Abstract
Background:
Patients treated with epidermal growth factor receptor inhibitors (EGFRIs) may develop dermatologic adverse drug reactions (ADRs) that may affect patients’ quality-of-life, require medical care, and may lead to substantial costs. This study assessed the economic burden of dermatologic ADRs in colorectal cancer (CRC), head and neck cancer (HNC), and non-small cell lung cancer (NSCLC) patients.
Methods:
Adult patients with ≥1 diagnosis for the study cancer initiated on EGFRIs indicated for CRC, HNC, and NSCLC were selected from a large commercial database (MarketScan Commercial Database [2000–2010]; Thomas Reuters, New York, NY). For each cancer type, patients were classified into two mutually exclusive cohorts: ‘ADR’ (patients with ≥1 ADR following EGFRI initiation) and ‘ADR-free’ (patients without any ADR). Patients were observed from the index date up to the end of continuous healthcare plan enrollment or 90 days after EGFRI discontinuation, whichever occurred first. For each cancer group, the proportion of patients and the incidence rate (IR) of experiencing ≥1 dermatologic ADR were reported. Incidence rate ratios for healthcare resource utilization and monthly incremental costs (2010 USD) were estimated using Poisson regression and generalized linear or two-part models, respectively.
Results:
Overall, the proportion of patients with ≥1 ADR ranged between 20.5–36.4% across cancer groups (IR ranged between 44.2–57.4 per 100 patient-years). After adjusting for confounders, in each cancer group, ADR patients had higher incidence of healthcare resource utilization, generally driven by higher incidence of emergency room visits and incurred incremental total monthly healthcare costs that ranged between $2284–$3210 across cancer groups.
Limitations:
There was no clinical measure of cancer staging and ADR severity in the database.
Conclusions:
Results suggest that patients with CRC, NSCLC, and HNC, who may benefit from EGFRI therapies, may also incur a substantial economic burden that is associated with dermatologic ADRs.
Background
Colorectal cancer (CRC) and non-small cell lung cancer (NSCLC) represent a significant portion of cancer cases diagnosed in the US each year, and they are two of the most common cancers and the leading causes of cancer-related death in both men and womenCitation1,Citation2. The annual age-adjusted incidences of CRC and lung and bronchus cancer, between 2005–2009, have been estimated at 46.3 and 62.6 per 100,000 persons, respectivelyCitation3,Citation4. In addition, head and neck cancers (HNC) account for ∼3% of all malignancies in the USCitation5. The prognosis for most afflicted with these tumors is poor and the associated economic burden is significantCitation1–5.
New biologic targeted therapies, such as epidermal growth factor receptor inhibitors (EGFRIs), have demonstrated significant improvement in survival and progression-free survival in patients with CRC, NSCLC, or HNCCitation6,Citation7. Their main mechanism of action is to block the downstream signaling of receptor activation that plays a key role in the development and proliferation of tumor cells. However, the use of EGFRI therapy has also been associated with dermatologic adverse drug reactions (ADRs), reactions that may affect the tolerability of treatment and lead to treatment discontinuationCitation8. These symptomatic dermatologic events are thought to be related to EGFR inhibition in the skin, although the precise mechanism by which EGFR inhibition leads to skin eruptions is unknown. A wide range of dermatologic toxicities associated with EGFRI therapies have been described in the literature, with the most common manifestations including acneiform skin rashes, xerosis, pruritus, paronychia, hair abnormalities, mucositis, and increased growth of the eyelashes or facial hairCitation9.
ADRs can negatively affect patients’ quality-of-life, often require medical care, and may pose a substantial financial burden on the patientCitation10,Citation11. A recent meta-analysis reported that the cost associated with the treatment of rashes in EGFRI patients varied between US$ 500 (grade 3 rash) to US$ 15,000 (grade 4 rash) per rash episodeCitation12. A study conducted on 10 NSCLC patients treated with erlotinib showed that the average total cost for the treatment of ADRs (all grades) was $2716 per patientCitation11. Further studies have confirmed these costs, including a study conducted on 132 patients treated with molecular targeted therapies showing a median cost of dermatologic toxicity management (treatment and diagnosis) of $674 per visit per patientCitation13. However, the literature on the economic burden of ADRs associated with EGFRI treatments, including the aforementioned studies, is usually based on relatively small patient samples and the cost burden generally limited to the direct costs of treating/managing the ADR symptoms. Nevertheless, dermatologic ADRs may be associated with an economic burden that goes beyond the cost of treating skin symptoms. For example, dermatologic ADRs may impact the subsequent course of EGFRI treatment and cancer management, which may in turn have economic consequences beyond the direct costs of managing skin symptoms.
This study aims to provide a more complete picture of the economic burden associated with a broad range of dermatologic ADRs in EGFRI-treated patients with CRC, NSCLC, and HNC by investigating costs beyond the management of skin symptoms, such as the costs of monitoring, diagnosing, and treating symptoms, as well as costs associated with potential recurrent events and follow-up management of cancer patients subsequent to the occurrence of dermatologic ADRs.
Methods
Data source
Data were extracted from the Thomson Reuters MarketScan® Commercial Database (01/2000–07/2010), that includes fully integrated patient-level data, including pharmacy and medical (inpatient [IP], outpatient [OP]) claims and associated diagnosis and procedure codes, and enrollment data from ∼25 million lives covered annually by self-insured employers and private health insurance plans in the US. For patients who receive supplemental Medicare benefits through employer-sponsored health plans, information on the employer-paid portion of Medicare-paid benefits and patients’ out-of-pocket expenses of their medical and pharmacy services were also available.
Patient selection and construction of cohorts
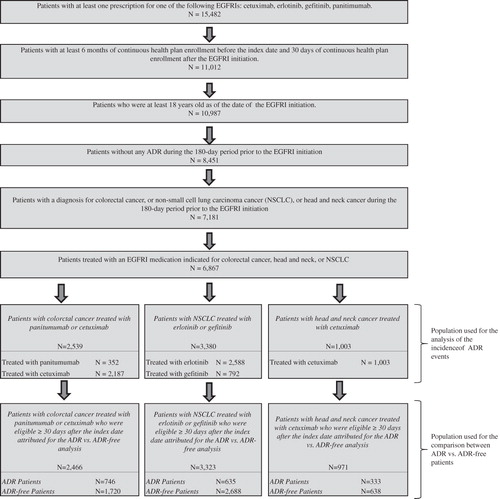
A retrospective cohort study design was used. Patients were included if they (1) had at least one prescription filled for one of the following EGFRIs: cetuximab, erlotinib, gefitinib, and panitumumab, regardless of whether or not the EGFRI was used as the primary systemic therapy; (2) were continuously enrolled in their plans for at least 180 days prior to and at least 30 days following the EGFRI initiation; (3) had at least one diagnosis of the following cancers: CRC (ICD-9-CM codes 153.xx–154.xx, 209.53–209.57, 211.3x, 230.3x), NSCLC (ICD-9 CM codes 162.xx), or HNC (ICD-9 CM codes 140.xx–148.xx 160.xx–161.xx) prior to the EGFRI initiation; (4) did not have any claim for a dermatologic ADR during this 180-day period prior to the EGFRI initiation (to allow analysis of incident ADR cases only); and (5) were at least 18 years old at the date of EGFRI initiation ().
Selected patients were classified into three groups based on the type of cancer diagnosed prior to the EGFRI initiation: (1) CRC patients treated with cetuximab or panitumumab, (2) NSCLC patients treated with erlotinib or gefitinib, and (3) HNC patients treated with cetuximab. For each of the three groups, patients were then classified into two mutually exclusive cohorts, ‘ADR cohort’ (those with at least one ADR) and ‘ADR-free cohort’ (those without any ADR), for the analysis of the burden of dermatologic ADRs in terms of differences in resource utilization and costs between the two cohorts.
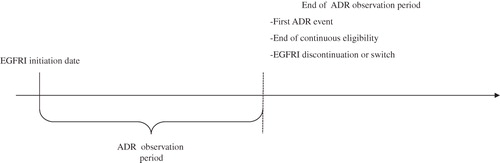
To measure the risk of developing an ADR, the ‘ADR observation period’ was defined as the period spanning from the EGFRI initiation to the date of first ADR event, or the end of continuous enrollment, or treatment discontinuation (or switch to another treatment), whichever occurred first ().
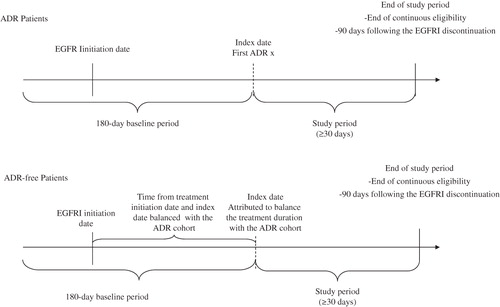
For the ‘ADR’ cohorts, the ‘index ADR date’ was defined as the date of the first ADR diagnosis. To enable comparisons of resource use and costs, a ‘pseudo-index date’ was randomly attributed to balance the treatment duration with the ADR cohort (). Only patients who were continuously enrolled in their healthcare plan for at least 30 days following the index date were included for the ADR burden analysis. The ‘study period’ for the comparisons of ADR burden between the two cohorts was defined as the period spanning from the index date up to the end of continuous enrollment, end of data availability, or 90 days following the EGFRI discontinuation, whichever occurred first. The baseline period was defined as the 180-day period prior to the index date.
Outcomes
The study outcomes included the incidence of dermatologic ADRs, healthcare resource utilization, and costs.
Incidence of dermatologic ADRs
A dermatologic ADR was defined as any medical claim associated with a diagnosis of one of the following: infections of skin and subcutaneous tissue (680.xx–686.xx), other inflammatory conditions of skin and subcutaneous tissue (690.xx–698.xx), other diseases of skin and subcutaneous tissue (700.xx–709.xx), symptoms involving skin and other integumentary tissue (782.xx), and symptoms involving the cardiovascular system for example gangrene (785.4x). For each cancer group, the proportion of patients and the incidence of experiencing at least one dermatologic ADR were reported for the overall population as well as stratified by EGFRI treatment. The incidence rate (IR) of experiencing a dermatologic ADR was defined as the total number of patients with at least one ADR divided by the total number of days over the ADR study period. The incidence rate of experiencing a dermatologic ADR was reported per 100 patient-years.
Healthcare resource utilization
For each cancer group, the incremental use of resource utilization associated with ADR events was estimated by comparing the resource utilization incurred during the study period by the ADR patients to that of the ADR-free patients.
Healthcare resource utilization was reported for the following categories: IP admissions, IP days, emergency room (ER) visits, OP visits, and other medical services (i.e., laboratory, radiology, or other ancillary services). Unadjusted and adjusted incidence rate ratios (IRR) with their respective 95% confidence intervals (CIs) and p-values were estimated using GLM regression models with a log link and a Poisson distribution.
Healthcare costs
For each cancer group, the incremental healthcare costs associated with ADR events were estimated by comparing healthcare costs incurred during the study period by ADR patients to those of ADR-free patients. Healthcare costs were adjusted for inflation using the consumer price index (CPI) for medical components (expressed in 2010 US dollars). As the observation periods varied across patients, costs were reported on a monthly basis. Average monthly healthcare costs were compared between ADR and ADR-free patients for the following categories: pharmacy (EGFRI drug costs, other pharmacy costs), medical services (IP, ER, OP, other medical service), and total costs (medical services and pharmacy costs). Unadjusted average monthly healthcare costs were compared between ADR and ADR-free patients using Wilcoxon rank-sum tests. Adjusted incremental cost differences, controlling for confounding factors, between ADR and ADR-free patients were estimated using generalized linear models (GLM) with a log link and gamma distribution or two-part models, where the first part is a logistic model and the second part is a GLM model with a log link and a gamma distribution, for cost components with a portion of zero values greater than 10%. p-values and 95% CIs were estimated using non-parametric bootstrap re-sampling techniques of 501 iterations.
Multivariate regression analyses
Multivariate regression analyses were conducted to control for differences in baseline patient characteristics. For all analyses and for all cancer groups, multivariate regression models controlled for baseline demographics (age and gender), CCI, and baseline characteristics that showed statistically significant differences between ADR and ADR-free patients with at least 5% of patients with the characteristic in each cohort, and for resource utilization and costs with significant differences at baseline.
Results
Among the 15,482 patients who initiated a new EGFRI therapy, 7181 patients were at least 18 years old, had no ADRs during the 180-day period prior to the EGFRI initiation, and had received a diagnosis for CRC, NSCLC, or HNC during the 180 days prior to the EGFRI initiation (). Among them, 2539 patients with CRC were initiated on an EGFRI for CRC and were included in the CRC group; 3380 patients with NSCLC were initiated on an EGFRI for NSCLC and were included in the NSCLC group; 1003 patients with HNC were initiated on an EGFRI for HNC and were included in the HNC group.
Incidence of dermatologic ADR events
Patients with CRC
Overall, 32.3% of the patients with CRC had experienced at least one ADR event following EGFRI treatment initiation (). More specifically, 32.2% of those who initiated cetuximab and 32.7% of those who initiated panitumumab had experienced at least one ADR. Incidence rates of ADRs were 55.7 and 70.9 per 100 patient-years for patients treated with cetuximab and panitumumab, respectively.
Table 1. Patients with at least one dermatologic ADR and incidence rate of dermatologic ADR by cancer type.
Patients with NSCLC
Among NSCLC patients 20.5% had experienced at least one ADR event following EGFRI treatment initiation (). More specifically, 15.4% of those who initiated gefitinib and 22.0% of those who initiated erlotinib had experienced at least one ADR. Incidence rates of ADRs were 32.0 and 48.2 per 100 patient-years for patients treated with gefitinib and erlotinib, respectively.
Patients with HNC
Overall, 36.4% of the patients with HNC treated with cetuximab had experienced at least one ADR event following EGFRI treatment initiation (). The incidence rate of ADRs was 56.1 per 100 patient-years.
Burden of dermatologic ADR events
Among the selected patients, 2466 patients in the CRC group, 3323 patients in the NSCLC group, and 971 patients in the cancer group were continuously enrolled in their healthcare plan for at least 30 days following the index date and were included for the analysis of the burden of dermatologic ADRs ().
Patients with CRC
The ADR patients with CRC were younger than ADR-free patients (54.7 vs 55.2, p = 0.0307) and had a higher CCI (7.34 vs 6.98, p = 0.0052) (). ADR patients also had a higher prevalence of mild diabetes (13.0% vs 9.7%, p = 0.0151), HNC (3.5% vs 1.5%, p = 0.0017), skin cancer (2.8% vs 1.2%, p = 0.0049), as well as higher OP resource utilization and costs at baseline. ADR patients also had lower prevalence of fluid electrolyte disorders (25.7 vs 29.8%, p = 0.0418) and pulmonary circulation disorders (2.5 vs 4.4%, p = 0.0265).
Table 2. Baseline characteristics—comparison between ADR and ADR-free patients.
Over the study period, after controlling for confounding factors, ADR patients with CRC had a higher incidence rate of ER visits (adjusted IRR = 1.18; 95% CI = 1.03–1.35) and OP visits (adjusted IRR = 1.12; 95% CI = 1.10–1.14) compared to ADR-free patients (). ADR patients also incurred higher healthcare costs compared to ADR-free patients, with average monthly total healthcare costs of $15,631 vs $13,170, respectively (). After controlling for confounding factors, ADR patients incurred significantly higher total healthcare costs by ∼$2313 per patient per month (PPM), which was mainly driven by an incremental OP cost of $876 PPM ().
Table 3. Comparison of healthcare resource utilization of ADR vs ADR-free patients by cancer type.
Table 4. Comparison of healthcare costs of ADR patients and ADR-free patients by cancer type.
Patients with NSCLC
The ADR patients were younger than ADR-free patients (56.2 vs 57.6, p = 0.0005) and included a higher proportion of females (59.2 vs 50.9, p = 0.0001) (). No significant differences in the CCI were found between the two groups. ADR patients had a higher prevalence of mild diabetes (11.0% vs 7.3%, p = 0.0018), hypothyroidism (6.6% vs 3.5%, p = 0.0003), and breast cancer (6.5% vs 4.5%, p = 0.0441) and a lower prevalence of fluid electrolyte disorders (15.0 vs 18.9%, p = 0.0195). As for baseline healthcare utilization and cost, ADR patients had lower IP and ER utilization and cost at baseline, but higher pharmacy costs.
Over the study period, among NSCLC cancer patients, after controlling for confounding factors, ADR patients had a statistically significantly higher incidence rate of ER visits (adjusted IRR = 1.28; 95% CI = 1.13–1.45), OP visits (adjusted IRR = 1.28; 95% CI = 1.25–1.30), and other medical services (adjusted IRR = 1.15; 95% CI = 1.11–1.19) compared to ADR-free patients (). Moreover, ADR patients also incurred higher average monthly total healthcare costs compared to ADR-free ($12,815 vs $9053) (). ADR patients incurred, after controlling for confounding factors, higher total healthcare costs by ∼$3210 PPM, mainly driven by incremental IP costs of $1111 and OP costs of $1872 in ADR patients ().
Patients with HNC
Patients were similar in terms of gender and age profiles. Compared to ADR-free patients, ADR patients had a higher CCI (6.50 vs 5.94, p = 0.0106) and a higher prevalence of lymphoma (7.2% vs 3.4%, p = 0.0089) (). No statistically significant differences in baseline healthcare utilization and cost were observed between the two groups.
Over the study period, after controlling for confounding factors, among patients with HNC, ADR patients had a statistically significantly higher incidence for each one of the medical services compared to ADR-free patients. More particularly, compared to ADR-free patients, patients with an ADR had an 85% higher incidence rate of ER visits (adjusted IRR = 1.85; 95% CI = 1.55–2.22) and a 46% higher incidence rate of IP days (adjusted IRR = 1.46; 95% CI = 1.38–1.54) (). In addition, ADR patients also incurred higher healthcare costs compared to ADR-free patients, with average monthly total healthcare costs of $12,539 vs $9684, respectively (). After controlling for confounding factors, ADR patients incurred a statistically significantly incremental total healthcare cost of $2284 PPM, mainly due to an incremental IP cost of $1702 PPM in ADR patients ().
Discussion
In this retrospective study of patients treated with EGFRIs, we found that ∼32% of CRC patients, 36% of HNC patients, and 21% of patients with NSCLC had a dermatologic ADR following the initiation of an EGFRI. Other studies have reported that skin toxicities generally occur in 45–100% of EGFRI-treated patients and that moderate-to-severe skin toxicities, leading to dose modification or treatment interruption, occurred in 8–17% of the patientsCitation11,Citation14,Citation15. However, these rates tend to vary depending on the method used, treatments considered, and the duration of the observation period. In our study, dermatologic ADRs were identified based on medical service claims associated with a diagnosis of the studied dermatologic ADRs. In comparison to clinical trial data and case reports where dermatologic ADRs were closely followed and evaluated, our study most likely under-estimates the incidence rate of dermatologic ADRs by only capturing events requiring medical attention. Nevertheless, considering that the incidence estimates of dermatologic ADRs in our study were more likely to be the lower bound, our findings are significant and suggest that a relatively high proportion of patients incurred an ADR that was severe enough to require medical attention.
The findings of our study also suggest that ADRs represent a significant economic burden for patients treated with EGFRI therapies for CRC, NSCLC, or HNC. Results showed that patients with at least one dermatologic ADR incurred significant higher healthcare resource utilization and costs compared to ADR-free patients. Overall, patients with ADR were associated with an incremental total healthcare monthly cost of $2313 in CRC patients, $3210 in NSCLC patients, and $2284 in HNC patients, representing an increase of 18–35% of the total costs. These incremental costs were explained by the higher utilization of medical services in patients with dermatologic ADRs.
Previous studies have also found that dermatologic ADRs in EGFRI-treated patients can be costly. For example, findings from a meta-analysis reported average costs of rash treatment between US$ 500 (grade 3 rash) to US$ 15,000 (grade 4 rash) per rash episode for severe dermatologic eventsCitation12. A study from Abraham et al.Citation11 showed that the average total cost for treatment of ADRs (all grades) was $2716 per patient. Another recent study from Borovicka et al.Citation13 showed a median total cost per visit per patient of $674 for the management of dermatologic toxicities in patients treated with molecularly targeted therapies. However, it is difficult to compare our findings to the existing literature. First, in our analysis, all dermatologic ADRs requiring medical attention, regardless of severity, were considered. Although our study captured ADRs that might not be limited to only very severe events, it may, however, have excluded very mild events. Also, in our study, the definition of dermatologic ADRs included a broad range of types of skin symptoms and manifestations, from localized rash to gangrene. Finally, in contrast to studies where only the cost of managing skin symptoms was included, by comparing healthcare resource utilization and costs over the entire EGFRI treatment period (up until 90 days after treatment discontinuation), our study included costs beyond the simple management of skin symptoms, such as the costs of monitoring, diagnosing, and treating symptoms, as well as costs associated with potential recurrent events and follow-up management of cancer patients subsequent to the occurrence of dermatologic ADRs. To the best of our knowledge, the present study is the first to estimate the economic burden of dermatologic ADRs beyond the direct costs of managing skin symptoms in CRC, NSCLC, and HNC patients treated with EGFRI therapies using a large retrospective US database.
Skin rashes are generally mild-to-moderate in severity and rarely threaten patients’ livesCitation14,Citation16. However, in addition to presenting a substantial economic burden, ADRs can also lead to serious infection and may compromise the course of treatment, by requiring dose reductions, treatment interruption, or complete discontinuation, which may have serious implications on the treatment efficacy and overall survivalCitation14,Citation16. Indeed, studies have reported that ADRs are generally dose-dependent and can be reversible with dose reduction or treatment discontinuationCitation14. Moreover, dermatologic ADRs may cause a substantial physical and psycho-social discomfort that might significantly reduce overall patient quality-of-life. Therefore, when choosing treatments for these types of cancer, it is essential for prescribers to consider the comparative efficacy of the treatment, but also the risk of ADRs and the associated increased burden to patients and payers.
The limitations of the study include the usual caveats of retrospective claims database analyses. First, there was no clinical measure of ADR severity in the database. Therefore, it was not possible to control for differences of ADR severity in our regression models or examine utilization and costs associated with varying levels of ADRs severity. Second, the data did not allow evaluation of the cancer stage as worsening ADRs may be strongly associated with more advanced stages of cancer. That said, the clinical application of these therapies is generally restricted to stage IV disease. Third, findings from clinical trial studies suggest that the severity of rash during treatment with EGFRIs can also be positively correlated with the efficacy of these agents and with improved patient’s survivalCitation17. However, our data source did not include clinical information that could have been used to determine whether or not patients who had developed dermatologic ADRs had better treatment response or were associated with better overall survival compared to patients who had not experienced ADRs during the study period. Fourth, patients’ baseline characteristics differed between cohorts. Although multivariate regression models were used to adjust for observables differences of baseline characteristics, an unobserved confounding effect may still exist such that the incremental costs of patients with ADRs may be due to an unobserved worse disease profile. Finally, retrospective databases are also subject to coding errors or data omissions; however, these are expected to affect all treatment cohorts to a similar extent and are unlikely to alter the conclusions. Nonetheless, claims data remain a valuable source of information, as they comprise a fairly valid, large sample reflecting real world practice patterns.
In addition to the above limitations that are mainly associated with the nature of the data source, this study is also subject to other general limitations related to the design of the study. First, some studies have showed that the use of EGFRIs in combination with pre-emptive or reactive skin treatments, such as topical antibiotics, can reduce the incidence or the risk of recurrence of skin toxicitiesCitation18. However, the current study did not adjust for the use of these medications. Further studies would be warranted to assess the impact of pre-emptive or reactive skin treatments on the incidence and the risk of recurrence of dermatologic ADRs in a clinical setting. Second, the current study focuses only on direct healthcare costs. However, dermatologic ADRs may also have indirect effects on patients’ lives, and it could also be of interest to examine the indirect costs of dermatologic ADRs. Further studies would be warranted to identify factors associated with the development of dermatologic ADRs and to better understand the additional cancer management challenges associated with these events. Finally, further research based on real-world data would also be warranted to compare the incidence of ADRs across all therapies available for the treatment of CRC, HNC, and NSCLC and to assert the economic burden of other non-cutaneous ADRs in CRC, HNC, and NSCLC patients.
Conclusions
This study found that patients with CRC, NSCLC, and HNC, who may benefit from EGFRI therapies, may also incur a substantial economic burden that is associated with dermatologic ADRs.
Transparency
Declaration of funding
Funding for this study was provided by Abbott Laboratories.
Declaration of financial/other relationships
A. Guérin, G. Gauthier, E. Q. Wu, and M. Cloutier are employees of Analysis Group, Inc., which has received consultancy fees from Abbott Laboratories. K. D. Holen, S. Ray, and V. Bonthapally are Abbott Laboratories employees and hold Abbott stock.
Acknowledgments
No additional contributors to acknowledge. No assistance in the preparation of this article is to be declared.
References
- National Cancer Institute. A snapshot of colorectal cancer. http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/snapshots. Accessed September 19, 2012
- National Cancer Institute, A Snapshot of Lung Cancer. 2012. http://www.cancer.gov/aboutnci/servingpeople/cancer-statistics/snapshots. Accessed September 19. 2012
- National Cancer Institute. SEER stat fact sheets: colon and rectum. 2012. http://www.cancer.gov/statfacts/html/colorect.html. Accessed September 19, 2012
- National Cancer Institute. SEER stat fact sheets: lung and bronchus. 2012. http://www.cancer.gov/statfacts/html/lungb.html. Accessed September 19, 2012
- National Cancer Institute. A snapshot of head & neck and thyroid cancers. 2012. http://www.cancer.gov/aboutnci/servingpeople/snapshots/head-neck.pdf. Accessed September 19, 2012
- Chang A, Parikh P, Thongprasert A, et al. Gefitinib (IRESSA) in patients of Asian origin with refractory advanced non-small cell lung cancer: subset analysis from the ISEL Study. J Thorac Oncol 2006;1:847-55
- Cutsem E, Peeters M, Siena S, et al. Open-Label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 2007;25:1658-64
- Hidalgo M, Siu LL, Nemunaitis J, et al. Phase I and pharmacologic study of OSI-774, an epidermal growth factor receptor tyrosine kinase inhibitor, in patients with advanced solid malignancies. J Clin Oncol 2001;19:3267-79
- Li T, Perez-Soler R. Skin toxicities associated with epidermal growth factor receptor inhibitors [abstract]. Target Oncol 2009;4:107-19
- Gridelli C, Maione P, Amoroso D, et al. Clinical significance and treatment of skin rash from erlotinib in non-small cell lung cancer patients: results of an Experts Panel Meeting. Crit Rev Oncol/Hematol 2008;66:155-62
- Abraham T, Rademaker West D, Lacouture M, et al. Economic impact in the management of dermatologic adverse drug reactions induced by the Epidermal Growth Factor Receptor Inhibitor (EGFRI) Erlotinib [abstract]. J Am Acad Dermatol 2009;66(3):AB55
- Mittmann N, Seung SJ. Rash rates with egfr inhibitors: meta-analysis. Curr Oncol 2011;18:e52-e63
- Borovicka J, Calahan C, Gandhi M, et al. Economic burden of dermatologic adverse events induced by molecularly targeted cancer agents. Arch Dermatol 2011;147:1403-9
- Tan EH, Chan A. Evidence-based treatment options for the management of skin toxicities associated with epidermal growth factor receptor inhibitors. Ann Pharmacother 2009;43:1658-66
- Lacouture M. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nature Rev Cancer 2006;6:803-12
- Mittman N, Seung SJ. Rash rates with EGFR Inhibitors: meta-analysis. Curr Oncol 2011;18:e54-e63
- Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res 2007;13:3913-21
- Melosky B, Burkes R, Rayson D, et al. Management of skin rash during EGFR-targeted monoclonal antibody treatment for gastrointestinal malignancies: Canadian recommendations. Curr Oncol 2009;16:14-24