Abstract
Objectives:
A cost-effectiveness model for rivaroxaban evaluated the cost-effectiveness of prophylaxis with rivaroxaban (a once-daily, orally administered Factor Xa inhibitor) vs enoxaparin in the prevention of venous thromboembolism (VTE) after total hip replacement (THR) and total knee replacement (TKR). This Canadian analysis was conducted using the Ontario Ministry of Health perspective over a 5-year time horizon. The model combined clinical data and builds upon existing economic models.
Methods:
The model included both acute VTE (represented as a decision tree) and long-term complications (represented as a Markov process with 1-year cycles) phases. The model allowed VTE event rates, quality-adjusted life expectancy and direct medical costs to be estimated over a 5-year time horizon, based on current approved practice patterns in Canada. A number of one-way sensitivity analyses were performed on the baseline assumptions, including a comparison of rivaroxaban with dalteparin, and probabilistic sensitivity analyses were performed to address any uncertainty concerning model inputs.
Results:
When comparing equal durations of therapy, rivaroxaban dominated enoxaparin in the prevention of VTE events in patients undergoing THR and TKR, providing more benefit at a lower cost. Rivaroxaban was cost-effective when comparing 35 days’ prophylaxis with 14 days’ prophylaxis with enoxaparin following THR. One-way and probabilistic sensitivity analyses demonstrated that the results of the economic analysis were robust to variations in key inputs. Rivaroxaban remained dominant during one-way sensitivity analyses comparing rivaroxaban with dalteparin after THR or TKR.
Limitations:
Although clinical trial data were used in the prophylaxis module, assumptions and values used in the post-prophylaxis and long-term complication (LTC) modules were based on several different literature sources; it was not always possible to source Canadian data.
Conclusions:
This economic analysis suggests that the use of rivaroxaban for the prophylaxis of VTE after THR or TKR in Canada was cost-effective.
Introduction
Venous thrombosis is a potentially serious condition that can result in sudden death or cause long-term morbidity with subsequent economic consequences. Deep vein thrombosis (DVT) and pulmonary embolism (PE)—together referred to as venous thromboembolism (VTE)—result in a major burden on healthcare systems. VTE is a common disease, with an estimated annual incidence of ∼5–12 persons per 10,000Citation1–3, and the impact of VTE is highlighted by the fact that ∼10% of all hospital deaths can be attributed to PECitation4.
Without prophylaxis, the risk of VTE in patients undergoing major surgery is 15–40%Citation5; this risk is particularly high in patients undergoing major orthopaedic surgeryCitation5–7. Despite prophylaxis, VTE remains a significant problem in patients undergoing surgery. Even with standard prophylaxis such as enoxaparin or warfarin up to 4 weeks after surgery, between 1.4–2.8% of patients develop symptomatic DVT after total knee replacement (TKR) or total hip replacement (THR) and PE occurs in up to 1.2% of patientsCitation8. The prognosis for patients with VTE is characterized by the risk of recurrent events or post-thrombotic syndrome (PTS). Over 5 years of follow-up in patients with DVT, the cumulative incidence of PTS ranged from 18.0% after 1 year to 29.6% after 5 years in a study by Prandoni et al.Citation9 Reported frequencies of DVT patients that develop PTS range from about one-third to one-half, with most cases occurring within 1–2 years of acute DVTCitation10.
In Canada, the number of patients undergoing elective THR and TKR has increased by more than 100% over the last 10 years, with 24,253 THR and 37,943 TKR operations in 2008/9Citation11. To minimize risk of VTE, the American College of Chest Physicians (ACCP) recommends that patients receive VTE prophylaxis for up to 35 days (minimum of 10 days) after THR and suggest up to 35 days of VTE prophylaxis (minimum of 10 days) after TKRCitation5. Prior to the launch of rivaroxaban, the most commonly used method of VTE prophylaxis in Canada was parenterally administered low molecular weight heparins (LMWHs) such as enoxaparin and dalteparinCitation11.
Rivaroxaban is a novel, once-daily, orally administered direct Factor Xa inhibitorCitation12,Citation13. Phase III clinical trials (REgulation of Coagulation in ORthopaedic surgery to prevent Deep vein thrombosis and pulmonary embolism RECORD1--3) have demonstrated that rivaroxaban offers superior efficacy and similar safety and bleeding, compared with enoxaparin in terms of reducing VTE events after THR and TKRCitation14–17. Rivaroxaban is indicated for the prevention of VTE in patients who have undergone elective THR or TKR surgery. This paper presents the results of additional Canadian analyses based on the cost-effectiveness model for rivaroxaban in VTE prophylaxisCitation18, using the Ontario Ministry of Health perspective. Whereas a previously-published economic model took a more clinical perspective by including only statistically significant differences in event ratesCitation18, the current analysis is based upon the observed event rates from the pivotal clinical trials, allowing less common events to be included. In addition, this analysis demonstrates the cost-effectiveness of rivaroxaban relative to both 35 days’ and 14 days’ enoxaparin prophylaxis after THR, while the model described in Diamantopoulos et al.Citation18 presented results of rivaroxaban vs 35 days’ enoxaparin prophylaxis only. The duration of enoxaparin and rivaroxaban prophylaxis for TKR is 14 days after surgery in both the current and the previously published analysesCitation18.
Management of VTE: Impact on health resources
Costs per VTE event can include costs for inpatient and/or outpatient care of a VTE event plus costs of anticoagulation therapy and associated monitoringCitation19. Published Canadian data for inpatient complication costs (for discharged patients) include C$10,316 and C$6716 for DVT and PE, respectively, with no major bleed, C$20,191 for DVT or PE with major bleed, C$13,997 for major bleed with no VTE and C$436 for post-discharge drug therapyCitation20. The development of PTS has also been highlighted as an important cost driverCitation21. The costs used in this analysis were based on the sources stated in , including Skedgel et al.Citation20 inflated to 2011 prices.
Table 1. Cost and resource use (95% CI where necessary)a.
Among patients who develop VTE following major orthopaedic surgery, there is a risk of recurrent VTE events. Data from the Computerized Registry of Patients with Venous Thromboembolism (RIETE), a prospective international registry of consecutive patients with objectively confirmed symptomatic acute VTE, reported the 3-month incidence of fatal PE, fatal bleeding and major bleeding as 1.3%, 0.8% and 2.3%, respectively, following VTE post-orthopaedic surgeryCitation31. In addition, the use of thromboprophylaxis was deemed sub-optimal in this study, with the recommendation that the use of adequate thromboprophylaxis should be strongly encouraged for all post-operative patients. This finding is supported by data from the multinational Global Orthopaedic Registry (GLORY), which evaluated the compliance of surgeons with the ACCP guidelines for VTE preventionCitation32. Although most patients in this study received some form of ACCP-recommended prophylaxis (THR patients: 95% in the US, 92% outside the US; TKR patients: 99% in the US, 96% outside the US), a large proportion of patients did not receive prophylaxis in accordance with the recommended start time, duration and dose/treatment intensity stated in the ACCP guidelines (THR patients: 53% in the US, 38% outside the US; TKR patients: 39% in the US, 31% outside the US)Citation32.
In addition to the direct medical costs, management of VTE impacts upon patient and carer time and transportation costs. One Canadian prospective cohort study investigating patient/family costs only (lost income, drug costs, transportation costs) showed these costs to range from C$219 among patients managed in the outpatient setting to C$403 among patients managed in the inpatient setting (based on per DVT treatment using LMWH)Citation33; such costs add to the overall burden of VTE. Guanella et al.Citation34 reported an average loss of 7.8 work days per patient in the 4 months following diagnosis of DVT. This number increased to 12.1 work days lost after 2 yearsCitation34, with loss of productivity (C$1543) and hospitalizations (C$1504, baseline and follow-up hospitalizations) cited as the largest components of DVT-related costs during the 2 years following diagnosisCitation34.
As an important and resource-intensive complication after THR and TKR, the potential impact of VTE on healthcare systems is substantial. There is, therefore, a clear need for more efficacious and cost-effective prophylaxis strategies to reduce the burden of VTE. Indeed, it has been previously recommended that every hospital needs to develop a formal strategy that addresses the prevention of VTECitation5.
Cost-effectiveness model: A Canadian analysis using the Ontario Ministry of Health Perspective
A decision-analytic model evaluated the cost-effectiveness of rivaroxaban, compared with enoxaparin as prophylaxis for VTE in patients undergoing THR and TKR from the perspective of the Canadian healthcare systemCitation18. This cost-effectiveness model compared rivaroxaban with the recommended practice of 35 days’ LMWH prophylaxis following THR, as outlined in ACCP guidelinesCitation5, and 14 days’ post-surgical prophylaxis following TKR. However, at the time of the introduction of rivaroxaban in Canada, prophylaxis after THR and TKR consisted of 14 days’ LMWH in many provinces. This analysis is designed to assess the cost-effectiveness of rivaroxaban (35 days) vs both 14 days’ (short-term) and 35 days’ (extended) LMWH prophylaxis after THR and 14 days’ (short-term) LMWH prophylaxis after TKR, in Canada using the Ontario Ministry of Health Perspective. Sensitivity analyses also compare rivaroxaban with dalteparin, a commonly used LMWH in Canada.
Methods
The economic analysis was developed to answer the question: What is the incremental cost-effectiveness of rivaroxaban 10 mg od, compared with enoxaparin 40 mg od in Canadian THR and TKR patients from the perspective of the Ontario Ministry of Health over a 5-year time horizon? The model (structurally the same as that published by Diamantopoulos et al.Citation18) included both acute VTE (represented as a decision tree) and long-term complications (LTCs) (represented as a Markov process with 1-year cycles) phases; this allowed VTE event rates, quality-adjusted life expectancy and direct medical costs to be estimated over a 5-year time horizon, based on current approved practice patterns in Canada.
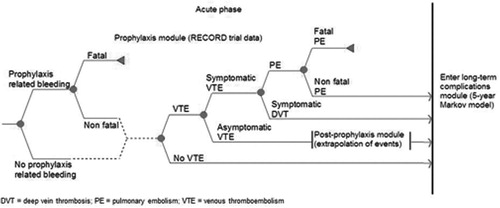
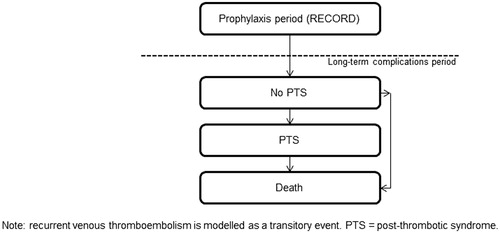
The decision-analytic model was based upon previously published modelsCitation6,Citation35,Citation36 that were extended to better incorporate the use of quality-adjusted life years (QALYs) LTCsCitation18. In addition, Quinlan et al.Citation37 previously reported a consistent relationship between asymptomatic DVT and symptomatic VTE beyond the 35-day period studied in clinical trials. This reflects the reality in clinical practice that patients will typically not receive venography or an ultrasound, so an asymptomatic VTE will remain untreated with an ongoing risk of becoming symptomatic. Data have previously shown that patients are at risk of developing VTE for up to 3 months following surgeryCitation35. Therefore, the decision-analytic model extrapolated data for the risk of VTE in patients who had asymptomatic VTE at the end of the prophylaxis period. The decision-analytic model includes the 3-month period following THR and TKR with the potential for acute VTE (prophylaxis period and post-prophylaxis period), which is represented as a decision tree (). This is followed by the period with potential for LTCs represented as a Markov process with 1-year cycles up to 5 years ().
The event rates used in the model are derived from RECORD1--3 clinical trials and are presented in . Data from The RECORD4 trial were not included in our model as this study used a different treatment regimen, which was not the focus of our research question. The previous published Canadian cost-effectiveness analysisCitation18 presents results based on event rates that had statistically significant differences between treatment arms. Because clinical trials are often not powered to show statistically significant differences in rare events, using the observed events rates provides some indication of the effect of the intervention on rare events. Therefore, the current baseline analyses used observed events from the clinical trials, with the probabilistic sensitivity analysis (PSA) addressing the uncertainty relating to non-statistically significant differences in event rates.
Table 2. Event probabilities used in the model (95% CI)a.
Canada’s national health insurance program is designed to ensure that all residents have reasonable access to medically necessary hospital and physician services, on a pre-paid basisCitation40. Instead of having a single national plan, Canada has a national program that is composed of 13 interlocking provincial and territorial health insurance plans, all of which share certain common features and basic standards of coverage. Roles and responsibilities for Canada’s healthcare system are shared between the federal and provincial-territorial governments. Provincial and territorial governments are responsible for the management, organization and delivery of health services for their residentsCitation40. Consequently, the costs for healthcare resources may differ between provinces. Because of the potential for slightly different costs between provinces, one jurisdiction (i.e., province) is typically used as the reference case for economic evaluations conducted in the Canadian setting. Wherever possible, Ontario costs were used in our base case analysis, as Ontario has the largest population of all the provinces. These costs were varied extensively in sensitivity analyses to explore the impact that differences in cost inputs may have on study results.
Costs relating to prophylaxis, VTE treatment, LTC and bleeding were included in the model (). Resource use data were based upon previous publications, while cost data were based on locally published cost data, as well as data from the Ontario Case Costing Initiative (OCCI) (not published, but can be accessed by request) (); costs were inflated to 2011 prices using Statistics Canada consumer price indexes for health and personal careCitation41,Citation42. VTE-related utilities reported in a previous cost utility studyCitation43 were used in the analysis. As it was unlikely that patients who had just had TKR or THR would have a utility of 1 (i.e., full health), data from Räsänen et al.Citation44 were utilized; these data showed patients who have recently undergone THR or a TKR to have utilities of 0.805 and 0.807, respectively. These utility values were used to weight the values for each of the events occurring in the prophylaxis and extrapolation periods. The utility values used in the model are shown in .
Table 3. Utility values.
In order to assess the cost-effectiveness of rivaroxaban, compared with enoxaparin, in the short-term, one-way sensitivity analyses based on time horizons of 35 days (prophylaxis period) and 3 months (post-prophylaxis period) were conducted. To assess the robustness of the model and examine the effect of potential variations in clinical practice, costs and event rates, a number of one-way sensitivity analyses were also performed on the baseline assumptions. Parameters tested included outpatient administration costs associated with enoxaparin and event treatment costs. A sensitivity analysis comparing rivaroxaban with dalteparin in THR and TKR was performed as dalteparin is a frequently used LMWH in Canada. This analysis assumed that the efficacy and safety of LMWHs did not differCitation46–48 and, therefore, used enoxaparin efficacy and safety inputs for the dalteparin arm.
Probabilistic sensitivity analyses were also performed to address any uncertainty concerning model inputs. A total of 2000 iterations of the base case were performed using a Monte Carlo simulation approach. Parameters of the model that were sampled include drug administration and monitoring costs, prophylaxis-related bleeding costs, diagnosis and treatment costs, DVT and PE treatment costs, LTC costs, utility values and event probabilities for the prophylaxis, post-prophylaxis and LTC modules. Distributions were generated using 95% confidence intervals (CIs), standard deviations and assumptions, depending upon the parameter and the availability of data.
Results
Rivaroxaban prophylaxis for 35 days was shown to be cost-effective following THR compared with either 14 days or 35 days of enoxaparin prophylaxis (). When compared with 35 days’ enoxaparin, rivaroxaban dominated enoxaparin with per-patient cost savings of C$296.95 over a 5-year period combined with improved QALYs and reduced symptomatic VTE events. The higher drug costs for rivaroxaban were offset by the reduced incidence and cost of symptomatic VTE and its LTCs and by the reduced need for assistance, compared with enoxaparin injections. When compared with 14 days’ enoxaparin, 35 days’ rivaroxaban is associated with an incremental cost of C$35.35 and a QALY gain of 0.0052 per patient; this corresponds to an incremental cost per QALY gained of C$6741.96, which is well below the frequently referenced Canadian threshold of $50,000/QALY.
Table 4. Costs and cost-effectiveness of rivaroxaban vs enoxaparin following THR.
In TKR, rivaroxaban dominated enoxaparin, providing more benefit at less cost, with a per-patient cost saving of C$150.44, combined with improved QALYs and reduced symptomatic VTE events (). This cost saving is primarily driven by the reduced incidence and cost of symptomatic VTE and its LTCs and by the reduced need for assistance, compared with enoxaparin injections.
Table 5. Costs and cost-effectiveness of thromboprophylactic intervention following TKR.
Rivaroxaban remained dominant during sensitivity analyses comparing rivaroxaban with dalteparin after THR or TKR ( and , respectively). A range of sensitivity analyses were conducted on the results of rivaroxaban vs 14 days’ and 35 days’ enoxaparin prophylaxis (). In the comparison of 35 days’ rivaroxaban with 35 days’ enoxaparin for the THR population, rivaroxaban remains dominant in each one-way sensitivity analysis—even when the analysis period is reduced to 90 days (cost saving C$286.75)—or when it is conservatively assumed that a relatively low proportion (8%) of patients receiving LMWH injection require assistance (cost saving C$112.12), as per the UK. This is consistent with the results of the PSA () in which nearly all of the simulations are in the lower right-hand quadrant showing improved health outcomes alongside cost savings with rivaroxaban. In the comparison of 35 days’ rivaroxaban vs 14 days’ enoxaparin prophylaxis for THR patients, the results remain consistent with the baseline finding of cost-effectiveness (). The sensitivity analyses show that the cost-effectiveness of rivaroxaban vs 14 days’ enoxaparin shows a position of dominance when 39% of enoxaparin patients receive assistance with injectionsCitation20; the finding of overall cost-effectiveness is supported by the cost-effectiveness acceptability curve (), which shows more than 90% of simulations below a cost-effectiveness threshold of C$50,000 per QALY gained.
Figure 3. (a) Cost-utility plane for economic evaluation comparing 35 days’ rivaroxaban 10 mg od vs 35 days’ enoxaparin 40 mg od following total hip replacement. (b) Cost-utility plane for economic evaluation comparing 35 days' rivaroxaban 10 mg od vs 14 days' enoxaparin 40 mg od following total hip replacemnt over a 5-year time horizon.
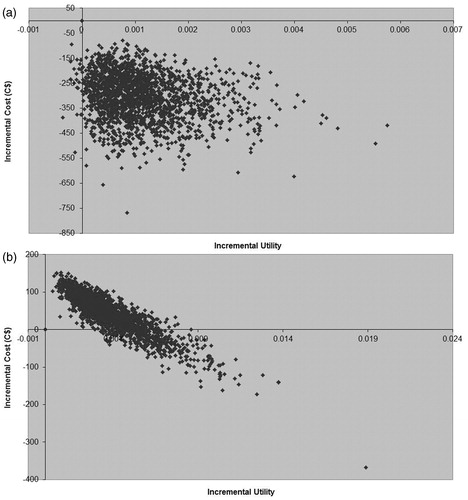
Table 6. One-way sensitivity analysis results: THR.
Table 7. One-way sensitivity analysis results: TKR.
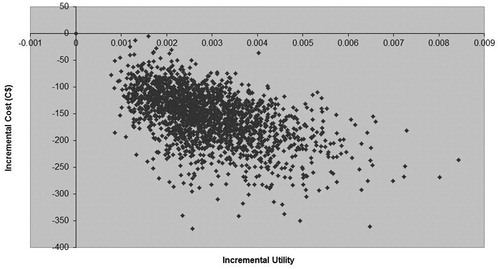
In the TKR population, the sensitivity analyses showed that rivaroxaban dominates enoxaparin, providing more benefit at less cost (). The dominance is clear even when the analysis period is reduced to 90 days (cost saving C$129.84) or when the lower proportion of patients receiving LMWH injection assistance as per the UK, 8%Citation6 is used (cost saving C$96.56). The results of the PSA further support the finding of dominance: 100% of simulations appeared in the lower right quadrant of the cost-utility analysis (CUA) plane (), indicating that 14 days of rivaroxaban were less costly and provided more benefit than 14 days of enoxaparin.
Figure 4. Cost-utility plane for economic evaluation comparing 14 days’ rivaroxaban 10 mg od vs 14 days’ enoxaparin 40 mg od following total knee replacement over a 5-year time horizon.
Overall, the one-way sensitivity analyses and the PSA suggest that the results of the economic analyses using the Canadian decision-analytic model were robust against variations in key inputs for both THR and TKR. Several factors were identified as drivers of cost-effectiveness for rivaroxaban. For example, cost-effectiveness was driven by improved efficacy in terms of fewer VTE events, leading to a higher number of QALYs gained. Also, the economic value of rivaroxaban was partially driven by the reduction of treatment-related monitoring needs and the reduction in LTCs that would impact upon healthcare resources.
Discussion
This Canadian economic evaluation assessed the cost-effectiveness of prophylaxis with rivaroxaban for the prevention of VTE after THR and TKR relative to enoxaparin 40 mg od from the perspective of the Ontario Ministry of Health over a 5-year time horizon. The province of Ontario was used as an example to represent the Canadian healthcare perspective.
The efficacy and safety of rivaroxaban have been demonstrated in large Phase III randomized controlled trials, where rivaroxaban showed a statistically significant improvement over enoxaparin in the primary end-point of total VTECitation14–17. In addition, the present cost-effectiveness model has demonstrated that rivaroxaban is cost-effective, compared with enoxaparin. Although rivaroxaban 10 mg od has a slightly higher drug acquisition cost than enoxaparin 40 mg od in Canada, it has been shown that prophylaxis with rivaroxaban in patients undergoing THR or TKR was associated with fewer symptomatic VTE events and overall cost savings (up to C$300 per patient), compared with enoxaparinCitation18.
Patients receiving LMWH have a lower risk of VTE events, compared with patients receiving no prophylaxisCitation6. The use of LMWH has also been shown to reduce the impact of VTE (including late VTE events) on healthcare resources. Huo et al.Citation49 recently reported that LMWHs were efficacious, associated with low rates of clinically-relevant bleeding complications and cost-effective in patients at high risk of VTE. Similarly, a comparison of 2-year outcomes and cost of prophylaxis in medical patients at risk of VTE reported that prophylaxis with enoxaparin was more effective and less costly than unfractionated heparinCitation50; this study considered all direct medical costs associated with VTE up to 2 years after an admission for acute illness.
Published data on utility values for symptomatic VTE are relatively scarce and the original source for published values can be difficult to uncover. This may lead to uncertainty regarding the results of economic evaluations that include utilities for VTE. A systematic literature review was conducted for this economic evaluation to capture all published VTE utilities. This analysis therefore uses mainly evidence and cost data based on published sources, with clinical expert opinion only used where published information was not available. The values used in the present economic evaluation are therefore consistent with those reported in the literature. To assess the sensitivity of the model results to variations in VTE utility values, sensitivity analyses were conducted using a range of symptomatic DVT and PE utilities from the literature. The cost-effectiveness results changed only minimally in these analyses, suggesting that the results presented in the base case analysis are robust against variations in VTE utilities.
The use of rivaroxaban may offer other additional benefits over other LMWHs. First, it is more efficacious than enoxaparin in preventing VTEs. Therefore, its use is associated with even greater savings to the healthcare system relative to LMWH. Furthermore, enoxaparin is administered as a parenteral injection while rivaroxaban is taken orally. This may lead to greater savings or improved quality-of-life for patients receiving rivaroxaban, compared with LMWH. For example, nursing time may be avoided if patients do not need to be trained to administer their own injections and the one-way sensitivity analyses showed rivaroxaban remained dominant regardless of whether high or low use of home care is considered. Furthermore, patients may have a preference or a higher utility for receiving oral vs injectable prophylaxis. The cost-effectiveness analysis conservatively assumed that no costs were incurred for the administration or monitoring of prophylaxis while in hospital. Furthermore, the analysis did not assume any disutility for injected vs orally-administered medications. Although this analysis has many strengths and includes extensive sensitivity analyses there are limitations with the approach taken using this model and these have been described previouslyCitation18. Briefly, although clinical trial data were used in the prophylaxis module, assumptions and values used in the post-prophylaxis and LTC modules were based on several different literature sources, it was not always possible to source Canadian data. Although rebound thromboembolic effects were not included specifically (as there was no evidence from the RECORD programme that rebound occurredCitation18), the post-prophylaxis period was assessed and long-term complications were considered for up to 5 years. Clinically relevant non-major bleeding and impact of health-related quality-of-life were excluded from the model as expert opinion suggested these parameters had low impact on resource useCitation18. Despite the limitations, one-way sensitivity analysis and PSA confirmed that variation of parameters did not affect the dominance of rivaroxaban for cost-effectiveness.
The Canadian Expert Drug Advisory Committee (2008) recommended that rivaroxaban be reimbursed for VTE prophylaxis for up to 14 days after THR and TKR in Canada, deeming this method of prophylaxis to be cost-effective when equal durations of prophylaxis were compared. In 2011, recognizing that many provinces were reimbursing LMWH for up to 35 days, this same committee recommended that rivaroxaban be reimbursed for the same duration as LMWHs (i.e., up to 35 days in provinces where 35 days of LMWH are reimbursed). Many provincial reimbursement agencies have listed rivaroxaban for up to 14 days following TKR and up to 35 days following THR. Rivaroxaban remains cost-effective for VTE prophylaxis when compared with the commonly used dalteparin during sensitivity analyses after THR or TKR and offers a potentially convenient alternative to injectable treatments.
Conclusions
Our cost-effectiveness analysis builds upon existing economic models evaluating VTE prophylaxis following THR and TKR and uses event data from the pivotal RECORD 1–3 clinical trials. The analysis suggests, when equal durations of therapy are compared, rivaroxaban dominates enoxaparin 40 mgod, providing more benefit at less cost relative to enoxaparin in THR and TKR patients. When 35 days of rivaroxaban are compared with 14 days of enoxaparin, rivaroxaban is still highly cost-effective relative to enoxaparin, with an incremental cost-effectiveness ratio of C$6741.96. The reduced incidence of symptomatic VTE with rivaroxaban, along with reduction in LTCs associated with symptomatic VTE, lead to substantial cost offsets. One-way analysis and PSA demonstrated that economic outputs for rivaroxaban are robust. Rivaroxaban remained dominant during sensitivity analyses comparing rivaroxaban with dalteparin after THR or TKR. Overall, the economic dominance of rivaroxaban over enoxaparin in THR and TKR when equal durations of prophylaxis are compared suggests that it is a cost-effective option for VTE prophylaxis in patients undergoing THR and TKR.
Transparency
Declaration of funding
The development of this manuscript was sponsored by Bayer HealthCare. The sponsor confirms that this manuscript is an accurate representation of the study results. The sponsor had input into the design of the study, collection, analysis and interpretation of data, writing of the manuscript and the decision to submit the manuscript for publication.
Declaration of financial/other relationships
Heather McDonald is an employee of Bayer Inc. Alex Diamantopoulos, Fiona Forster and Jaithri Ananthapavan provided consulting services to Bayer Pharma AG for which they received consultation fees. Philip Wells has received honoraria for presentations made for Bayer Pharma AG. Michael Lees was employed by Bayer plc at the time of the study. Kerstin Folkerts is an employee of Bayer Pharma AG.
Acknowledgements
The authors would like to acknowledge Matthew Joynson and Claire Lavin (Rx Communications, Mold, UK) for medical writing assistance (funded by Bayer Pharma AG). Additional editorial and project management assistance was provided by Rx Communications, Mold, UK (funded by Bayer Pharma AG).
References
- Fowkes FJI, Price JF, Fowkes FGC. Incidence of diagnosed deep vein thrombosis in the general population: systematic review. Eur J Vasc Endovasc Surg 2003;25:1-5
- Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001;86:452-63
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4-18
- Geerts WH, Pineo GF, Heit JA, et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126 (3 Suppl):338S-400S
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines, 8th edn. Chest 2008;133:381S-453S
- National Clinical Guideline Centre. Venous thromboembolism: reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. National Clinical Guideline Centre – Acute and Chronic Conditions. 2010. http://www.nice.org.uk/nicemedia/live/12695/47920/47920.pdf. Accessed March 12, 2011
- Blann AD, Lip GY. Venous thromboembolism. BMJ 2006;332:215-9
- Edelsberg J, Ollendorf D, Oster G. Venous thromboembolism following major orthopedic surgery: review of epidemiology and economics. Am J Health Syst Pharm 2001;58(2 Suppl):S4-13
- Prandoni P, Villalta S, Bagatella P, et al. The clinical course of deep-vein thrombosis. Prospective long-term follow-up of 528 symptomatic patients. Haematologica 1997;82:423-8
- Kahn SR. Frequency and determinant of the postthrombotic syndrome after venous thromboembolism. Curr Opin Pulm Med 2006;12:299-303
- Canadian Joint Replacement Registry. Hip and knee replacements in Canada 2008–2009 Annual Report. Canadian Institute for Health Information. http://secure.cihi.ca/cihiweb/dispPage.jsp?cw_page=services_cjrr_e. Accessed December 3, 2011
- Perzborn E, Kubitza D, Misselwitz F. Rivaroxaban. A novel, oral, direct factor Xa inhibitor in clinical development for the prevention and treatment of thromboembolic disorders. Hamostaseologie 2007;27:282-9
- Graff J, von Hentig N, Misselwitz F, et al. Effects of the oral, direct factor Xa inhibitor rivaroxaban on platelet-induced thrombin generation and prothrombinase activity. J Clin Pharmacol 2007;47:1398-407
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 2008;358:2765-75
- Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet 2008;372:31-9
- Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med 2008;358:2776-86
- Eriksson BI, Kakkar AK, Turpie AGG, et al. Oral rivaroxaban for the prevention of symptomatic venous thromboembolism after elective hip and knee replacement. J Bone Joint Surg Br 2009;91:636-44
- Diamantopoulos A, Lees M, Wells PS, et al. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. Thromb Haemost 2010;104:760-70
- Bullano MF, Willey V, Hauch O, et al. Longitudinal evaluation of health plan cost per venous thromboembolism or bleed event in patients with a prior venous thromboembolism even during hospitalization. J Manage Care Pharm 2005;11:663-73
- Skedgel C, Goeree R, Pleasance S, et al. The cost-effectiveness of extended-duration antithrombotic prophylaxis after total hip arthroplasty. J Bone Joint Surg Am 2007;89:819-28
- MacDougall DA, Feliu AL, Boccuzzi SJ, et al. Economic burden of deep vein thrombosis, pulmonary embolism, and post-thrombotic syndrome. Am J Health Syst Pharm 2006;63(20 Suppl 6):S5-15
- Ontario Ministry of Health and Long-Term Care. Government of Ontario, Canada. 2012. https://www.healthinfo.moh.gov.on.ca/formulary/index.jsp. Accessed February 16, 2012
- Harrison L, McGinnis J, Crowther M, et al. Assessment of outpatient treatment of deep-vein thrombosis with low-molecular-weight heparin. Arch Intern Med 1998;158:2001-3
- Anonymous. Ontario Association of Community Care Access Centres (OACCAC). 2010
- Ontario Case Costing Initiative (OCCI). Acute Inpatient Databases for 2005/06 and 2006/07. Toronto: Ministry of Health and Long-Term Care, 2007
- Ontario Schedule of Benefits. Physician Services under the Health Insurance Act (September 1, 2011). Toronto: Ministry of Health and Long-Term Care, 2011
- Government of British Columbia. Medical Services Commission Payment Schedule. British Columbia: Government of British Columbia. 2011. http://www.health.gov.bc.ca/msp/infoprac/physbilling/payschedule/index.html Accessed February 16, 2012
- Ontario Drug Benefit Formulary 2008. Ministry of Health and Long-Term Care. Toronto: Government of Ontario, Canada, 2008. http://www.health.gov.on.ca/english/providers/program/drugs/odbf_mn.html Accessed January 17, 2008
- Caprini JA, Botteman MF, Stephens JM, et al. Economic burden of long-term complications of deep vein thrombosis after total hip replacement surgery in the United States. Value Health 2003;6:59-74
- X-RatesTM. http://www.x-rates.com/. Accessed March 17, 2008
- Arcelus JI, Monreal M, Caprini JA, et al. Clinical presentation and time-course of postoperative venous thromboembolism: results from the RIETE Registry. Thromb Haemostat 2008;99:546-51
- Friedman RJ, Gallus AS, Cushner FD, et al., Global Orthopaedic Registry Investigators. Physician compliance with guidelines for deep-vein thrombosis prevention in total hip and knee arthroplasty. Curr Med Res Opin 2008;24:87-97
- Rodger MA, Gagné-Rodger C, Howley HE, et al. The outpatient treatment of deep vein thrombosis delivers cost savings to patients and their families, compared to inpatient therapy. Thromb Res 2003;112:13-8
- Guanella R, Ducruet T, Johri M, et al. Economic burden and cost determinants of deep vein thrombosis during 2 years following diagnosis: a prospective evaluation. J Thromb Haemost 2011;9:2397-405
- Sullivan SD, Kahn SR, Davidson BL, et al. Measuring the outcomes and pharmacoeconomic consequences of venous thromboembolism prophylaxis in major orthopaedic surgery. Pharmacoeconomics 2003;21:477-96
- Gordois A, Posnett J, Borris L, et al. The cost-effectiveness of fondaparinux compared with enoxaparin as prophylaxis against thromboembolism following major orthopedic surgery. J Thromb Haemost 2003;1:2167-74
- Quinlan DJ, Eikelboom JW, Dahl OE, et al. Association between asymptomatic deep vein thrombosis detected by venography and symptomatic venous thromboembolism in patients undergoing elective hip or knee surgery. J Thromb Haemost 2007;5:1438-43
- White RH, Romano PS, Zhou H, et al. Incidence and time course of thromboembolic outcomes following total hip or knee arthroplasty. Arch Intern Med 1998;158:1525-31
- Kearon C, Ginsberg JS, Hirsh J. The role of venous ultrasonography in the diagnosis of suspected deep venous thrombosis and pulmonary embolism. Ann Intern Med 1998;129:1044-9
- Health Canada 2012. Canada’s Health Care System. http://www.hc-sc.gc.ca/hcs-sss/medi-assur/index-eng.php. Accessed March 9, 2012
- Statistics Canada. Consumer Price Index, health and personal care, by province. http://www.statcan.ca/english/freepub/62-001-XIE/2007012/tablesectionlist.htm. Accessed 2008
- Statistics Canada. Consumer Price Index, health and personal care, by province. http://www40.statcan.gc.ca/l01/cst01/econ161a-eng.htm. Accessed February 16, 2012
- Haentjens P, De Groote K, Annemans L. Prolonged enoxaparin therapy to prevent venous thromboembolism after primary hip or knee replacement. A cost-utility analysis. Arch Orthop Trauma Surg 2004;124:507-17
- Räsänen P, Paavolainen P, Sintonen H, et al. Effectiveness of hip or knee replacement surgery in terms of quality-adjusted life years and costs. Acta Orthop 2007;78:108-15
- Lenert LA, Soetikno RM. Automated computer interviews to elicit utilities: potential applications in the treatment of deep venous thrombosis. J Am Med Inform Assoc 1997;4:49-56
- Anderson DR, O’Brien B, Nagpal S, et al. Economic evaluation comparing low molecular weight heparin with other modalities for the prevention of deep vein thrombosis and pulmonary embolism following total hip or knee arthroplasty. Ottawa: Canadian Coordinating Office for Health Technology Assessment (CCOHTA), 1998
- Boucher M, Rodger M, Johnson JA, et al. Shifting from inpatient to outpatient treatment of deep vein thrombosis in a tertiary care center: a cost minimization analysis. Pharmacotherapy 2003;23:301-9
- Hooper WC, Evatt BL. The role of activated protein c resistance in the pathogenesis of venous thrombosis. Am J Med Sci 1998;316:120-8
- Huo MH, Muntz J. Extended thromboprophylaxis with low-molecular-weight heparins after hospital discharge in high-risk surgical and medical patients: a review. Clin Ther 2009;31:1129-41
- Deitelzweig SB, Becker R, Lin J, et al. Comparison of the two-year outcomes and costs of prophylaxis in medical patients at risk of venous thromboembolism. Thromb Haemost 2008;100:810-20