of age and/or attending day care); (3) 32–35 wGA, ≤6 months CA with 2006 AAP risk factors (any two of the following: exposure to environmental air pollutants, congenital abnormalities of the airways, severe neuromuscular disease, school-age siblings, day care attendance); and (4) 32–35 wGA, ≤6 months CA with ≤1 risk factor as described by Sampalis et al.Citation37. We examined full dosing of palivizumab (≤5 doses depending on month of birth with respect to the RSV season start, consistent with the FDA-approved labeling). Our model did not examine the 2009 AAP recommendation of a maximum of three doses for infants of 32–34 wGA because the clinical efficacy associated with this dosing administration is unknown. We excluded infants and children who were pre-term and who had CLDP or hemodynamically significant CHD from our analysis. Although premature infants with CLDP and children with CHD are included in the labeled indication for palivizumab, we only examined premature infants without these conditions to control for the additional effect of comorbidity on the risk of RSV hospitalization. CA was determined at the start of the RSV season (November 1).
Model structure
As shown in , the infants of interest received either prophylaxis with palivizumab or no prophylaxis. The clinical pathway simulated various clinical events associated with serious RSV disease. Based on a Medicaid study of healthcare utilization and costs among RSV premature infants conducted by Palmer et al.Citation45 and Shi et al.Citation24, the RSV hospitalization was estimated to occur on average 2.4 months after birth. Among those infants with an RSV hospitalization, there is a probability that a proportion of them will require care in the pediatric intensive care unit (ICU). Of those infants in the pediatric ICU, the outcomes are either death during the hospital stay or survival. In our model, mortality is incorporated for pediatric RSV ICU patients only. In accordance with guidance from the US Panel on Cost-Effectiveness in Health and MedicineCitation29, we analyzed a lifetime horizon. The model rolls back the life expectancy (77.45 yearsCitation46), after discounting quality-of-life adjustments at 3% annually. We varied the discount rate from 0–5% in sensitivity analyses.
Model assumptions
We made several assumptions in our economic analysis of palivizumab. We assumed risk of RSV infection only in the first RSV season, defined in our model as November 1 to March 31, although the start and length of the RSV season can vary across geographic regions in the US. Infants received ≤5 monthly doses of palivizumab throughout the RSV season, consistent with the dosing regimen in the registration trials for palivizumab. Infants were assumed to have received prophylaxis in the outpatient setting during a periodic preventive medicine visit. We assumed that the first dose of prophylaxis for infants born during the RSV season was administered during the birth hospitalization before discharge. We assumed 100% compliance with palivizumab and 5% drug wastageCitation47. Similar to economic models for vaccines, our model uses a baseline utility of 1.0 for infantsCitation29,Citation48–52. Life expectancy after surviving RSV hospitalization for premature infants was assumed to be equivalent to the life expectancy for the general US population and unchanged across infant gestational age categories. Three caregiver hours were lost owing to administration of palivizumab; caregiver time lost owing to RSV hospitalization per day was assumed to be 8 h. We did not examine prophylaxis in home health settings, which may be associated with lower costsCitation53,Citation54. Because the IMpact-RSV Clinical Study did not show a statistically significant difference in reported adverse events, we did not incorporate adverse events–related costs in the analysis. Some cases of anaphylaxis, including fatal cases, have been reported in post-marketing reporting.
Respiratory syncytial virus and its complications
The background rates for RSV hospitalization are expected to vary according to the risk profiles of each of the four populations of interest (). We first estimated the overall background rates of serious RSV disease in the general population and then adjusted these rates based on the data that suggest that the rates of RSV hospitalization are high among Medicaid and ethnic/minority populationsCitation1,Citation4–10. For infants <32 wGA and ≤6 months CA, we used RSV hospitalization data from a recent meta-analysis of 10 studies examining prophylaxis with palivizumab vs no prophylaxisCitation55. Checchia et al.Citation55 found a 10.6% RSV hospitalization background rate for pre-term infants ≤32 wGA. The meta-analysis, however, did not specify rates for the other cohorts in our model. Therefore, we used the estimate of 7.1% for 32–34 wGA infants with 2009 AAP risk factors and 32–35 wGA infants with 2006 AAP risk factors, and a 1.7% RSV hospitalization rate was used for 32–35 wGA infants with ≤1 risk factor based on the moderate- and low-risk groups from the PICNIC study in CanadaCitation37.
Table 1. Probabilities used in the model by palivizumab prophylaxis and no prophylaxis.
Because Medicaid infants use hospitalization services frequentlyCitation56,Citation57, we adjusted the expected background RSV hospitalization rates for the four groups of premature infants by converting the original background RSV hospitalization rates (10.7%, 7.1%, 1.7%) to odds (O = P/[1 − P], where O = odds and P = probability). We then multiplied the baseline odds by 2.03 (95% CI = 1.99–2.06), which represented the increased odds of RSV hospitalization among Medicaid compared with non-Medicaid infants and children in CaliforniaCitation9. Few studies have compared the rates of RSV hospitalization among Medicaid-insured infants compared with commercially-insured infants. Frogel et al.Citation16 also found increased odds of RSV hospitalization among Medicaid vs non-Medicaid infants (odds ratio = 1.76; p < 0.0001), although this particular study was a prospective cohort registry enrolling those who received ≥1 dose of palivizumab during any RSV season (2000–2004) in the US. These revised odds ratios were then transformed back to probabilities of RSV hospitalization for Medicaid infants using the formula P = O/(1 + O). The odds of RSV hospitalization were 18.5% (<32 wGA), 13.4% (32–34 wGA with 2009 AAP risk factors and 32–35 wGA with 2006 AAP risk factors), and 3.4% (32–35 wGA with ≤1 risk factor) in our Medicaid population.
Intensive care unit admission
We used data from a recently published study that examined the characteristics of RSV hospitalizations among high-risk infantsCitation58 to inform our model on the rates of ICU admission among those hospitalized with RSV. Specifically, Forbes et al.Citation58 examined the average cost of RSV hospitalization across several RSV seasons and characterized the RSV hospitalization (length of stay, ICU admission, readmissions within 30 days) among premature infants compared with full-term infants. Approximately 20% of premature infants hospitalized for RSV required an ICU admissionCitation58. For infants <32 wGA, we used a 19.5% ICU admission rate based on the rates among early–pre-term infants (<33 wGA) in the Forbes et al.Citation58 study. We used a 21.9% ICU admission for infants of 32–34 wGA with 2009 AAP risk factors, 32–35 wGA with 2006 AAP risk factors, and 32–35 wGA with ≤1 risk factor based on the rates among late–pre-term infants in the Forbes et al.Citation58 study (33–36 wGA). This is lower than the 30–48.4% rates of ICU admission among premature infants with RSV lower respiratory tract infection (LRI) previously described by Horn and SmoutCitation59. Using probabilities from Forbes et al.Citation58 instead of Horn and SmoutCitation59 is more conservative because it biases the model towards no prophylaxis.
Case fatality in intensive care unit
We modeled a case fatality rate in the ICU of 6.1% for infants of 32–34 wGA with 2009 AAP risk factors, 32–35 wGA with 2006 AAP risk factors, and 32–35 wGA with ≤1 risk factor and 4.3% for <32 wGA as per Gunville et al.Citation60 This study examined 271 patients who were admitted to the pediatric ICU with respiratory distress, the majority with RSV LRI. These data are also consistent with an analysis by Thorburn et al.Citation61 who observed an RSV pediatric ICU case fatality rate of 4.1% among premature infants hospitalized in the UK. We chose the point estimates from Gunville et al.Citation60 because they are more recent, germane to pre-term populations of interest, and are US data. We only modeled mortality for those requiring an ICU stay. By choosing to incorporate mortality only in an ICU setting and not incorporating mortality for all other patients, we are using a conservative approach.
Palivizumab effectiveness
The relative risk reduction (RRR) for RSV hospitalization with palivizumab used in the model was from the post-hoc analysis of infants of ≤35 wGA without CLDP from the IMpact-RSV Clinical StudyCitation3,Citation20. Specifically, we used a 75.1% RRR in RSV hospitalization rates for infants ≤32 wGA (1.78% palivizumab; 7.14% placebo). We used an 82.2% RRR (1.78% palivizumab; 10% placebo)Citation3,Citation20 for infants of 32–34 wGA with 2009 AAP risk factors, 32–35 wGA with 2006 AAP risk factors, and 32–35 wGA with ≤1 risk factor.
Relative risk reduction on intensive care unit admission among hospitalized infants
We incorporated a 63% RRR (11.1% palivizumab [2/13] vs 30% placebo [12/31]) in the incidence of ICU admissions among those who were hospitalized with RSV based on post-hoc analysis of infants ≤35 wGA without CLDP from the IMpact-RSV Clinical StudyCitation3,Citation20. The 63.0% RRR in ICU admissions was used across all cohorts in the model.
Costs
Costs were categorized as palivizumab prophylaxis costs and RSV-related direct costs and indirect costs (). Palivizumab prophylaxis costs included prophylaxis acquisition and administration costs, direct non-medical costs (i.e., transportation costs), and indirect costs (i.e., caregiver time lost from work and usual activities attributable to prophylaxis). RSV direct costs were specific to a Medicaid population and based on an analysis of total healthcare costs within 12 months of an initial RSV episode using a national Medicaid claims databaseCitation24. RSV indirect costs consisted of caregiver time lost from work and usual activities attributable to severe RSV. Costs have been adjusted to US 2010 dollars, when necessary, using the medical care services component of the consumer price indexCitation62.
Table 2. Cost of drug administration and RSV-related outcomes adjusted to 2010.
The recommended dosage of palivizumab is 15 mg/kg delivered intramuscularly monthly during the RSV season. We modeled dosing regimens that started in November lasting through the end of March. To determine the cost of prophylaxis, the model determined the number of doses an infant received based on their month of birth and predicted their corresponding chronologic and gestation-adjusted age at each monthly administration of prophylaxis for the general population of infants. Dosing stopped at the end of March.
The amount of each dose (15 mg/kg) depends on the infant’s weight at prophylaxis administration, which is predicted by the chronologic and gestation-adjusted age (i.e., the infant’s CA minus the number of weeks of prematurity) at prophylaxis administration using gestational age-adjusted infant growth curvesCitation63. More specifically, the 50th percentile growth curve for very low birth weight infants (defined as birth weight ≤1500 g) was used to predict patient weight at time of administration for infants <32 wGACitation63. The 50th percentile growth curve for low-birth weight infants (defined as birth weight 1501–2500 g) was used to predict patient weight for moderately premature infantsCitation63. To adjust CA for prematurity, the mean gestational age for each cohort is required. Using the 2006 US Natality dataCitation64, our estimate for the mean gestational age for infants <32 wGA was 28.6 weeks. The mean gestational age was 33.3 wGA for infants 32–34 wGA with 2009 AAP risk factors. The mean gestational age was 34.2 wGA for infants 32–35 wGA with 2006 AAP risk factors and 32–35 wGA with ≤1 risk factor. Because of the weight-based dosing of palivizumab, the model calculations are based on a cost-per-milligram value that is derived from the average cost per milligram for 100-mg and 50-mg vials of palivizumab, respectively.
The model includes an estimated average public payer spend, which was calculated by taking the average reimbursement methodology of the top 10 palivizumab-using states in 2010 under Medicaid programs (ingredient cost and dispense fee) minus the unit rebate amount from the fourth quarter of 2010Citation20,Citation27. Providers who dispense outpatient drugs to Medicaid patients are reimbursed by the state Medicaid based on a formula determined by the individual state program. The reimbursement formula varies from state to state and often contains two components that are denoted as ingredient cost and dispensing fee, which are combined to produce a single total reimbursement to the provider. In addition, federal law mandates that state Medicaid programs receive a significant rebate directly from the drug manufacturer for each unit of product dispensed.
RSV-related costs
Direct costs associated with RSV were based on a recent analysis of utilization and cost data from a national Medicaid database, including healthcare data from patients across 12 statesCitation24,Citation45. A retrospective cohort study was conducted among a population of Medicaid-insured premature infants (33–36 wGA) with RSV LRI (n = 9180) and without RSV LRI (n = 19,110) during two RSV seasons (2003–2005). The primary outcome was average total healthcare cost within the 1-year period following the first RSV LRI diagnosis date. In this population of Medicaid-insured infants who were hospitalized with RSV, the total direct medical costs within 1 year after an RSV LRI hospitalization episode was $22,288 and significantly higher compared with the 1-year costs among a comparison group of infants without RSV LRI ($4823), representing an incremental cost difference of $17,465 (p < 0.001)Citation24,Citation45.
Our cost-effectiveness model of palivizumab was from the societal perspective and, therefore, included indirect costs. Indirect costs associated with RSV included caregiver time lost from work or usual activities (8 h per hospitalization day) resulting from RSV hospitalization. The cost of caregiver time off work was based on the hourly compensation from the US Department of LaborCitation62. Transportation costs were taken from Luce et al.Citation65
Quality-adjusted life-years and utilities
Health utilities were applied in our economic analysis. Similar to economic models for vaccines, our model used a baseline utility of 1.0 for infantsCitation29,Citation48–52. The utility decrement for RSV hospitalization and post-hospitalization recovery period was estimated in a prospective controlled study of children and parents with and without RSV hospitalizationCitation66 (). To estimate total QALYs lost, we used the hospital length of stay for estimating the QALYs lost from an RSV hospitalization and a 60-day period for estimating QALYs lost from the post-hospitalization recovery periodCitation66.
Table 3. Health utilities incorporated in the cost-effectiveness model.
Analyses
The primary outcome was the ICER, calculated as the difference between the average costs for each group divided by the difference between the average QALYs for each group. An ICER within the range of other pediatric preventative therapies such as currently recommended vaccines ($0–$157,000 QALY)Citation49,Citation67–74 was considered cost-effective.
Sensitivity analyses
Extensive one-way and probabilistic sensitivity analyses were conducted to ascertain which variables were most influential in the model. Variables that had a substantial impact on the model results included background risk of RSV hospitalization, societal cost of palivizumab in the public sector, and palivizumab efficacy. The parameter ranges (minimum and maximum values) used were identified from the literature or through post-hoc analysis of palivizumab clinical trial studies wherever possible.
The background risk for RSV hospitalization was an influential variable in previous economic models. There are no Medicaid studies that have examined the risk of RSV hospitalization among the exact cohorts in our model; therefore, the minimum and maximum values for the background rates of RSV hospitalization used in sensitivity analysis were based on extrapolation on the available evidence in the literature. The minimum background risk of RSV hospitalization for infants <32 wGA, ≤6 months CA was taken from the Boyce et al.Citation15 study, which examined rates of RSV hospitalization among infants and children in Tennessee Medicaid. Boyce et al. found that the rate of RSV hospitalization among infants of ≤28 wGA (0 to <6 months) was 9.4%. We used a maximum value (34.2%) from Boyce et al.Citation15, that reflects the average rates of RSV hospitalization between CLDP and CHD infants (0–6 months). We assumed that the upper-bound parameter estimate of RSV hospitalization for infants <32 wGA could be similar to rates among infants with comorbidities given that extremely premature infants have immature lung development and function. In addition, a majority of these infants are exposed to mechanical ventilation which introduces additional trauma to the lungs. The minimum background rate of RSV hospitalization for infants of 32–34 wGA with 2009 AAP risk factors and 32–35 wGA with 2006 AAP risk factors (8%) was taken from Boyce et al.Citation15 and represented the RSV hospitalization rate of infants 33 to <36 wGA (0–6 months). We also used the RSV hospitalization rate of 34.2% from Boyce et al.Citation15 to represent the upper-bound parameter estimate of RSV hospitalization. For 32–35 wGA with ≤1 risk factor, we used 1.7% from Sampalis et al.Citation37 to represent the lower-bound parameter estimate and 6.6% from Wegner et al.Citation23 to represent the upper-bound parameter estimate. The 6.6% RSV hospitalization rate was among infants 32–35 wGA who did not receive RSV prophylaxis under North Carolina Medicaid. The minimum and maximum values for RRR of RSV hospitalization were taken from the 95% CI of the post-hoc analysis of the IMpact-RSV Clinical StudyCitation3,Citation20.
Data regarding case fatality rates among RSV infants in the ICU setting are few. For a lower-bound parameter estimate, we examined a 3.0% mortality rate among RSV hospitalized infants (CHD infants <2 years of age) based on Yount et al.Citation75 For an upper-bound parameter estimate, we used an 8.11% mortality rate after RSV hospitalization among premature infants born at 32–35 wGA from Sampalis et al.Citation76
The estimated societal public spend for palivizumab was an influential variable in the model. We calculated ±20% of the estimated societal public spend for palivizumab. Furthermore, we examined the 2010 wholesale acquisition cost (WAC) for palivizumab to represent an upper-bound parameter estimate in sensitivity analysis (100-mg vial, $2027.80; 50-mg vial, $1073.88)Citation77. The values for other variables in one-way sensitivity analyses are presented in .
Probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA)Citation78 was also conducted across all model parameters simultaneously. Monte Carlo techniques were used according to pre-specified model distribution assumptions (beta, gamma, normal, and lognormal) based on the characteristics of each parameterCitation78. The background risk of RSV hospitalization, the background risk of ICU admission, case fatality rate in the ICU, drug wastage, and discount rates were based on beta distributions. The RRR of RSV hospitalization and RRR of ICU admission were based on log-normal distributions. Cost data were based on gamma distributions because these take into account the likely skew and variability with these parameters. The estimation of the distribution ranges was based on the published data or, if not reported, a confidence interval was assumed to equal 20% of the deterministic value and the standard errors were calculated. Results of the probabilistic sensitivity analysis were based on 1000 Monte Carlo simulations.
Results
Palivizumab prophylaxis was cost-effective compared with no RSV prophylaxis among high-risk infants. In the base-case analysis, infants <32 wGA and ≤6 months CA who received prophylaxis with palivizumab had lower average costs of care compared with those without prophylaxis, resulting in a cost savings of $2339 (). The QALY gained for infants <32 wGA was 0.046. Therefore, among infants <32 wGA and ≤6 months CA, palivizumab was a dominant strategy compared with no prophylaxis (i.e., saved costs and improved QALY). Infants of 32–34 wGA and ≤3 months CA with 2009 AAP risk factors who received RSV prophylaxis had higher average costs of care compared with those not receiving prophylaxis for a net cost increase of $864 per infant. Infants with palivizumab prophylaxis had, on average, fewer hospitalization costs than infants without palivizumab prophylaxis ($519 vs $2921, respectively). Prophylaxis with palivizumab resulted in an additional 0.0539 QALY gained per infant. The incremental cost per (additional) QALY gained was $16,037. For infants 32–35 wGA, ≤6 months CA with 2006 AAP risk factors, there was a net increase cost of $2060 and a net QALY gained of 0.0539, resulting in an ICER of $38,244. In contrast, for infants 32–35 wGA, ≤6 months with ≤1 risk factor, there was a net increase cost of $3853 and a net QALY gained of 0.014, resulting in an ICER of $281,892. Results for these populations are presented in the context of ICERs for various vaccines in the USCitation49,Citation70–74 ().
Figure 2. Benchmark of palivizumab cost-effectiveness results with those of vaccines. AAP = American Academy of Pediatrics; DTaP = diptheris, tetanus, and acellular pertussis vaccine; HPV = human papillomavirus vaccine; RF = risk factor; TIV = trivalent inactivated influenza vaccine; wGA = weeks gestational age.
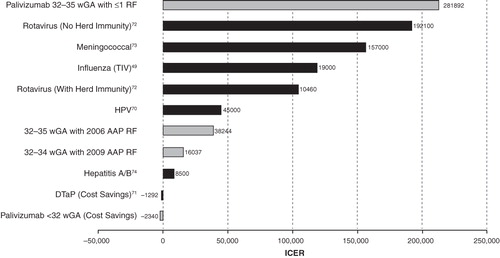
Table 4. Net costs and QALYS between palivizumab prophylaxis and no prophylaxis among the 4 cohorts.
One-way and probabilistic sensitivity analyses
One-way sensitivity analysis determines the impact of each variable on baseline cost-effectiveness results ( in the Appendix). Overall, the baseline results for palivizumab remained robust in one-way sensitivity analyses across all high-risk cohorts. Greater variability in sensitivity analysis results were observed among infants of 32–35 wGA with <1 risk factor. In infants <32 wGA, ≤6 months CA, palivizumab prophylaxis was either dominant or within accepted CEA thresholds in one-way sensitivity analyses. Palivizumab was cost-effective in infants 32–34 wGA with 2009 AAP risk factors and infants 32–35 wGA with 2006 AAP risk factors when the background RSV hospitalization rate was decreased from 13.4% to 8% (i.e., the minimum parameter estimate). Baseline cost-effectiveness results were also within CEA thresholds when using 2010 WAC palivizumab costs (i.e., the maximum parameter estimate without incorporating manufacturer rebates). Overall, the background risk of RSV hospitalization, the cost of palivizumab, and the efficacy of palivizumab were some of the most influential variables on the base case results across cohorts.
Table 5. One-way sensitivity analysis for infants <32 wGA, ≤6 months CA.
Table 6. One-way for infants 32–34 wGA, ≤3 months CA with 2009 AAP RF.
Table 7. One-way for infants 32–35 wGA, ≤6 months CA with 2006 AAP RF.
Table 8. One-way sensitivity analysis for infants 32–35 wGA, ≤6 months CA with ≤1 RF.
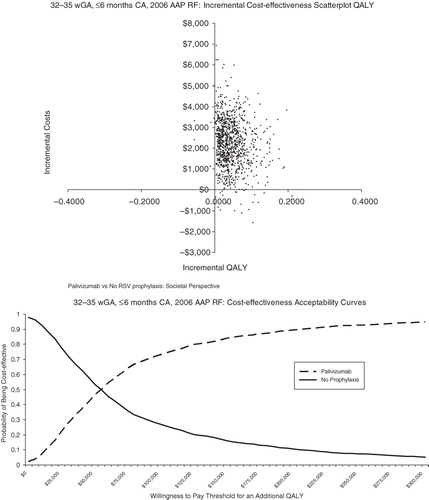
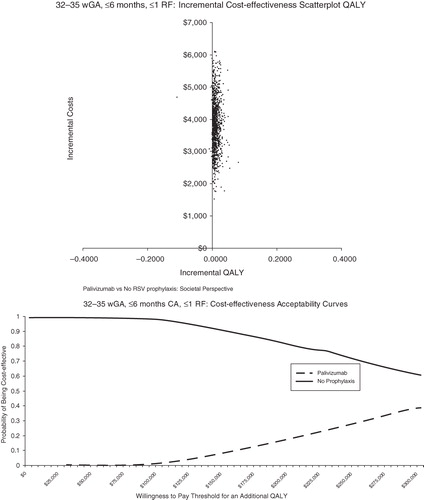
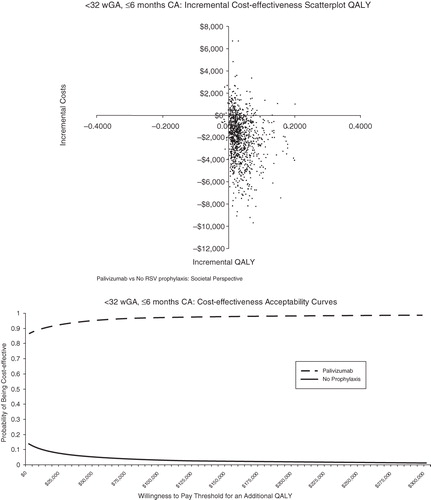
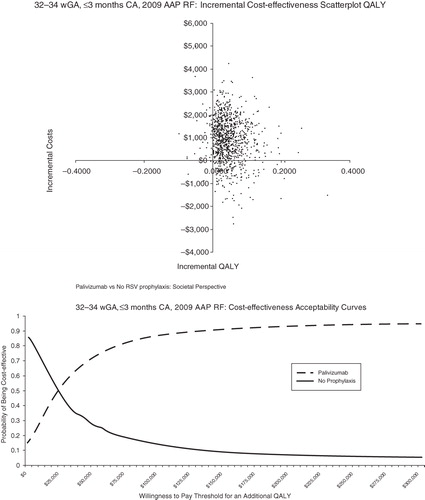
Probabilistic sensitivity analysis was conducted to allow the joint effect of parameter uncertainty to be simultaneously assessed ( in the Appendix). The scatterplot reflects the incremental cost and incremental QALYs generated for palivizumab compared with no prophylaxis for each run of the probabilistic sensitivity analysis. The cost-effectiveness acceptability curves present the probability of cost-effectiveness at different willingness-to-pay thresholds for an additional QALY. For <32 wGA infants, 6 months CA, palivizumab was found to be cost-effective at all willingness-to-pay thresholds for an additional QALY ($0–$300,000 QALY). For infants 32–34 wGA with 2009 AAP risk factors and infants 32–35 wGA with 2006 AAP risk factors, palivizumab was found to be cost-effective the majority of the time at relatively low willingness-to-pay thresholds for an additional QALY. For infants 32–35 wGA with ≤1 risk factor, palivizumab was generally not cost-effective.
Figures 3 and 4. Probabilistic analyses <32 wGA, ≤6 months CA. CA = chronologic age; QALY = quality-adjusted life-year; wGA = weeks gestational age.
Figures 5 and 6. Probabilistic analyses for 32–34 wGA, ≤3 months CA with AAP 2009 RF. AAP = American Academy of Pediatrics; CA = chronologic age; QALY = quality-adjusted life-year; RF = risk factor; wGA = weeks gestational age.
Discussion
To our knowledge, this is the first cost-utility analysis of palivizumab in a Medicaid population. It has been conducted according to the guidelines set forth by the US Panel on Cost-Effectiveness in Health and MedicineCitation29. We found that palivizumab was cost-effective among various high-risk premature infants without CLDP or CHD in a Medicaid population. Palivizumab was cost-effective compared with no RSV prophylaxis among infants <32 wGA, ≤6 months CA; infants 32–34 wGA, ≤3 months CA with 2009 AAP risk factors; and infants 32–35 wGA, ≤6 months CA with 2006 AAP risk factors. Palivizumab was not cost-effective among infants 32–35 wGA, ≤6 months CA with ≤1 risk factor. Results were marginally favorable among infants with 32–34 wGA, ≤3 months CA with 2009 AAP risk factors compared with 32–35 wGA, ≤6 months CA with 2006 AAP risk factors because the former cohort of infants weigh less than their older counterparts, resulting in lower palivizumab costs. Our cost-effectiveness analysis of palivizumab is the first to examine 2006 and 2009 AAP eligibility guidelines for premature infants within the 32–35 wGA group. Our findings indicate that palivizumab is cost-effective in both a broader (2006 AAP) and narrower (2009 AAP) group of 32–35 wGA infants covered by Medicaid who have multiple risk factors. Given the favorable cost-effectiveness findings for both groups, future policy should focus on identifying an algorithm that captures most high-risk infants in this GA group.
The methodological approach used in previous economic analyses of palivizumab in Medicaid populations focused on measuring cost per RSV hospitalization avoided and did not estimate QALYs. These studies concluded that RSV prophylaxis was not cost-effective in their respective Medicaid populationsCitation21–23. These studies, however, did not examine the impact of ICU care and other direct medical costs associated with severe RSV disease. A recent systematic review of cost-effectiveness analyses of palivizumab conducted by Smart and colleaguesCitation79 found that palivizumab was cost-effective when health utilities were incorporated in the model, whereas studies that used observational data instead of clinical trial data and did not incorporate health utilities tended to have lower measured study quality using criteria based on the Quality of Health Economic StudiesCitation79. A cost-effectiveness result represented by QALY is the recommended methodology by health economists that allows comparisons of the value of a drug or treatment across multiple disease areasCitation28,Citation29.
Previous economic models of palivizumab estimated the burden of serious RSV disease using claims data and examined International Classification of Diseases, Ninth Edition, RSV-specific codes onlyCitation21. This methodology underestimates the rate of RSV hospitalization because most of these hospitalizations are billed in claims data as unspecified bronchiolitis or unspecified pneumonia. Flaherman et alCitation80 recently published RSV testing rates from Kaiser Permanente in Northern California and estimated that 65% to 85% of severe RSV disease is underdiagnosed. Low rates of RSV testing are not surprising given that the American Academy of Pediatrics does not routinely recommend RSV testing for clinically diagnosed bronchiolitis because the knowledge gained from such testing rarely alters management decisions or outcomesCitation18.
Furthermore, many of the previous economic studies did not incorporate federally mandated manufacturer rebates under the Medicaid program. A task force of health economists from the International Society of Pharmacoeconomics suggests that with public health programs, “the drug cost must represent the actual acquisition costs, incorporating any rebates or discounts”Citation26.
The background rate of RSV hospitalization was an influential variable in our economic analysis of palivizumab. Evidence suggests that the background risk of RSV hospitalization is higher among ethnic minorities or Medicaid infantsCitation1,Citation4–10. Some studies have found increased prematurity rates and low-birth weight among minorities, including African AmericansCitation81, and prematurity status increases the risk of severe RSV disease. Ethnic minorities also face increased exposure to potential risk factors for RSV, such as tobacco exposure, as reported in the 1998 Surgeon General’s Report on Tobacco Use Among U.S. Racial/Ethnic Minority GroupsCitation82. African Americans (37.6%) and American Indians and Alaska natives (36.95%) were more likely than other groups to report exposure to environmental tobacco smoke at home. The burden of RSV hospitalization among Medicaid infants may also be due to issues related to low compliance rates with RSV prophylaxisCitation83.
The cost-effectiveness results of RSV prophylaxis in our base-case analysis for AAP guideline-eligible, high-risk infants were comparable with those of recent pediatric vaccine intervention studies. Our results for <32 wGA, <6 month CA are similar to DTaP vaccine, ie, both are cost-saving and increase health outcomes compared with no intervention. Ray et alCitation84 examined the benefits of pneumococcal conjugate vaccination among children ≤23 months of age and found cost-effectiveness ratios of $112,000 per life-year saved without herd immunity and $7500 with herd immunity. Similarly, Goldie et alCitation85 found that the most cost-effective strategy for human papillomavirus 16/18 vaccine was starting vaccination at 12 years of age combined with triennial conventional cytologic screening beginning at 25 years of age, which had a cost-effectiveness ratio of $60,000 per QALY. Finally, Shepard et alCitation86 examined the strategy of universal conjugate meningococcal vaccination of all US adolescents starting at 11 years of age and found the cost-effectiveness ratio to be $121,000 per life-year saved, whereas the cost-effectiveness of meningococcal vaccine (2 doses, all 11- and 16-year-olds) was found to be $157,000 cost per QALY gainedCitation73. Our palivizumab cost-effectiveness results for infants 32–34 wGA, ≤3 months CA with 2009 AAP risk factors and infants 32–35 wGA, ≤6 months CA with 2006 AAP risk factors are similar to the lower ICER results of a number of recommended vaccines by both the Advisory Committee on Immunization Practices and the AAP.
Our analysis makes several important conservative assumptions. First, we did not attribute a reduction in hospital length of stay resulting from palivizumab prophylaxisCitation87. Second, the model did not include RSV disease presenting in outpatient settings. The incidence of outpatient RSV LRI visits and associated costs can be substantial on the healthcare systemCitation45,Citation88,Citation89. Third, we conservatively did not assume a mortality risk reduction due to palivizumabCitation55 and modeled a background case fatality rate among RSV ICU patients only. Fourth, we did not value parents’ quality-of-life decrements when their child was hospitalized with RSVCitation66. Some have advocated that pediatric cost-effectiveness analyses should value the effect on both the parents and child because these outcomes are real for both parties.Citation90 Fifth, per the Panel on Cost-Effectiveness in Health and Medicine, we did not value the lifetime earnings lost (indirect costs) of infants because we include infant quality of life. Sixth, we did not assume a reduction in recurrent wheezingCitation91. Should any of these aforementioned conservative assumptions be included in the analysis, the cost-effectiveness profile of RSV prophylaxis with palivizumab would likely improve.
There are several limitations to this economic analysis. More evidence on RSV-specific mortality with and without palivizumab prophylaxis is needed. Non-compliance with palivizumab is a common problem in Medicaid populations, and future economic analyses can determine the cost-effectiveness of noncompliance versus compliance with palivizumab. Our economic model did not incorporate other strategies to prevent RSV infection, such as the effect of parent education, but they are likely to be consistent between prophylaxis and no-prophylaxis groups. Therefore, inclusion of these strategies should not have a major impact on the results. Our results are not generalizable to commercially insured infant populations or infants outside of the US. Despite these limitations, the strengths of our model include using Medicaid-specific healthcare data and incorporating federally mandated rebates in estimating the societal public spend for palivizumab. We were able to ascertain the important drivers of cost-effectiveness for RSV prophylaxis for each subpopulation of interest in our sensitivity analysis. Finally, we specifically utilized the efficacy in non-CLDP premature infants (75.1%–82.2% RRR)Citation3,Citation20.
In sum, the morbidity and associated healthcare needs of Medicaid-insured infants and children remain a public health priority in the US. This vulnerable population includes a disproportionate number of premature infants, those born with low birth weight, racial/ethnic minorities, and infants with increased exposure to environmental risk factors for RSV disease, such as tobacco exposure. Additional data are needed regarding the burden of RSV disease among the Medicaid populations in various geographic regions of the US, access to care with palivizumab, and long-term health outcomes.
CONCLUSIONS
This is the first cost-utility analysis of palivizumab in a Medicaid population. Using conservative assumptions, we conclude that RSV prophylaxis was highly cost-effective for a population of Medicaid infants <32 wGA and ≤6 months CA (palivizumab was a dominant strategy compared with no prophylaxis); 32–34 wGA, ≤3 months CA with 2009 AAP risk factors; and 32–35 wGA, ≤6 months CA with 2006 AAP risk factors. RSV prophylaxis was not cost-effective for infants 32–35 wGA, ≤6 months CA with ≤1 risk factor. Incorporating risk factor data favorably changes the cost-effectiveness profile of RSV prophylaxis because the risk of RSV hospitalization increases among infants who have >1 risk factor. Palivizumab is cost-effective in a population of Medicaid infants owing to the combination of lower cost structure for healthcare items and services, manufacturer rebates, and higher rates of RSV disease. Cost-effectiveness results for palivizumab among high-risk infants in a Medicaid population were found to be in the range of those for vaccines recommended by Advisory Committee on Immunization Practices and AAP. The de facto assumption that RSV prophylaxis is not cost-effective should be reexamined. Palivizumab administration with full-season dosing among high-risk infants in the Medicaid population was cost-effective.
Transparency
Declaration of funding:
The study was funded by MedImmune, LLC
Declaration of financial/other relationships:
PM and AM have disclosed that they are employees of MedImmune LLC and own shares in the company. MP and LW have disclosed that they have served as consultants for MedImmune for this economic analysis. MP is on MedImmune’s speakers bureau for palivizumab. CMRO Peer Reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments:
Editorial assistance for this manuscript was provided by Susan Myers, MSc, and Gerard P. Johnson, PhD, of Complete Healthcare Communications, Inc., Chadds Ford, PA, USA, and was funded by MedImmune.
REFERENCES
- Leader S, Kohlhase K. Recent trends in severe respiratory syncytial virus (RSV) among US infants, 1997 to 2000. J Pediatr 2003;143; S127-32
- Feltes TF, Cabalka AK, Meissner HC, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr 2003;143; 532-40
- The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics 1998;102; 531-7
- Bockova J, O'Brien KL, Oski J, et al. Respiratory syncytial virus infection in Navajo and White Mountain Apache children. Pediatrics 2002;110; e20
- Bulkow LR, Singleton RJ, Karron RA, et al. Risk factors for severe respiratory syncytial virus infection among Alaska native children. Pediatrics 2002;109; 210-6
- Holman RC, Curns AT, Cheek JE, et al. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics 2004;114; e437-44
- Karron RA, Singleton RJ, Bulkow L, et al. Severe respiratory syncytial virus disease in Alaska native children. RSV Alaska Study Group. J Infect Dis 1999;180; 41-9
- La Via WV, Grant SW, Stutman HR, et al. Clinical profile of pediatric patients hospitalized with respiratory syncytial virus infection. Clin Pediatr (Phila) 1993;32; 450-4
- Sangaré L, Curtis MP, Ahmad S. Hospitalization for respiratory syncytial virus among California infants: disparities related to race, insurance, and geography. J Pediatr 2006;149; 373-7
- Singleton RJ, Petersen KM, Berner JE, et al. Hospitalizations for respiratory syncytial virus infection in Alaska Native children. Pediatr Infect Dis J 1995;14; 26-30
- Wadhwa PD, Culhane JF, Rauh V, et al. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol 2001;15 Suppl 2, 17-29
- Kramer MS, Goulet L, Lydon J, et al. Socio-economic disparities in preterm birth: causal pathways and mechanisms. Paediatr Perinat Epidemiol 2001;15 Suppl 2, 104-23
- Moutquin JM. Socio-economic and psychosocial factors in the management and prevention of preterm labour. BJOG 2003;110 Suppl 20, 56-60
- StateHealthFacts.org. Medicaid coverage rates for the nonelderly by race/ethnicity, states (2009–2010), U.S. (2010) Available at: http://www.statehealthfacts.org/comparetable.jsp?ind=163&cat=3&sub=42 [Accessed January 23, 2012.]
- Boyce TG, Mellen BG, Mitchel EF, Jr et al. Rates of hospitalization for respiratory syncytial virus infection among children in medicaid. J Pediatr 2000;137; 865-70
- Frogel M, Nerwen C, Cohen A, et al. Prevention of hospitalization due to respiratory syncytial virus: results from the Palivizumab Outcomes Registry. J Perinatol 2008;28; 511-7
- Synagis® (palivizumab). Full Prescribing Information, MedImmune, LLC., Gaithersburg, MD, 2011
- American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Red Book: 2009 Report of the Committee on Infectious Diseases. Elk Grove Village, IL: American Academy of Pediatrics, 2009 560-69
- American Academy of Pediatrics. Fact sheet. Medicaid and children. Available at: http://www2.aap.org/research/factsheet.pdf [Accessed February 6, 2012.]
- Data on File. MedImmune, LLC., Gaithersburg, MD
- Hampp C, Kauf TL, Saidi AS, et al. Cost-effectiveness of respiratory syncytial virus prophylaxis in various indications. Arch Pediatr Adolesc Med 2011;165; 498-505
- Shireman TI, Braman KS. Impact and cost-effectiveness of respiratory syncytial virus prophylaxis for Kansas medicaid's high-risk children. Arch Pediatr Adolesc Med 2002;156; 1251-5
- Wegner S, Vann JJ, Liu G, et al. Direct cost analyses of palivizumab treatment in a cohort of at-risk children: evidence from the North Carolina Medicaid Program. Pediatrics 2004;114; 1612-9
- Shi N, Palmer L, Chu B-C, et al. Association of RSV lower respiratory tract infection and subsequent healthcare use and costs: A Medicaid claims analysis in early-preterm, late-preterm, and full-term infants. J Med Econ 2011;14; 335-40
- Levinson DR. Medicaid Brand-Name Drugs: Rising Prices Are Offset By Manufacturer Rebates. Office of Inspector General. Available at: http://oig.hhs.gov/oei/reports/oei-03-10-00260.pdf [Accessed January 17, 2012.]
- Mullins CD, Seal B, Seoane-Vazquez E, et al. Good research practices for measuring drug costs in cost-effectiveness analyses: Medicare, Medicaid and other US government payers perspectives: the ISPOR Drug Cost Task Force report–Part IV. Value Health 2010;13; 18-24
- Government Printing Office. United States Code, Title 42 – The Public Health and Welfare; Section 1396r-8-payment for covered outpatient drugs. Available at: http://www.gpo.gov/fdsys/pkg/USCODE-2010-title42/html/USCODE-2010-title42-chap7-subchapXIX-sec1396r-8.htm. [Accessed March 12, 2012.]
- Stratton KR, Durch JS, Lawrence RS, et al, eds. Vaccines for the 21st Century: A Tool for Decisionmaking: The National Academies Press, 2000
- Gold MR, Siegal JE, Russell LB, et al. Cost-effectiveness in health and medicine. Oxford University Press, 1996
- Figueras-Aloy J, Carbonell-Estrany X, Quero J. Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J 2004;23; 815-20
- Law BJ, Langley JM, Allen U, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J 2004;23; 806-14
- Figueras-Aloy J, Carbonell-Estrany X, Quero-Jimenez J, et al. FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J 2008;27; 788-93
- Stensballe LG, Fullarton JR, Carbonell-Estrany X, et al. Population based external validation of a European predictive model for respiratory syncytial virus hospitalization of premature infants born 33 to 35 weeks of gestational age. Pediatr Infect Dis J 2010;29; 374-6
- Rietveld E, Vergouwe Y, Steyerberg EW, et al. Hospitalization for respiratory syncytial virus infection in young children: development of a clinical prediction rule. Pediatr Infect Dis J 2006;25; 201-7
- Lanctot KL, Masoud ST, Paes BA, et al. The cost-effectiveness of palivizumab for respiratory syncytial virus prophylaxis in premature infants with a gestational age of 32-35 weeks: a Canadian-based analysis. Curr Med Res Opin 2008;24; 3223-37
- Simoes EA. RSV disease in the pediatric population: epidemiology, seasonal variability, and long-term outcomes. Manag Care 2008;17; 3-6, discussion 18-9
- Sampalis JS, Langley J, Carbonell-Estrany X, et al. Development and validation of a risk scoring tool to predict respiratory syncytial virus hospitalization in premature infants born at 33 through 35 completed weeks of gestation. Med Decis Making 2008;28; 471-80
- Paes B, Cole M, Latchman A, et al. Predictive value of the respiratory syncytial virus risk-scoring tool in the term infant in Canada. Curr Med Res Opin 2009;25; 2191-6
- Paes B, Steele S, Janes M, et al. Risk-Scoring Tool for respiratory syncytial virus prophylaxis in premature infants born at 33–35 completed weeks' gestational age in Canada. Curr Med Res Opin 2009;25; 1585-91
- Tam DY, Banerji A, Paes BA, et al. The cost effectiveness of palivizumab in term Inuit infants in the Eastern Canadian Arctic. J Med Econ 2009;12; 361-70
- Roeckl-Wiedmann I, Liese JG, Grill E, et al. Economic evaluation of possible prevention of RSV-related hospitalizations in premature infants in Germany. Eur J Pediatr 2003;162; 237-44
- Farina D, Rodriguez SP, Bauer G, et al. Respiratory syncytial virus prophylaxis: cost-effective analysis in Argentina. Pediatr Infect Dis J 2002;21; 287-91
- Simpson S, Burls A. A systematic review of the effectiveness and cost-effectiveness of palivizumab (Synagis) in the prevention of respiratory syncytial virus (RSV) infection in infants at high risk of infection. Department of Public Health & Epidemiology, University of Birmingham. Available at: http://www.rep.bham.ac.uk/2001/Palivizumab_final_post_panel.pdf [Accessed January 17, 2012.]
- Detsky AS, Laupacis A. Relevance of cost-effectiveness analysis to clinicians and policy makers. JAMA 2007;298; 221-4
- Palmer L, Hall CB, Katkin JP, et al. Healthcare costs within a year of respiratory syncytial virus among Medicaid infants. Pediatr Pulmonol 2010;45; 772-81
- Centers for Disease Control and Prevention. US Vital Statistics. Available at: http://www.cdc.gov/nchs/nvss.htm [Accessed January 17, 2012.]
- Wills S, Simpson JH, Coutts J. Cost minimisation of RSV prevention with palivizumab. Arch Dis Child 2006;91; 717
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008;359; 821-32
- Prosser LA, Bridges CB, Uyeki TM, et al. Health benefits, risks, and cost-effectiveness of influenza vaccination of children. Emerg Infect Dis 2006;12; 1548-58
- Chesson H. HPV Vaccine Cost-effectiveness Updates and Review. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf [Accessed January 17, 2012.]
- National Network for Immunization Information. Vaccine Economics. Available at: http://www.immunizationinfo.org/issues/immunization-policy/vaccine-economics. [Accessed March 12, 2012.]
- Moore L, Remy V, Martin M, et al. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010;8; 7
- Blais LR, Williams LB, Lamoureux VA. A novel home-care based program for palivizumab prophylaxis reduces RSV hospitalization. Pediatr Res 2004;55; 589A
- Goldenring J. Give Synagis via home health. Pediatrics 2007;119; 219
- Checchia PA, Nalysnyk L, Fernandes AW, et al. Mortality and morbidity among infants at high risk for severe respiratory syncytial virus infection receiving prophylaxis with palivizumab: a systematic literature review and meta-analysis. Pediatr Crit Care Med 2011;12; 580-8
- Finkelstein JA, Barton MB, Donahue JG, et al. Comparing asthma care for Medicaid and non-Medicaid children in a health maintenance organization. Arch Pediatr Adolesc Med 2000;154; 563-8
- Peterson TH, Peterson T, Armon C, et al. Insurance-associated disparities in hospitalization outcomes of Michigan children. J Pediatr 2011;158; 313-8 e1-2
- Forbes ML, Hall CB, Jackson A, et al. Comparative costs of hospitalisation among infants at high risk for respiratory syncytial virus lower respiratory tract infection during the first year of life. J Med Econ 2010;13; 136-41
- Horn SD, Smout RJ. Effect of prematurity on respiratory syncytial virus hospital resource use and outcomes. J Pediatr 2003;143; S133-41
- Gunville CF, Sontag MK, Stratton KA, et al. Scope and impact of early and late preterm infants admitted to the PICU with respiratory illness. J Pediatr 2010;157; 209-14 e1
- Thorburn K. Pre-existing disease is associated with a significantly higher risk of death in severe respiratory syncytial virus infection. Arch Dis Child 2009;94; 99-103
- US Department of Labor. Bureau of Labor Statistics for 2010 (May to Aug) Available at: http://www.dol.gov/ [Accessed April 20, 2011.]
- Guo SS. Growth in weight, recumbent length, and head circumference for preterm low-birth weight infants during the first three years of life using gestation-adjusted ages. Early Hum Dev 1997;47; 305-25
- Centers for Disease Control and Prevention. Natality public use file. Available at: http://www.cdc.gov/nchs/births.htm [Accessed April 20, 2011.]
- Luce BR, Zangwill KM, Palmer CS, et al. Cost-effectiveness analysis of an intranasal influenza vaccine for the prevention of influenza in healthy children. Pediatrics 2001;108; E24
- Leidy NK, Margolis MK, Marcin JP, et al. The impact of severe respiratory syncytial virus on the child, caregiver, and family during hospitalization and recovery. Pediatrics 2005;115; 1536-46
- Azimi NA, Welch HG. The effectiveness of cost-effectiveness analysis in containing costs. J Gen Intern Med 1998;13; 664-9
- Elhassan NO, Sorbero ME, Hall CB, et al. Cost-effectiveness analysis of palivizumab in premature infants without chronic lung disease. Arch Pediatr Adolesc Med 2006;160; 1070-6
- Ubel PA, Hirth RA, Chernew ME, et al. What is the price of life and why doesn't it increase at the rate of inflation? Arch Intern Med 2003;163; 1637-41
- Kulasingam SL, Myers ER. Potential health and economic impact of adding a human papillomavirus vaccine to screening programs. JAMA 2003;290; 781-9
- Purdy KW, Hay JW, Botteman MF, et al. Evaluation of strategies for use of acellular pertussis vaccine in adolescents and adults: a cost-benefit analysis. Clin Infect Dis 2004;39; 20-8
- Shim E, Galvani AP. Impact of transmission dynamics on the cost-effectiveness of rotavirus vaccination. Vaccine 2009;27; 4025-30
- Ortega-Sanchez IR. Cost-Effectiveness of Meningococcal Vaccination in Infants and Toddlers in the United States. Available at: http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-oct11/03-MCV-Ortega-Sanchez.pdf [Accessed January 12, 2012.]
- Jacobs RJ, Saab S, Meyerhoff AS. The cost effectiveness of hepatitis immunization for US college students. J Am Coll Health 2003;51; 227-36
- Yount LE, Mahle WT. Economic analysis of palivizumab in infants with congenital heart disease. Pediatrics 2004;114; 1606-11
- Sampalis JS. Morbidity and mortality after RSV-associated hospitalizations among premature Canadian infants. J Pediatr 2003;143; S150-6
- Thomson Healthcare, Inc. Red Book Drug Topics. Montvale, NJ: PDR Network, LLC, 2010
- Briggs AH, Claxton K, Sculpher MJ. Decision modelling for health economic evaluation. Oxford: Oxford University Press, 2006
- Smart KA, Lanctot KL, Paes BA. The cost effectiveness of palivizumab: a systematic review of the evidence. J Med Econ 2010;13; 453-63
- Flaherman V, Li S, Ragins A, et al. Respiratory syncytial virus testing during bronchiolitis episodes of care in an integrated health care delivery system: a retrospective cohort study. Clin Ther 2010;32; 2220-9
- Mays VM, Cochran SD, Barnes NW. Race, race-based discrimination, and health outcomes among African Americans. Annu Rev Psychol 2007;58; 201-25
- Tobacco use among U.S. racial/ethnic minority groups—African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. A Report of the Surgeon General. Executive summary. MMWR Recomm Rep 1998;47; v-xv, 1-16
- Frogel MP, Stewart DL, Hoopes M, et al. A systematic review of compliance with palivizumab administration for RSV immunoprophylaxis. J Manag Care Pharm 2010;16; 46-58
- Ray GT, Whitney CG, Fireman BH, et al. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J 2006;25; 494-501
- Goldie SJ, Kohli M, Grima D, et al. Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 2004;96; 604-15
- Shepard CW, Ortega-Sanchez IR, Scott RD, 2nd, et al. Cost-effectiveness of conjugate meningococcal vaccination strategies in the United States. Pediatrics 2005;115; 1220-32
- Gooch K, Busch K, Khong H. Length of Hospital Stay for Respiratory Syncytial Virus in Prophylaxed Versus Non-Prophylaxed Premature Infants. Presented at: European Society for Paediatric Infectious Diseases; June 7-11, 2011; The Hague, The Netherlands
- Paramore LC, Ciuryla V, Ciesla G, et al. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics 2004;22; 275-84
- Paramore LC, Mahadevia PJ, Piedra PA. Outpatient RSV lower respiratory infections among high-risk infants and other pediatric populations. Pediatr Pulmonol 2010;45; 578-84
- Mittmann N, Trakas K, Risebrough N, et al. Utility scores for chronic conditions in a community-dwelling population. Pharmacoeconomics 1999;15; 369-76
- Simoes EA, Groothuis JR, Carbonell-Estrany X, et al. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr 2007;151; 34-42, 42.e.1