Abstract
Objective:
To characterize treatment patterns and measure the economic burden associated with metastatic (mHNC) and recurrent, locally-advanced head and neck cancer (rHNC).
Methods:
Administrative claims from Medicare- and privately-insured individuals during 2004–2008 were used in this retrospective database study of patients with advanced HNC. Patients diagnosed with HNC were matched 1:1 to cancer-free controls to measure the incremental economic burden of HNC. Outcomes of interest were measured during the 6 months following the date of a secondary tumor diagnosis for metastatic patients or the date of a diagnosis indicating rHNC. To assess treatment patterns, HNC patients were evaluated for the use frequency of treatments (radiotherapy, chemotherapy and surgery). Costs were reported in 2008 US$ from a third-party payer perspective and were analyzed using generalized linear models and two-part regression models adjusting for differences in age and baseline Charlson Comorbidity Index (excluding cancer diagnoses) between the HNC and control cohorts. Components of cost included inpatient, outpatient and other medical services as well as pharmacy costs.
Results:
The mHNC cohort consisted of 1042 patients and the rHNC cohort included 324 patients. The most common treatments for mHNC patients were supportive care (90.2%), radiation therapy (48.5%), surgery (41.9%) and chemotherapy (38.3%). Patients with rHNC frequently received HNC-related supportive care (71.0%), radiation therapy (67.9%) and chemotherapy (27.2%); HNC-related surgery was infrequent (12.7%) during the study period. The 6-month incremental adjusted total costs were $60,414 per patient for mHNC and $21,141 per patient for rHNC (p < 0.0001). Approximately 46–58% of the incremental cost was attributable to outpatient visits, 27–37% to inpatient costs and 11–13% to pharmacy, depending on the HNC cohort.
Limitations:
The identification of mHNC/rHNC was based on diagnosis codes and treatment patterns with the limitation of the claims database.
Conclusions:
Metastatic and recurrent, locally-advanced HNC patients frequently receive cancer-related treatments and incur substantial economic burden.
Introduction
Head and neck cancer (HNC) encompasses a diverse group of cancers that affect the oral cavity, pharynx and larynx. More than 90% of these cancers are of squamous cell histologyCitation1. The dominant risk factors for HNC are tobacco and alcohol use, though a significant portion (approximately one-quarter) of HNC is attributable to human papillomavirusCitation2. Worldwide, ∼635,000 new cases of HNC are diagnosed annually and 358,000 people die from HNC each yearCitation3. In the US, cancers of the oral cavity, pharynx and larynx account for 3.2% (49,260) of all new cancers and 2.0% (11,480) of all cancer deathsCitation4.
Survival among HNC patients in the US has improved over the past 30 years. The 5-year relative survival rate was 52.7% during 1982–1986 and increased to 65.9% during 2002–2006. Survival varies considerably by tumor site; for example, the 5-year survival is estimated to be 97.4% for lip cancer, 62.9% for cancer of the oral cavity and 42.2% for cancer of the oropharynxCitation5. Survival also varies by the stage of disease at initial diagnosis, with 5-year survival estimated at 82% for early stage HNC (stage I/II), 52% for locally-advanced HNC (stage III/IVA/IVB) and 27% for metastatic HNC (stage IVC). Local recurrence is the most common cause of death (more common than metastatic HNC) and is associated with a 1-year survival rate of 33%Citation6.
Specific tumor site, stage of the disease and pathological findings influence treatment decisions in clinical practice. In general, the 30–40% of patients who present with early stage disease receive single-modality treatment consisting of surgery or radiotherapy. Individuals presenting with a more advanced stage of HNC typically receive combined modality therapy including concurrent radiotherapy and chemotherapyCitation7. Patients with metastatic or recurrent HNC are administered chemotherapy for the purpose of extending overall survival or palliation. Generally, the prognosis for patients with advanced stage disease is poor. Recent developments have focused on the use of agents that target the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor receptor (VEGFR). For example, cetuximab is a monoclonal antibody inhibitor of EGFR that has been shown to provide survival benefits in combination with platinum–fluorouracil chemotherapy as first-line treatment in patients with metastatic or recurrent HNC or as monotherapy in patients with advanced HNC for whom prior platinum-based therapy has failedCitation6,Citation8.
Although HNC has the fifth highest incidence of all cancers worldwide, the impact of HNC on healthcare utilization and cost has not been studied extensivelyCitation9. A review of the literature published between 1990–2002 revealed only three studies that have measured the per-patient burden of HNC in the US; two were published only as abstracts and the other report was published in 1990Citation10. More recently, a study from the UK measured the per-patient cost of managing HNC based on 147 case reviews at two hospitals; in the first year after diagnosis the cost ranged from $3443 for pre-cancer patients to $24,890 for stage IV cancer patients (2006 US dollars)Citation9. A study by Lang et al. quantified the resource use and costs associated with elderly patients (≥65 years) in the US diagnosed with HNC between 1991–1993 and estimated the total incremental cost of HNC to be $25,542 over a 5-year follow-up periodCitation11.
The present study aimed to enhance the literature on the treatment patterns and the incremental healthcare resource utilization and economic cost burden of HNC in the US. Using recent retrospective administrative claims data, this study provides a comprehensive and nationally representative picture of real-world treatment patterns and burden among patients with metastatic and recurrent, locally-advanced HNC.
Patients and methods
Data source
This study was based on administrative claims data extracted from Thomson Reuters MarketScan Databases from January 2004–December 2008. Two datasets were obtained: (1) the Commercial Claims and Encounters Database, which contains healthcare claims information from ∼100 third-party payers, including self-insured employers and health insurance plans; and (2) the Medicare Supplemental and Coordination of Benefits Database, which includes healthcare claims for patients aged 65 and older who have employer-sponsored Medicare plans. These databases include 69 million unique patients since 1996 and cover all census regions in the US, with predominance in the South and North Central (Midwest) regions. Data elements include information on patient demographics, enrollment history, claims for inpatient and outpatient medical services and pharmacy claims. Data are de-identified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act (HIPAA).
Study design and sample selection
This retrospective cohort study analyzed the treatment patterns, resource utilization and costs of patients with metastatic HNC or recurrent locally-advanced HNC, compared to cohorts of cancer-free control patients. The cohort of patients with metastatic HNC had at least one claim with a diagnosis code for HNC, which was identified based on International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes for certain malignant neoplasms: 141.xx (malignant neoplasm of tongue), 143.xx (gum), 144.xx (floor of mouth), 145.xx (other and unspecified parts of mouth), 146.xx (oropharynx), 148.xx (hypopharynx) and 161.xx (larynx). The date of the first HNC claim was defined as the initial HNC diagnosis date. To be included in the sample, patients were required to be free from any other malignancy prior to the initial HNC diagnosis date in the database. After the initial HNC diagnosis date, metastatic HNC patients had a secondary tumor diagnosis (ICD-9-CM codes 197.xx or 198.xx). The first date for secondary tumor diagnosis was defined as the index date for this cohort. Patients were also required to have continuous health plan eligibility for 6 months before and 6 months after the index date and to be 18 years or older as of the index date.
Similarly to the metastatic cohort, patients with recurrent, locally-advanced HNC had at least one HNC claim; the date of the first HNC claim was defined as the initial HNC diagnosis date. Patients were also required to be free from any other malignancy prior to the initial HNC diagnosis date in the database. In addition, patients had at least two HNC claims that occurred at least 180 days apart; the date of the latter HNC claim was defined as the index date and is considered as indicating recurrence of HNC. The minimum of 180 days between two HNC claims was required to ensure that the patients had recurrent cancer. Eligible patients also received at least one HNC-related treatment (including chemotherapy, targeted therapy or radiation therapy) within a 60-day window around the index date. Patients could not have a claim for metastatic cancer (i.e., secondary tumor ICD-9-CM codes 197.xx and 198.xx) for the period between the initial HNC diagnosis date and 6 months following the index date. Eligibility and age criteria were the same as described for the cohort of patients with metastatic HNC.
Patients in both HNC cohorts (i.e., metastatic HNC and recurrent, locally-advanced HNC) were each matched 1:1 to cancer-free control patients based on geographic region, gender and age group. Patients in the HNC cohorts who could not be matched were excluded from the sample. Eligible control patients consisted of individuals who were 18 years or older and did not have a claim for any type of cancer. Control patients were attributed the same index year as their matched pair. Their index dates were randomly selected from all eligible days within the attributed index years.
Outcome measures and statistical analysis
Baseline characteristics were compared between patients in each HNC cohort and its respective cancer-free control group. The baseline period was defined as the 6 months prior to the index date. The summarized demographic characteristics included patient age, gender, region of residence, insurance type, index year and Charlson Comorbidity Index (CCI). CCI is a validated predictor of a patient’s 1-year mortality risk based on the presence or absence of major comorbidities, where each comorbid condition is assigned a score ranging from 1–6 depending on the associated risk of dyingCitation12; cancer diagnoses were excluded from the computation of CCI in the present study. Baseline characteristics were statistically compared between HNC groups and their respective control groups using Wilcoxon rank-sum tests for continuous variables and McNemar’s tests for categorical variables.
Disease characteristics and treatment patterns were assessed within each HNC cohort. For the cohort of patients with metastatic HNC, disease characteristics included primary HNC tumor sites (defined based on the ICD-9 codes observed on the initial HNC diagnosis date), secondary malignancy sites (defined based on the malignancy codes observed on the index date) and time from the initial HNC diagnosis to the date of metastatic HNC (i.e., the index date). For the cohort of patients with recurrent, locally-advanced HNC, disease characteristics included primary HNC tumor sites and time from the initial HNC diagnosis to the date of recurrence (i.e., the index date). Treatment patterns for HNC-related surgery, radiation therapy, chemotherapy and supportive care (i.e., rehabilitation, nutrition therapy and drugs for treating cancer-associated symptoms such as cancer pain and cancer-related infections and events induced by cancer treatments such as vomiting, nausea and anemia) were assessed during the 6-month study period following the index date. Chemotherapy treatment was further stratified by individual drugs and whether it was targeted chemotherapy (e.g., cetuximab) or platinum-based chemotherapy.
All-cause healthcare resource utilization during the 6-month study period following the index date was compared between each HNC cohort and its corresponding cancer-free control group. Outcomes of interest included inpatient visits, outpatient visits, emergency room (ER) visits, supportive care and other uses of healthcare services not otherwise categorized (e.g., home visits, skilled nursing facility services, etc., but excluding pharmacy-related resource use). The outcomes were measured and expressed in two ways. The first metric was the proportion of patients with at least one claim in a given utilization category. The second metric, the frequency of utilization, reflects the average number of claims per patient in each utilization category during the 6-month study period. Multivariate regression modeling was also performed to compare utilization outcomes between the cohorts with adjustment for between-group differences in age and baseline CCI. The multivariate comparison of the proportion of patients with at least one claim was based on a logistic regression model and was expressed as odds ratios (OR). Negative binomial regression models were used for frequency of utilization and the comparison was expressed using incidence rate ratios (IRR).
The economic burden of HNC was assessed by quantifying the all-cause, per-patient costs associated with medical and pharmacy-related services. Medical costs were disaggregated into categories for inpatient, outpatient, ER, supportive care and other medical costs. Pharmacy costs reflect prescription drug purchases at retail pharmacies. These all-cause costs were computed for each HNC cohort and its respective control group. Multivariate regression models were used to compute adjusted cost differences, which may be interpreted as the incremental costs associated with HNC after controlling for age and baseline CCI differences between the HNC and control cohorts. Because healthcare costs have a non-normal, skewed-positive distribution, generalized linear models and two-part regression models were used to analyze cost outcomes. Costs represent reimbursed amounts from a third-party payer perspective and are inflation-adjusted to 2008 US$.
Results
Metastatic HNC
A total of 1042 patients were selected into the metastatic HNC cohort. The average age was 62 years and 72.4% of patients were male (). The HNC and control groups were similar with respect to geographic location and insurance type. The baseline CCI value was higher in the HNC cohort compared to the control group (0.6 vs 0.3, p < 0.0001).
Table 1. Baseline characteristics of metastatic HNC patients and their controls during the 6-month baseline period.
More than two-thirds of the primary tumors were in three sites, the larynx (27.9%), tongue (22.2%) and oropharynx (19.5%). The respiratory and digestive system was the most common secondary tumor site (location of metastatic disease) in 32.3% of patients. The average time between initial HNC diagnosis and metastasis was 334 days. Metastatic HNC patients were most commonly treated with supportive care (90.2%), radiation therapy (48.5%), surgery (41.9%) and chemotherapy (38.3%). Platinum-based chemotherapies were used by 27.8% of patients, while targeted chemotherapies were used by 10.3%. Disease characteristics and treatment patterns for the metastatic HNC cohort are presented in .
Table 2. Disease characteristics and treatment patterns of metastatic HNC patients.
Medical healthcare resource utilization was substantially higher in the metastatic HNC cohort compared to the control group (). A greater proportion of metastatic HNC patients had at least one claim in each utilization category compared to the controls: 59.0% vs 4.9% for inpatient visits, 97.7% vs 71.2% for outpatient visits, 25.3% vs 6.8% for ER visits, 27.6% vs 1.8% for supportive care services and 57.7% vs 17.4% for other visits. The differences for all measures were statistically significant. After controlling for age and baseline CCI in the multivariate regression model, a higher proportion of HNC patients had at least one claim for inpatient visits (OR = 26.4), outpatient visits (OR = 35.3), ER visits (OR = 4.4), supportive care (OR = 16.7) and other medical claims (OR = 6.2) (all p < 0.0001).
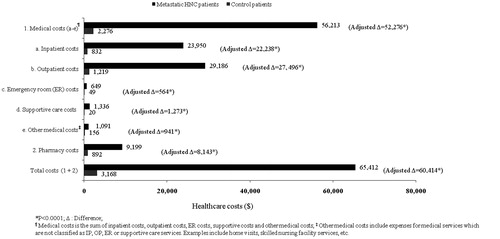
Figure 1. Healthcare resource utilization of metastatic HNC patients and their controls during the 6-month study period.
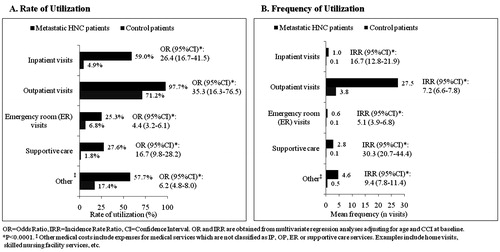
The mean frequency of medical visits over the 6-month study period was also significantly higher among patients with metastatic HNC compared to their matched controls. Patients in the metastatic HNC cohort had an average of 1.0 inpatient visits (vs 0.1 visits for the control group), 27.5 outpatient visits (vs 3.8 visits), 0.6 ER visits (vs 0.1 visits), 2.8 claims for supportive care (vs 0.1 claims) and 4.6 claims for other medical services (vs 0.5 claims) (all p < 0.0001). In the regression-adjusted analysis, metastatic HNC continued to be associated with significantly higher numbers of inpatient visits (IRR = 16.7), outpatient visits (IRR = 7.2), ER visits (IRR = 5.1), supportive care visits (IRR = 30.3) and other medical claims (IRR = 9.4) over 6 months (all p < 0.0001).
The total 6-month cost for HNC patients was observed to be $65,412 vs $3168 for patients in the control group, an unadjusted difference of $62,245 per patient. Controlling for age and baseline CCI, the adjusted total incremental cost for metastatic HNC patients was estimated to be $60,414 (p < 0.0001) over the 6-month study period. The total incremental cost was mainly attributed to incremental outpatient costs (46% of the adjusted total), inpatient costs (37%) and pharmacy costs (13%). The costs associated with ER, supportive care and other medical services comprised the remaining 4% of the adjusted total incremental costs. The comparison of healthcare costs of the metastatic HNC patients and their control group is presented in .
Recurrent, locally-advanced HNC
The cohort of recurrent, locally-advanced HNC consisted of 324 patients. Baseline characteristics of this HNC cohort and its control group are shown in . The average age was 67 years and the sample was predominantly male (71.6%). The HNC cohort and the controls were largely similar in baseline characteristics; however, mean CCI values during the baseline period indicated significantly greater comorbidity burden among patients with HNC than in the control group (0.7 vs 0.4, p < 0.0001).
Table 3. Baseline characteristics of recurrent locally advanced HNC patients and their controls during the 6-month baseline period.
The primary tumor sites in this HNC sample were the larynx (43.2%), tongue (20.7%), other and unspecified parts of the mouth (13.9%) and the oropharynx (12.3%), which together comprise ∼90% of the total (). On average, recurrence occurred 577 days following the initial HNC diagnosis. Patients with recurrent, locally-advanced HNC frequently received HNC-related supportive care (71.0%) and radiation therapy (67.9%). Chemotherapy was administered to 27.2% of patients, with platinum-based and targeted chemotherapies used by 9.6% and 4.6% of patients, respectively. HNC-related surgery was infrequent (12.7%) during the study period.
Table 4. Disease characteristics and treatment patterns of recurrent locally advanced HNC patients.
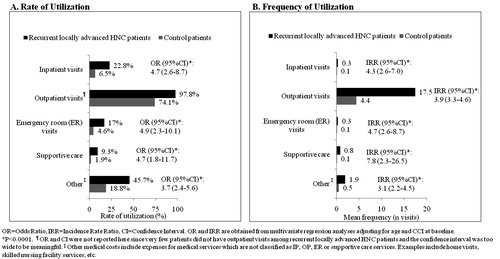
Medical healthcare utilization in this HNC cohort was statistically significantly higher than in the control group for all categories except for outpatient visits. HNC patients demonstrated higher rates of medical service use (i.e., at least one claim) compared to their controls in terms of inpatient visits (22.8% vs 6.5%), outpatient visits (97.8% vs 74.1%), ER visits (17% vs 4.6%), supportive care (9.3% vs 1.9%) and other medical services (45.7% vs 18.8%) during the 6-month study period. After adjustment for age and CCI, similar utilization differences were observed. OR values ranged from 3.7–4.9 across the utilization categories (all p < 0.0001).
Recurrent, locally-advanced HNC was similarly associated with higher frequencies of medical visits across all utilization categories and all differences were at least 3-fold (all p < 0.0001). In the multivariate regression analysis, HNC was associated with more frequent inpatient visits (IRR = 4.3), outpatient visits (IRR = 3.9), ER visits (IRR = 4.7), supportive care (IRR = 7.8) and other medical services (IRR = 3.1) (all p < 0.0001) ().
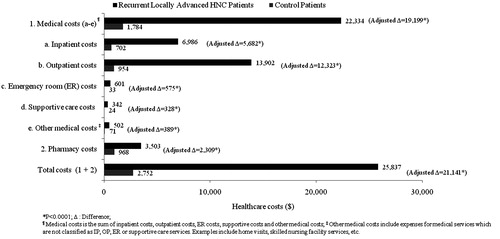
Figure 3. Healthcare resource utilization of recurrent locally advanced HNC patients and their controls during the 6-month study period.
The healthcare costs of patients with recurrent, locally-advanced HNC were substantially higher than costs incurred by the matched controls. The total cost for HNC patients over the 6-month study period was ∼9-times higher than that of the controls ($25,837 vs $2752) (). After adjusting for between-group differences in baseline CCI and age, the total 6-month incremental cost of HNC was calculated to be $21,141 (p < 0.0001). Over half of the incremental total cost was attributable to outpatient costs ($12,323). Incremental inpatient costs also accounted for a large portion of the overall total cost (over 25%), while incremental pharmacy costs represented a smaller portion (11%) (all p < 0.0001).
Discussion
There has been a scarcity of research focusing on the treatment patterns, medical resource utilization and economic burden of advanced HNC in the US. This retrospective study is aimed to provide up-to-date information on these outcomes of interest using recent data (2004–2008) that are nationally representative in the US.
The treatment patterns observed in the present study reveal the current practice patterns in patients with advanced HNC. The current study indicated that ∼50% of metastatic HNC patients in the US received radiation therapy, while chemotherapy and HNC-related surgery were each used in ∼40% of patients. Treatment patterns for patients with recurrent, locally-advanced HNC differed, with radiation therapy being more common (∼70%) and chemotherapy and HNC-related surgery being less commonly used (∼30% and 10%, respectively). These patterns were somewhat different from those observed in a prior study by Lang et al.Citation11, which evaluated treatment patterns of HNC patients by cancer stage in the first 4 months after diagnosis. Their study included Medicare-covered patients diagnosed with HNC during 1991–993, used a 5-year follow-up period and was restricted to patients ≥ 65 years old. In that study, radiotherapy was administered to ∼70% of the patients with regional or distant HNC and surgery to ∼50%, while chemotherapy was rarely used (13–18% of patients)Citation11. These differences might be attributed to different study populations of the two studies (a younger population being used in the current study who might require more aggressive treatment, and the two study populations having different insurance coverage), different study period (4 vs 6 months) and the increased role of chemotherapy in treating these patients.
A wide range of values for healthcare resource use among HNC patients was reported in the literature. Lang et al.Citation11 stated that ∼82% of HNC patients were hospitalized during the 5-year follow-up period; and Murphy et al.Citation13 found that 37% of patients were hospitalized over the course of treatment in a prospective 6-week study based on 75 patients. Healthcare resource use in the present study was substantial as well. In the 6 months after HNC had metastasized, 59% of patients were hospitalized. Metastatic HNC patients had an average of 27.5 outpatient visits, 1.0 inpatient visits and 0.6 ER visits over 6 months. For patients with recurrent, locally-advanced HNC, utilization of outpatient, inpatient and ER services was lower than utilization by metastatic patients. For both cohorts of HNC patients, utilization was significantly higher than for those in the cancer-free control groups.
In addition to the extensive resource use among the HNC patients, the cost for HNC is correspondingly high. Adjusting for age and CCI, 6-month total incremental cost (cost difference between HNC patients and their controls) was $60,414 for patients with metastatic HNC and $21,141 for those with recurrent, locally-advanced HNC. For both groups of HNC patients, the majority of the adjusted incremental costs were attributable to outpatient, inpatient and pharmacy costs. The distribution of the costs differed between the HNC cohorts, with inpatient costs accounting for a greater proportion of costs in patients with metastatic HNC vs recurrent, locally-advanced HNC (37% vs 27%). Outpatient costs comprised a relatively smaller portion of costs in the metastatic population vs the recurrent, locally-advanced population (46% vs 58%), while pharmacy costs on a percentage basis were roughly similar across HNC cohorts.
The economic burden of HNC patients estimated in the current study was in line with prior researchCitation11,Citation14,Citation15. Lang et al.Citation11 analyzed the economic costs associated with HNC patients in the US. That study found that patients with squamous cell carcinoma of the head and neck used significantly more healthcare resources than matched cancer-free controls. On average, patients with distant stages of squamous cell carcinoma of the head and neck incurred $2547 total costs per month (1998 US dollars)Citation11, which translates into a 6-month cost of ∼$19,750 (2008 US$). This is comparable to the present study’s 6-month cost estimate for recurrent, locally-advanced HNC ($25,837, 2008 US$) but much lower than the estimate for metastatic HNC ($65,412, 2008 US$). The two studies differ in a number of ways, but the divergence in these cost estimates may be due in part to the fact that (1) current treatment patterns have evolved in the roughly 10 years that separate the data in the studies and (2) the present study included pharmacy costs that were not covered by Medicare in the 1990s. Additionally, Lang et al.Citation11 pointed out that patients with regional and distant HNC incur 1.3–1.4 times more costs than patients with localized cancer ($58,387 and $53,741 vs $42,698). In another study, Epstein et al.Citation14 found that median total cost of HNC patients during the first year of treatment also differed by cancer stage. Patients in the early-stage treatment group incurred $22,658 compared to $27,665 in the late-stage group. Another study also highlighted the high cost of treatment-related side-effects (mucisitis), bringing attention to the expenses associated with resource use of HNC patientsCitation15. In addition, a comprehensive analysis of Medicare-SEER database estimated the annual costs for all types of cancers, including HNC. This study used claims data before 2006, then inflated the costs to 2010 US$ and provided estimated costs for each major type of cancer by age group and gender. For HNC, the study estimated the initial annualized costs in the range of $40,000–50,000 per year, depending on the age and genderCitation16. Although the study included all head/neck cancer patients and not specifically metastatic and locally-advanced cases, its annualized costs were also in line with the estimate in the present study. Mariotto et al.Citation16 estimated costs for 13 types of cancers for men and 16 cancers for women and found the range from $5000 for melanoma to $130,000 per year for brain cancer. We can see that the HNC costs are about in the middle of this range, similar to kidney cancer.
The present study is subject to several limitations. First, in the absence of clinical information, identification of metastatic and recurrent locally-advanced HNC patients was based on diagnosis codes and thus is subject to misclassification. Future research on the burden of HNC would be enhanced with the use of clinical data containing histology/pathology records to confirm the disease stage. Second, recurrence of HNC was defined as a gap of at least 180 days between two diagnoses of HNC. Using this method, the number of recurrent locally-advanced HNC patients may have been under-estimated as patients may have regular check-ups that did not signify recurrent disease with an interval of less than 180 days. Third, the analysis is based on retrospective claims data and these data may be affected by measurement error associated with variable coding practices and inaccuracies in coding. With the use of the claims database, this study only estimates the total direct costs to the payer. However, for burden of illness, it is important also to consider the costs incurred to other parties, including both direct and indirect costs, in order to estimate the total costs to the society. The societal costs would need to include patients’ loss of productivity (i.e. presenteeism and absenteeism), as well as caregivers’ time loss. These indirect costs will be important to explore in a future study. In addition, information used in the current study is limited to the claims that are available throughout the patients’ enrollment in the database. These usual limitations of claims data, however, are unlikely to affect the cohorts differentially. Nonetheless, claims data have the advantage of being a valid, large-sample source of real-world practice data. Finally, the subjects in this study belonged to commercial health insurance plans and their characteristics may not be generalizable to populations with different socioeconomic or demographic characteristics.
Conclusion
This study provides new insight into the healthcare resource utilization and cost burden of metastatic and recurrent, locally-advanced HNC using retrospective claims data from the US during 2004–2008. Compared to a sample of cancer-free control patients, individuals with HNC were found to have much higher levels of healthcare utilization. Over the 6 months following HNC metastases, patients incurred a total, adjusted incremental cost of $60,414 per patient for medical and pharmacy expenses. The 6-month incremental cost of care following recurrence of locally-advanced HNC was $21,141. Metastatic HNC was associated with an ∼20-fold increase in total direct healthcare costs relative to cancer-free patients, while recurrent locally-advanced HNC was linked to an ∼9-fold cost increase. Thus, healthcare resource utilization and economic burden of patients with metastatic or recurrent, locally-advanced HNC are substantial.
Transparency
Declaration of funding
Eli Lilly and Company provided an unrestricted grant to Analysis Group, Inc to conduct this study.
Declaration of financial/other relationships
T. Kim Le and Katherine B. Winfree are employees of Eli Lilly and Company and T. Kim Le owns stock/stock options in the company. Hongbo Yang, Maryna Marynchenko, Andrew P. Yu, Christian Frois and Eric Q. Wu are employees of Analysis Group, Inc.
Acknowledgments
Presented at the International Society for Pharmacoeconomics and Outcomes Research, 16th Annual International Meeting, Baltimore, MD, May 21–25, 2011.
References
- Marur S, Forastiere AA. Head and neck cancer: changing epidemiology, diagnosis, and treatment. Mayo Clin Proc 2008;83:489–501
- Kreimer AR, Clifford GM, Boyle P, et al. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75
- Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr. Accessed
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277–300
- Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist 2010;15:994–1001
- Gold KA, Lee HY, Kim ES. Targeted therapies in squamous cell carcinoma of the head and neck. Cancer 2009;115:922–35
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers. V.2. 2010. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed November 4, 2010
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 2008;359:1116–27
- Menzin J, Lines LM, Manning LN. The economics of squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2007;15:68–73
- Lee JM, Turini M, Botteman MF, et al. Economic burden of head and neck cancer: a literature review. Eur J Health Econ 2004;5:70–80
- Lang K, Menzin J, Earle CC, et al. The economic cost of squamous cell cancer of the head and neck: findings from linked SEER-Medicare data. Arch Otolaryngol Head Neck Surg 2004;130:1269–75
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epi 1992;45:613–9
- Murphy BA, Beaumont JL, Isitt J, et al. Mucositis-related morbidity and resource utilization in head and neck cancer patients receiving radiation therapy with or without chemotherapy. J Pain Symptom Manage 2009;38:522–32
- Epstein JD, Knight TK, Epstein JB, et al. Cost of care for early- and late-stage oral and pharyngeal cancer in the California Medicaid population. Head Neck 2008;30:178–86
- Peterman A, Cella D, Glandon G, et al. Mucositis in head and neck cancer: economic and quality-of-life outcomes. J Natl Cancer Inst Monogr 2001;29:45–51
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the Cost of Cancer Care in the U.S.: 2010-2020. J Natl Cancer Inst 2011;103:117–28