Abstract
Objectives:
This study evaluated patient and prescriber characteristics, treatment patterns, average daily dose (ADD), and glycemic control of patients initiating glucagon-like peptide 1 (GLP-1) receptor agonists in Germany.
Methods:
The LifeLink™ EMR-EU database was searched to identify patients initiating exenatide twice daily (BID) or liraglutide once daily (QD) during the index period (January 1, 2009–April 4, 2010). Eligible patients had ≥180 days pre-index history, ≥90 days post-index follow-up, and a pre-index type 2 diabetes diagnosis. Univariate tests were conducted at α = 0.05.
Results:
Six hundred and ninety-two patients were included (exenatide BID 292, liraglutide QD 400): mean (SD) age 59 (10) years, 59% male. Diabetologists prescribed liraglutide QD to a larger share of patients (65% vs 35% exenatide BID) than non-diabetologists (51% vs 49%). GLP-1 receptor agonist choice was not associated with age (p = 0.282), gender (p = 0.960), number of pre-index glucose-lowering medications (2.0 [0.9], p = 0.159), pre-index HbA1c (8.2 [1.5%], p = 0.231) or Charlson Comorbidity Index score (0.45 [0.78], p = 0.547). Mean (SD) ADD was 16.7 mcg (9.2, label range 10–20 mcg) for exenatide BID and 1.4 mg (0.7, label range 0.6–1.8 mg) for liraglutide QD. Among patients with post-index HbA1c tests, mean unadjusted values did not differ between cohorts. Exenatide BID patients were more likely than liraglutide QD patients to continue pre-index glucose-lowering medications (67.1% vs 60.3%, p = 0.027) or to start concomitant glucose-lowering medications at index (32.2% vs 25.0%, p = 0.013); exenatide BID patients were less likely to augment treatment with another drug post-index (15.8% vs 22.5%, p = 0.027).
Limitations:
Results may not be generalizable. Lab measures for clinical outcomes were available only for a sub-set of patients.
Conclusions:
Results suggested that some differences exist between patients initiating exenatide BID or liraglutide QD, with respect to prescribing physician specialty and pre- and post-index treatment patterns. Both GLP-1 receptor agonists showed comparable post-index HbA1c values in a sub-set of patients.
Introduction
Diabetes is a group of diseases measured by high levels of blood sugar caused by imperfections in insulin production by the pancreas, insulin resistance, incretin defect, or a combination. Globally, at least 171 million people are estimated to have diabetes, a figure likely to more than double by 2030Citation1. In Germany, ∼8–12% of adults are estimated to have diabetes, and type 2 diabetes (T2D; resulting from insulin resistance, sometimes combined with an absolute insulin deficiency) accounts for 90% of all diagnosed casesCitation2–4. The major risk factors for T2D are being overweight, inactivity, family history, age, and raceCitation1.
To maintain good glycemic control, patients with T2D require systematic, individualized, and progressive interventions involving different oral and/or subcutaneously administered therapiesCitation5. Glucagon-like peptide 1 (GLP-1) receptor agonists represent a newer class of glucose-lowering medications that avoid some of the limitations of earlier-generation medications through their glucose-dependent mechanism of action and other non-glycemic effects. Subcutaneous GLP-1 receptor agonists are not associated with edema, have a low incidence of hypoglycemia, and have been shown to promote weight loss in patientsCitation6–9.
A recent consensus statement by the American Diabetes Association and European Association for the Study of Diabetes (ADA/EASD) recommends use of GLP-1 receptor agonists to reduce glycosated hemoglobin (HbA1c) in patients where hypoglycemia is undesirable and/or promotion of weight loss is crucial, and the HbA1c level is close to target (<8.0%; target <7%)Citation7. The published treatment algorithm places GLP-1 receptor agonists as possible second-line pharmacotherapy after metformin failure, alongside alternative agents; pioglitazone, sulfonylureas, and basal insulinCitation7.
As of June 2011, two GLP-1 receptor agonists had been approved for improving glycemic control in adults with T2D: exenatide (Amylin and Lilly, USA), approved in the US in 2005 and in Europe in 2006, and liraglutide (Novo Nordisk, Denmark), approved in Europe in 2009 and in the US in 2010. This study provides insight on the patient demographics, clinical characteristics, and treatment patterns in patients treated with either GLP-1 receptor agonist. The cohort design compared exenatide, taken twice daily (BID), and liraglutide, taken once daily (QD), from a database of electronic medical records in Germany.
The primary objective of this study was to describe the demographic and clinical characteristics of patients with T2D treated with either exenatide BID or liraglutide QD. Secondary objectives were to evaluate clinical outcomes such as HbA1c values and body mass index (BMI), and treatment patterns including use of oral glucose-lowering agents, use of insulin, concurrent treatments, treatment modification (discontinuation, switching, up-titration, down-titration, and augmentation), and time to treatment modification.
Patients and methods
German data from July 1, 2008 through July 31, 2010 were obtained from the LifeLink™ EMR-EU database (LifeLink), which is comprised of longitudinal patient-level data from physician–practice data systems of office-based physicians in France, Germany, and the UK. The rationale to examine German data stemmed from the country’s early marketing authorization of liraglutide.
The index date was defined as the date of the first prescription for a GLP-1 receptor agonist. The index window for exenatide BID was January 1, 2009 to April 30, 2010 and for liraglutide QD was July 1, 2009 to April 30, 2010. The study follow-up period was variable and terminated on the last date of activity in the database or at the end of the study period, whichever occurred first.
Patients in Germany were selected from the LifeLink database if they met the following inclusion criteria: had at least one physician visit in which exenatide BID or liraglutide QD was prescribed during the index window, had a pre-index period of ≥180 days and post-index period of ≥90 days, were ≥18 years old, and had a diagnosis for T2D (International Statistical Classification of Diseases [ICD] 10 codes: E11–E14) recorded during the pre-index period (). Patients were excluded from the study if they had a claim for a GLP-1 receptor agonist in the pre-index period, or had unknown/missing fields for age or gender.
Table 1. Attrition of sample size.
Data were collected from participating specialists as well as primary and secondary care (specialists) physicians. Patient data included gender, age, height, weight, BMI, smoking status, diabetes-related comorbidities, Charlson Comorbidity Index (CCI) scoreCitation10 (specified ICD-9-CM diagnosis codes were translated to ICD-10 codes), HbA1c value, and insurance status. Details regarding physician-written prescriptions, including medication, formulation, and daily dose were also collected.
Treatment patterns of anti-hyperglycemic agents (medication classes and number of medications) leading up to the index date were reported using data from the pre-index period and the index date.
Treatment patterns in the post-index period were reported for the available follow-up period. Treatment modification included up-titration, down-titration, discontinuation, switching, and augmentation. Up-titration of dose was defined as two consecutive prescriptions with doses greater than the index dose, and measured as on-label up-titration and off-label up-titration. On-label up-titration for exenatide and liraglutide was based on the package insertCitation11,Citation12. According to the exenatide package insert, the expected initial dose for exenatide is 10 mcg/day and is increased to 20 mcg/day after at least 1 month based on glycemic response; on-label up-titration for exenatide was defined as prescriptions with daily doses of up to 20 mcg/dayCitation11. Exenatide doses above 25 mcg/day were considered off-label up-titration, allowing minor dosing fluctuations due to timing of refills. According to the liraglutide product label, the starting dose is 0.6 mg/day for 1 week and is increased to 1.2 mg/dayCitation12. Some patients are expected to benefit from an increase in dose from 1.2 to 1.8 mg. Daily doses higher than 1.8 mg are not recommended. Per-label up-titration for liraglutide was defined as prescriptions with doses up to 2.1 mg daily, and off-label up-titration was defined as doses >2.1 mg daily, again allowing a dosing margin due to timing of refills. Down-titration was defined as two consecutive prescriptions with doses lower than the previous dose.
Discontinuation was the occurrence of a gap in a series of successive prescriptions that was ≥2-times the expected duration of drug. The day after the expected duration of the prescription ends was defined as the date of discontinuation.
Switching was defined as the occurrence of a new non-index GLP-1 receptor agonist prescription, within 30 days before or after discontinuation of the patient’s index treatment. The date of the new non-index prescription was defined as the date of the switch.
Augmentation (adding new prescriptions to their GLP-1 receptor agonist regimen) was measured during the follow-up period as the occurrence of one or more prescriptions for a new post-index T2D treatment, starting more than 30 days before the index GLP-1 receptor agonist discontinuation date (concurrent use).
The daily dose field in the LifeLink database was not populated for most patients. Therefore, the average daily dose (ADD) was calculated based on the quantity of pens prescribed and time between prescriptions. The calculated ADD was used to determine the overall ADD, index ADD, and monthly ADD, and was calculated by dividing the total amount of drug prescribed (calculated using package size, package count, and strength) by the time (days) between consecutive prescriptions.
GLP-1 receptor agonists prescribed after a patient’s discontinuation date were excluded from ADD calculations. To avoid inflation of ADD from duplicate prescriptions or ambiguous up-titration, we excluded patients with consecutive GLP-1 receptor agonist prescriptions ≤14 days apart. An exception to this rule was granted if the amount of overlap between GLP-1 receptor agonist prescriptions was matched by a gap of equal length before the next observed prescription.
Calculated ADD values were sensitive to small gaps and overlaps in available prescriptions. For clarity, we grouped ranges of ADD values in categories consistent with labeled use and doses dispensed from the syringes. For exenatide BID, a calculated ADD of 5–15 mcg = 10 mcg, > 15–25 mcg = 20 mcg, and >25 mcg = dose above label. For liraglutide QD, a calculated ADD of 0.6–1.5 mg = 1.2 mg, >1.5–2.1 mg = 1.8 mg, and >2.1 = dose above label.
Concurrent treatment was defined as the occurrence of a non-GLP-1 receptor agonist prescription starting on index and throughout the follow-up period. Concurrent use of medications were categorized based on therapeutic class (i.e., insulin, biguanide, sulfonylurea, oral combination, alpha glucosidase inhibitor, thiazolidinedione, DDP-4 inhibitor, and other), and were reported as the number of medication classes prescribed in the follow-up period.
Time-to-event measures were calculated from the index date to the date of any of the treatment modifications. Each time-to-event was quantified by the number of days to each event. Time to up-titration for liraglutide QD was not assessed for the index prescription, because the product label recommends up-titration within the first prescription.
Clinical outcomes were measured and reported where data were available for pre-index HbA1c levels, HbA1c levels by time to index medication, BMI, and referral to a specialist. HbA1c levels during pre-index were measured to evaluate differences in blood glucose levels between cohorts before GLP-1 receptor agonists were initiated. HbA1c levels were then measured during the follow-up period to assess differences in blood glucose after treatment with GLP-1 receptor agonists. Referred to a specialist (yes/no) applied only to patients first seen by a primary care physician. Referrals back to a primary care physician are not captured in the dataset.
The primary cohort analysis compared demographic and clinical characteristics, and treatment patterns of new exenatide BID patients vs new liraglutide QD patients. The sub-cohort analysis of patients who started exenatide BID before and after liraglutide QD launch compared demographic and clinical characteristics, and treatment patterns of patients. For all other sub-group comparisons, only the demographic and clinical characteristics were compared, and no treatment patterns were assessed.
The demographic and clinical characteristics of study patients were described using frequency and percentage distributions for categorical variables. The normally distributed continuous and count variables were described using a mean (standard deviation [SD]) and median, while in the case of non-normal distribution, following demonstration by the Shapiro-Wilk testCitation13, interquartile ranges were used in place of SD. The statistical significance of differences in the characteristics of patients in each group was ascertained using Student t-tests and Wilcoxon rank sum tests for continuous variables that were found to be normally and non-normally distributed, respectively; chi-square tests and Fisher’s exact tests were used to test categorical variables, as appropriate. All univariate tests were performed at 95% confidence, without family-wise adjustment of Type I error rates. In all formal statistical comparisons, a p-value less than 0.05 was considered statistically significant.
Logistic regression models were performed to examine characteristics associated with receiving exenatide BID or liraglutide QD. When an association had an effect size equivalent to at least 0.2 SDs the association between the index medication use and patterns of treatment (up-titration, discontinuation, switch, augmentation, and concurrent) were examined. Additionally, the difference in time to treatment modification between treatment cohorts was assessed using the Cox Proportional Hazard model, and Kaplan-Meier survival curves with log-rank tests were also reported for treatment modification.
Results
A total of 692 patients were included in the final sample (exenatide BID = 292 and liraglutide QD = 400) (). Baseline patient characteristics are summarized in . In general, new GLP-1 receptor agonists users in Germany who appear in the LifeLink database since January 2009 were male (58.7%), aged 50 or older (82.1%), and had a mean (SD) BMI of 37.3 (7.2) kg/m2. With a few exceptions, the demographic profile of patients (age, gender, mean BMI, and smoking status) who received exenatide BID and liraglutide QD did not differ significantly. BMI data were missing for about a quarter of German patients. Diabetologists prescribed liraglutide more frequently than exenatide BID (65% vs 35%); non-diabetologists prescribed approximately equal numbers of each (51% vs 49%). The mean post-index follow-up period was significantly longer for exenatide BID than liraglutide QD (335 vs 209 days), perhaps as a consequence of the longer index period. The overall mean (SD) unadjusted HbA1c level in the pre-index period was 8.2 (1.5) with median 8.0. The unadjusted pre-index HbA1c levels did not differ significantly between exenatide BID and liraglutide cohorts (8.1 [1.5] vs 8.3 [1.6]). Mean (SD) and median days between HbA1c and index were 46.2 (43.3) and 36 for exenatide BID and 39.2 (37.2) and 28.5 for liraglutide QD.
Table 2. Demographic and clinical characteristics.
describes the pre-index treatment patterns across exenatide BID and liraglutide QD cohorts. For the majority of patients in Germany, GLP-1 receptor agonists were not used as first-line treatment for T2D. The mean (SD) total number of different glucose-lowering treatment classes utilized in the 180-day pre-index period was 1.92 (0.91) for exenatide BID and 1.96 (0.90) for liraglutide QD (p = 0.159). The most commonly used oral anti-hyperglycemic drug (OAD) was a biguanide (metformin), for both exenatide BID and liraglutide QD patients (68.5% vs 61.5%, p = 0.058). Metformin was the most recently used OAD; ∼40% of the patients were prescribed metformin immediately before their GLP-1 receptor agonist prescription. The mean dose of metformin was similar in both exenatide and liraglutide cohorts (1796 mg vs 1845 mg), and was also aligned with the recommended therapeutic dose of metforminCitation14.
Table 3. Pre-index treatment patterns.
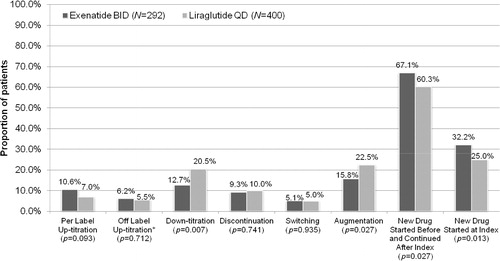
describes treatment patterns across study cohorts in the post-index period. The proportion of patients with per-label up-titration (from 10 mcg to 20 mcg daily for exenatide, from 1.2 mg to 1.8 mg daily for liraglutide) did not differ significantly between the exenatide and liraglutide cohorts (10.6% vs 7.0%, p = 0.093); however, time to per-label up-titration was significantly longer for exenatide users as compared with liraglutide users (62.5 [53.7, median = 42] days vs 45.1 [32.3, median = 35] days). The proportion of patients with off-label up-titration of their index GLP-1 receptor agonist (>20 mcg daily for exenatide, >1.8 mg daily for liraglutide) did not differ across the treatment cohorts (6.2% vs 5.5%, p = 0.712). Time to off-label up-titration was also similar across the study cohorts. Down-titration of index medication was significantly higher among patients starting with liraglutide as compared with those starting with exenatide (20.5% vs 12.7%, p = 0.007). Time to down-titration was longer for patients starting on exenatide; however, the difference was not statistically significant (47.2 [32.8, median = 31] days vs 34.1 [22.2, median = 27] days). The proportions of patients who discontinued or switched their index GLP-1 agent, and time to discontinuation or switch were similar across the study cohorts, despite longer mean follow-up for exenatide BID. In terms of augmentation, more liraglutide users augmented their treatment with other glucose-lowering agents (22.5% vs 15.8%, p = 0.027) during follow-up, compared with exenatide users. The most common medications used during augmentation were biguanides and insulins among both exenatide BID (5.1% and 3.8%) and liraglutide patients (8.5% and 5.0%). More exenatide BID patients than liraglutide patients continued their pre-index glucose-lowering treatment during follow-up (67.1% vs 60.3%, p = 0.027), and more started a new glucose-lowering agent (other than the GLP-1 receptor agonist) on the index date (32.2% vs 25.0%, p = 0.013) ().
Figure 1. Post-index treatment patterns: Rate of treatment modifications. *Cut-points used for defining off-label up-titration: exenatide dose >25 mcg and liraglutide dose >2.117 mg. BID, taken twice daily; QD, taken once daily.
The mean overall calculated ADD for exenatide BID and liraglutide QD was 16.7 mcg and 1.43 mg, and the mean calculated days supply per prescription was 53 days and 51 days, respectively. The calculated ADD was also reported by days from index. Overall, exenatide BID patients tended to start with a lower dose (mean = 15.7 mcg daily) and up-titrate after 60 days (mean = 18.8 mcg daily), followed by stable dosing until 240 days after the index date, after which the mean dose for exenatide BID increased to 20.9 mcg daily, possibly due to smaller sample size in these categories. Each dose of exenatide comes in a pre-filled pen containing either 5 or 10 mcg. Therefore, based on a twice-daily administration, dose can only be 10, 15, or 20 mcg. Liraglutide is a self-dialing dose so it can (in theory) be dosed between 0.6–1.8 mg. Liraglutide QD patients tended to start with a higher than expected dose (mean = 1.4 mg daily) followed by down-titration after 60 days (mean = 1.32 mg daily) from the index date; liraglutide doses remained between 1.3–1.5 mg in each subsequent month.
We constructed Cox proportional hazards regressions to compare the hazard of modifying treatment regimens across treatment cohorts. Treatment modification included treatment discontinuation, switch, and/or augmentation of the index GLP-1 receptor agonists. Kaplan-Meier curves () and Cox proportional regression analyses () were carried out for patients who had HbA1c data available in the dataset (n = 423). After adjusting for HbA1c value, sex, age, relationship to insured, prescriber specialty, BMI, smoking status, pre-index comorbidities (cardiovascular diseases, obesity, retinopathy, nephropathy, diabetic foot ulcers, and depression), and pre-index diabetes medications (biguanides, insulin, sulfonylureas, oral combinations, alpha glucosidase inhibitors, thiazolidinediones, and DPP-4 inhibitors) time to treatment modification was comparable for exenatide and liraglutide patients (hazard ratio = 1.06, 95% CI: 0.67–1.66, p = 0.816).
Figure 2. Kaplan-Meier curves. After adjusting for pre-index HbA1C values along with other covariates, time to treatment modification was shorter for the exenatide cohort; results were not statistically significant (hazard ratio = 1.06, 95% CI: 0.673–1.655, p = 0.82).
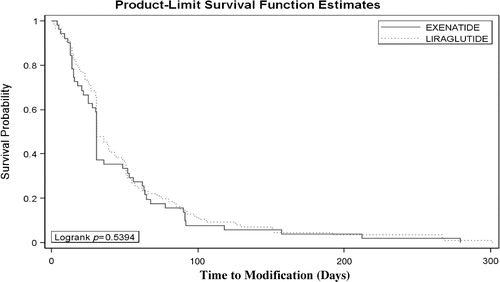
Table 4. aCox proportional hazard for time (days) to index treatment modificationb (n = 423).
Clinical outcomes in the post-index period included HbA1c values, total number of unique glucose-lowering prescription medication classes, and BMI (). While the unadjusted post-index HbA1c values did not differ significantly between the exenatide BID and liraglutide QD study cohorts (7.78 [1.53] and 7.93 [1.51], p = 0.293), time to HbA1c tests were significantly longer for exenatide BID patients. There were no significant differences between the cohorts for other clinical outcomes examined in this study.
Table 5. Observed post-index clinical outcomes.
describes results for sub-cohorts of exenatide patients categorized based on the launch of liraglutide. The objective of these sub-cohort analyses was to examine changes in patient characteristics, if any, receiving exenatide before and after the launch of liraglutide in the German market in July 2009.
Table 6. Characteristics of patients receiving exenatide before and after liraglutide launch.
Overall, the profiles of patients receiving exenatide did not differ significantly between these sub-cohorts, except for the prescribing physician and nephropathy as a pre-index comorbidity. After the liraglutide launch, a lower proportion of diabetologists prescribed exenatide (48.6% before vs 28.7% after) as compared with non-diabetologists (25.4% before vs 34.7% after) (p = 0.004). Among patients with nephropathy as a pre-index comorbidity, a significantly lower number of patients were prescribed exenatide after the liraglutide launch (4.9% vs 0.7%, p = 0.026). The post-index exenatide BID treatment patterns were similar before and after the liraglutide QD launch except for the discontinuation rate for exenatide BID, which was significantly higher before liraglutide QD was launched (14.8% vs 4.0%, p = 0.002), although this could be due to the longer period pre-liraglutide launch (2006–2009) vs post-liraglutide launch (2009–2010).
Discussion
In 2006, exenatide BID was approved in Europe as the first GLP-1 receptor agonist for improving glycemic control in adults with T2D through its glucose-dependent mechanisms of action. In 2009 liraglutide QD became the second therapeutic option in this class of medication to be approved for use in Europe. This retrospective cohort study aimed to describe and understand the demographic and clinical characteristics of patients with T2D treated with either exenatide BID or liraglutide QD in Germany. Exenatide once weekly formulation was approved in Europe in 2011 and was thus not included in this analysis.
Analysis of the patients receiving the two therapies showed little separation in terms of demographic and clinical characteristics. In general, patients were male, had a mean age of 58.9 years, mean HbA1c of 8.2%, and BMI of 37.3 kg/m2. The patient population in this study is similar to populations in the AC2993: Diabetes Management for Improving Glucose Outcomes (AMIGO, for exenatide BID) randomized clinical trials and the Liraglutide Effect and Action in Diabetes 6 randomized clinical trial (LEAD-6, for liraglutide QD). In the AMIGO trials, exenatide BID patients had a mean age of 52–56 years, mean HbA1c of 8.2–8.6%, and BMI of 33–34 kg/m2, and in the LEAD-6 study liraglutide patients had a mean age of 56.3 years, mean HbA1c of 8.2%, and BMI of 32.9 kg/m2Citation15–18. Most patients used at least two medications before starting a GLP-1 receptor agonist. Variations were observed in physician prescribing preference (with diabetologists favoring liraglutide since its launch) and the timing of concomitant medications.
Clinical trials of exenatide BID and liraglutide QD demonstrated that GLP-1 therapies reduced HbA1c by 0.5–1.5%, with significant weight loss in many patients and with no increased risk of hypoglycemia (unless used concomitantly with a sulfonylurea)Citation11,Citation12. Recent reviews have suggested that the anticipated glucose-lowering effect of the GLP-1 class of medications in clinical practice is 0.5–1% improvement in HbA1c, with anticipated weight loss and reduction in systolic blood pressureCitation19. It is difficult to understand the ‘real life’ effect of exenatide or liraglutide in the current analysis due to the high number of patients with few post-index lab values, including HbA1c, which occurred at irregular intervals. However, patients appeared to be experiencing comparable effective reduction in mean HbA1c with the two drugs, with similar proportions of patients receiving low-dose and high-dose regimens of each.
More liraglutide QD patients down-titrated and augmented with a new treatment compared with exenatide BID patients. In contrast, during the follow-up period, exenatide BID patients tended to continue using the glucose-lowering medication they started in the pre-index period. In addition, significantly more exenatide BID patients started a new OAD on index compared with liraglutide QD. In terms of post-index dosing overall, calculated ADD during follow-up was aligned with label-recommended dosing for both exenatide BID and liraglutide QD. Post-index treatment patterns in both cohorts did not suggest the use of dose escalation to achieve HbA1c reductions. Clinical outcomes between the two comparators showed that HbA1c levels and BMI were similar throughout the follow-up period.
Liraglutide was introduced several years after exenatide BID. The Association of British Clinical Diabetologists (ABCD) conducted large scale real-life audits of exenatide BID (2007–2009)Citation20 and liraglutide (2009–2010)Citation21,Citation22. The audits indicated that liraglutide was associated with a larger reduction in HbA1c, but exenatide was associated with larger reductions in weight. The ABCD audits found that insulin dose was reduced less and insulin was discontinued less in the liraglutide auditCitation21,Citation22. The researchers concluded that these differences may be due to increased confidence of clinicians in continuing glucose-lowering medications, particularly insulin, when initiating liraglutideCitation21,Citation22. The researchers also concluded, from their comparison of the ABCD nationwide audits on exenatide and liraglutide, that HCPs prescribing liraglutide may have learned from previous use of exenatide, and adapted their prescribing practices of liraglutide accordinglyCitation22.
In our study, the introduction of liraglutide QD did not change the profile, outcomes, or prescribing patterns of exenatide BID patients. No significant differences were found between exenatide BID patients in terms of pre- and post-index treatment patterns after the launch of liraglutide, except that pre-index thiazolidinedione use was higher in exenatide BID patients before the liraglutide QD launch. This may be attributable to Food and Drug Administration and European Medicines Agency warnings on the use of rosiglitazone during 2009 and 2010.
The study population was restricted to those registered in the LifeLink EMR-EU database in Germany; therefore, the findings of this study may not be generalizable in other patient cohorts. Patients who seek care outside the EMR practice setting would not have utilization recorded in the database. The results are generalizable only to office-based physicians and are not representative of all physicians in the country. Further, prescriber bias should be taken into account when interpreting study results. There was the possibility that patients could be duplicated as they transferred from primary to secondary care (or vice versa) when a different patient identifier was assigned to the same patient for each setting of care. However, since the German dataset only represents ∼2% of all practices in Germany, the probability of duplicate patients among LifeLink practices is low. The database also contains prescriptions written by the participating physician, but not actual prescription fills. Consequently, persistence with the prescribed medication could only be accessed through the timing of subsequent prescriptions written for the same medication, and the necessary assumption that the patient actually filled each prescription and took the medication could not be tested. Prescriptions with numerous refills are not standard practice in Germany (the mean calculated days supply per prescription was 53 days and 51 days), so the data source did support persistence analyses based on patients returning for repeat prescriptions from their providers. Lab measures for clinical outcomes were available only for a sub-set of patients in the database, so conclusions regarding clinical effectiveness could not be made for the entire patient population. In assessing potential differences in time to treatment modification between the two GLP-1 agonists, selection bias from measured confounders was addressed by incorporating relevant covariates in the model. Due to the observational nature of this research, the potential for bias due to unmeasured confounders cannot be excluded. Other unadjusted post-index outcomes were presented to help describe the observed clinical results in cohorts treated with GLP-1 agonists. The results presented in this manuscript are meant to help describe (a) how the drugs are being used and (b) the clinical outcome of those uses. The study was not conducted to compare the causal effects from the use of the two GLP-1 agonists, which would require further modeling, evaluation, and adjustment for selection bias and sensitivity analyses.
Conclusion
The patient population in this clinical practice study was similar to populations enrolled in the randomized phase III program for both liraglutide QD and exenatide BID. Results suggested that few differences in demographic and clinical characteristics existed between patients receiving liraglutide QD and exenatide BID in clinical practice. However, differences in prescribing physician specialty and pre- and post-index treatment patterns existed between German patients initiating exenatide BID or liraglutide QD. Both GLP-1 receptor agonists showed comparable post-index HbA1c values in a sub-set of patients.
Transparency
Declaration of funding
This research was funded by Amylin Pharmaceuticals, Inc., San Diego, CA, and by Eli Lilly and Company, Indianapolis, IN.
Declaration of financial/other relationships
L.-A.M. was employed by Eli Lilly and Company, Indianapolis, IN, USA while working on this study. C.B. is employed by IMS Health. IMS Health has ongoing consulting and research relationships with Eli Lilly and Company and Amylin Pharmaceuticals, Inc. A.Z. is employed and receives stock options by Eli Lilly and Company, Indianapolis, IN, USA. M.B. was employed by IMS Health while working on this study. M.R. is employed and receives stock options by Eli Lilly and Company, UK. V.S. is employed by IMS Health. D.B. was employed and received stock options from Eli Lilly and Company, Indianapolis, IN, USA while working on this study.
The peer reviewers on this manuscript have disclosed that they have no relevant financial relationships.
Acknowledgments
The authors thank Rebecca McCracken, MSPH, of PharmaNet/i3, part of the inVentiv Health Company, for her contributions to the writing preparation of the manuscript, and Teri Tucker and Casey Brackney for their editorial contributions to the preparation of this manuscript. The authors also thank Jennie Best of Amylin Pharmaceuticals, Inc. for comments on the manuscript.
References
- World Health Organization. Diabetes Action Now. WHO and IDF, 2004. http://www.who.int/diabetes/actionnow/en/DANbooklet.pdf. Accessed March 1, 2012
- International Diabetes Federation and Federation of European Nurses in Diabetes. Diabetes -- The Policy Puzzle: is Europe making progress? 2nd edn. IDF and FEND, 2008. http://www.idf.org/regions/europe/publications/diabetes-policy-audit. Accessed March 1, 2012
- International Diabetes Federation. IDF Diabetes Atlas: prevalence estimates of diabetes mellitus (DM). 5th edn. IDF, 2011. http://www.diabetesatlas.org/content/prevalence-estimates-diabetes-mellitus-dm-2010. Accessed March 1, 2012
- Hauner H, Bramlage P, Lösch C, et al. Overweight, obesity and high waist circumference: regional differences in prevalence in primary medical care. Dtsch Arztebl Int 2008;105:827-33
- Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004;291:335-42
- Gallwitz B. Glucagon-like peptide-1-based therapies for the treatment of type 2 diabetes mellitus. Treat Endocrinol 2005;4:361-70
- Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009;32:193-203
- Lind M, Jendle J, Torffvit O, et al. Glucagon-like peptide 1 (GLP-1) analogue combined with insulin reduces HbA1c and weight with low risk of hypoglycemia and high treatment satisfaction. Prim Care Diabetes 2012;6:41-6
- Jendle J, Nauck MA, Matthews DR, et al. Weight loss with liraglutide, a once-daily human glucagon-like peptide-1 analogue for type 2 diabetes treatment as monotherapy or added to metformin, is primarily as a result of a reduction in fat tissue. Diabetes Obes Metab 2009;11:1163-72
- Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075-9
- Byetta® [package insert]. San Diego, CA: Amylin Pharmaceuticals, Inc.; 2010
- Victoza® [package insert]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2011
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples). Biometrika 1965;52:591-611
- GLUCOPHAGE® [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2009
- Defronzo RA, Ratner RE, Han J, et al. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care 2005;28:1092-100
- Buse JB, Henry RR, Han J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628-35
- Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care 2005;28:1083-91
- Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomized, parallel-group, multinational, open-label trial (LEAD-6). Lancet 2009;374:39-47
- McGill JB. Selecting among ADA/EASD tier 1 and tier 2 treatment options. J Fam Pract 2009;58(9 Suppl Treating):S26-34
- Thong KY, Jose B, Sukumar N, et al. Safety, efficacy and tolerability of exenatide in combination with insulin in the Association of British Clinical Diabetologists (ABCD) nationwide exenatide audit. Diabetes Obes Metab 2011;13:703-10
- Ryder REJ, Thong KY, Cull ML, et al. The Association of British Clinical Diabetologists (ABCD) nationwise exenatide and liraglutide audits. Poster. Association of British Clinical Diabetologists Meeting. London, England. November 10–11, 2011
- Differences in response between exenatide and liraglutide in the Association of British Clinical Diabetologists (ABCD) nationwide audits. Abstract 00470. International Diabetes Federation World Diabetes congress. Dubai, United Arab Emirates. December 4–8, 2011