Abstract
Objective:
A recent expert study (RAND Appropriateness Method (RAM)) including a panel of 12 European urologists reported that the PCA3 score may be instrumental in taking appropriate prostate biopsy (PBx) decisions, mainly for repeat PBx. This study determined the cost/benefit balance of introducing PCA3 in the decision-making for PBx in France.
Methods:
Two RAM models, without and with PCA3, were retrospectively applied to a sample of 808 French men who had PBx in 2010 (78% first, 22% repeat). Outcome measures included the proportion of PBx that could have been avoided (i.e., judged inappropriate) in the French sample according to both RAM models, and the estimated impact of application of these models on the annual number of PBx and associated costs for France (based on most recent published data).
Results:
Complete profiles were available for 698 men. In the model without PCA3, 2% of PBx were deemed inappropriate. Knowledge of PCA3 would have avoided another 7% of PBx. Repeat PBx would have been avoided in 5% of cases without PCA3 and in 37% with PCA3. For France, application of the RAM model including PCA3 would result in 18,345 fewer repeat PBx. It would be budget-neutral in the unlikely hypothesis of no complications or no costs incurred by complications and would save €1.7 million for a mean cost for complications of €100/procedure or €5 million for a mean cost for complications of €280/procedure, calculated based on US and Canadian data.
Limitations:
Limitations of the study are the theoretical nature of the analysis and the fact that PCA3 distributions had to be derived from other sources.
Conclusions:
Adoption of RAM expert recommendations including PCA3 for repeat PBx decisions in clinical practice in France would reduce the number of repeat PBx and control costs.
Introduction
Prostate cancer (PCa) is in France the second most prevalent cancer in males, with an estimated age-standardized incidence rate of 178.7 per 100,000 and a mortality rate of 22.9 per 100,000Citation1. Although it exhibits sub-optimal predictive values, serum prostate specific antigen (PSA) is often instrumental in the decision to take prostate biopsies (PBx). Studies have shown that, in men with a PSA 2.5–10 ng/mL, only one in four random systematic PBx evidence cancer, suggesting that most patients undergo random PBx unnecessarilyCitation2,Citation3. Because of the fear of missing clinically significant PCa, a negative initial PBx often results in one or more repeat PBxCitation4, although most of them (65–90%Citation5–7) will again be negative.
The low predictive value of PSA and digital rectal examination (DRE) and the associated high percentage of negative initial and repeat PBx are most concerning in view of the discomfort, pain, anxiety and complications associated with the procedure and the resulting costsCitation8–12. In ∼4% of men, complications require hospitalization, mainly due to infections (72%), bleeding (19%) and retention (9%) and nine out of 10,000 men may die following the procedureCitation8. In a recent series up to 1.2% of men experienced urosepsis after PBxCitation9.
One field of improvement would be to better identify those patients most likely to benefit from PBx, in terms of presence of a significant PCa. In that perspective, the PROGENSA® Prostate CAncer gene 3 (PCA3) Assay was proven to have clinically significant predictive value for PCa in men with previously negative PBxCitation13–17 and in PBx-naïve patients with intermediate values of serum PSA ≥ 2.5–10 ng/mL.
Here we assessed, in a real-life cohort of 808 patients, the consequences of the adoption of the PCA3 assay. In addition, we determined the cost/benefit balance of introducing PCA3 in the decision-making for PBx in France.
Patients and methods
The goal of this study was to estimate the costs that the French healthcare system would incur if PCA3 would be introduced in the decision process for indicating PBx.
The budget impact model was constructed by applying the decision rules proposed by a panel of European experts from a RAND appropriateness Method (RAM) study to a cohort of 808 men undergoing PBx in five French hospitals in 2010.
The design and results of the RAM experts recommendations have been described in detail in a previous publicationCitation18. Briefly, a panel of 12 European urologists with broad expertise in PCa developed recommendations on the appropriateness of PBx for a systematically constructed set of 108 hypothetical patient profiles. These profiles were unique combinations of clinical variables predicting PCa risk encompassing life expectancy, DRE outcome, PSA, prostate volume, and number of prior negative PBx. For each of these clinical profiles, the appropriateness of a PBx was judged for five different scenarios (PCA3 test not preformed, PCA3 Score <20, 20–35, 35–50, and >50), yielding 540 different theoretical patient cases. Generally, a PCA3 Score <20, ≥2 negative PBx, and a life expectancy <10 years decreased PBx appropriateness; a PCA3 score >50 and a PSA ≥ 3 ng/mL increased it.
In the current study, the experts’ recommendations were applied to the individual profiles of a cohort of French men undergoing PBx in three academic and two non-academic French public hospitals. All centres were highly experienced in PCa. Each centre retrospectively collected data from at least 150 consecutive men within a period of 4 months in 2010 starting with the most recent cases. Men with prior positive PBx or under any treatment for PCa were excluded from the study. An electronic data capture system was used to collect clinical variables, including age, life expectancy, outcome of latest PBx (if positive: clinical stage, Gleason sum, number of cores, number of positive cores, treatment following last PBx), DRE outcome, PSA level, prostate volume and number of prior negative PBx. In three centres, PBx decisions were based on age, life-expectancy, PSA level, DRE outcome and prostate volume. In two centres, PBx was not performed in men with a PSA < 3 or 4 ng/mL, and in one centre PBx was not performed in men >75 years. PCA3 scores were not routinely tested in the present cohort and individual values are not included in the present dataset. However, because PCA3 scores are unrelated to other clinical variables such as prostate volume, age or PSA, a theoretical PCA3 score distribution could be calculated from the distribution observed in three pivotal European studiesCitation13,Citation16,Citation19.
For each individual case we verified whether taking PBx was warranted according to the experts’ recommendations. The same analysis was repeated including the theoretical PCA3 score distribution. The budget impact of implementing the expert’s recommendations, both with and without PCA3 testing, in France was then calculated based on the proportion of PBx deemed appropriate/inappropriate according to these recommendations in the French cohort. Introducing systematic PCA3 testing before recommending PBx would increase the costs of the initial evaluation. However, some PBx would be spared, therefore reducing the risks of complications and the costs of management in the whole cohort.
The budget impact model covered the first year after PBx and took into account the relative proportion of initial and repeat PBx (derived from the French cohort) and the number of PBx performed per year in France, the mean cost of PBx, the mean cost of complications per PBx (derived from literature) and the cost of PCA3 testing in 2010. The economic valorization in Euros of the procedure and of the afferent complications was estimated from the healthcare system perspective. The total number of PBx performed and the cost of PBx were based on data from the French OPEPS (Office parlementaire d’évaluation des politiques de santé) report from 2009 showing an estimate of 200,000–250,000 PBx performed per year in France since 2005 (p. 264)Citation20. The mean price of a prostate PBx (private or public) for 2007 was calculated at €700 exclusive costs for pathology tests (OPEPS report, p. 264). Costs of pathology tests were estimated at €75 in the case of a negative outcome and €90 in the case of a positive outcome (p. 264). For the budget impact model, the default cost of a PBx in 2010 was therefore set at €800. In view of the lack of literature pertaining to the prevalence and costs of PBx complications in France we considered three separate scenarios; (1) there are no complications or the management of complications does not entail significant costs, (2) on average, the costs of complications are €100 per PBx, (3) the costs of complications in France are comparable to those reported for the US and Canada, that is €280 on average per PBx. The latter estimate was derived from information on the incidence of PBx complications reported in three cohort studiesCitation10,Citation21,Citation22, and the cost per complication in three other studiesCitation23–25. Cost estimates per serious PBx-related complication derived from these studies were $159 for acute urinary retention (incidence 2%), $2525 for hematospermia (incidence 1%), $969 for anal pain (incidence 2%), $1111 for anal bleeding (incidence 1%) and $2525 for hematuria (incidence 12%) (converted from Canadian $ and inflated to 2010 US $)Citation23; the costs for erectile dysfunction (incidence 16%) and sepsis (incidence 3%) within 5 days were estimated at $159 and $5259, respectively (inflated to 2010 US $)Citation24,Citation25. When these data were projected on the total US population, the costs for treatment of complications were estimated at 35% of the cost of a PBxCitation26. This cost (i.e., 35% of €800 or €280) was used in the third hypothesis. The cost of PCA3 testing was set at €300 per test.
This study was performed in accordance with the principles outlined in the Declaration of Helsinki.
Results
Patient demographics and baseline characteristics
The characteristics of 808 men undergoing PBx in three academic (n = 543) and two non-academic public hospitals (n = 265) were analyzed; 632 (78%) underwent an initial PBx and 176 (22%) a repeat PBx (≥1 previous negative PBx); 328 (52%) of the initial PBx and 45 (26%) of the repeat PBx were found positive for cancer. shows the baseline characteristics of the 698 men with a complete profile.
Table 1. Baseline characteristics of all men with a complete profile (n = 698) included in the French retrospective chart review.
Impact of PCA3 testing on decision of PBx
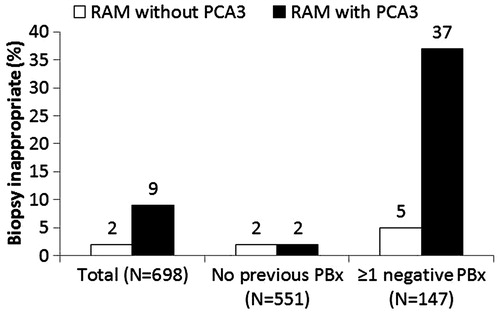
The appropriateness of a PBx according to RAM experts’ recommendations with and without testing for PCA3 was assessed for the 698 men with a complete profile. Taking PBx was considered inappropriate in 2% of men in the absence of PCA3 testing and in 9% if PCA3 was included in the model. This difference was mainly related to the group of men who underwent a repeat PBx. Repeat PBx was found inappropriate in 5% of men without PCA3 and in 37% of men with PCA3 ().
Budget impact of PCA3 testing
shows the default information included in the budget impact model in line with the 2010 figures in France. The proportion of initial PBx used in the analysis (i.e., 78%) was derived from the present retrospective chart population. The distribution of PCA3 scores was derived from the European initial and repeat PBx studiesCitation10,Citation13,Citation16. In addition, the model took into account the estimated total number of PBx in France, the cost of PCA3 testing, and the costs associated with PBx. The latter included the procedure itself, costs of pathology tests of the biopsied tissue, and management of complications resulting from PBx. This information was derived from published material (see Patients and methods).
Table 2. Default information included in the budget impact model for France.
shows the annual numbers of initial and repeat PBx and the associated costs under the current regimen without PCA3 testing and for systematic PCA3 testing before repeat PBx. The budget impact of PCA3 testing before any PBx (initial and repeat) was not assessed, as in the present retrospective chart population PCA3 results did not impact the decision to recommend initial PBx (). From the retrospective chart population we estimated that introducing systematic PCA3 testing before recommending repeat PBx would reduce the number of repeat PBx by 37%.
Table 3. Annual numbers of initial and repeat prostate biopsy (PBx) and associated costs under the current regimen (without PCA3 testing), and for the strategy assuming PCA3 testing before each planned repeat PBx (RAM + PCA3) for three different scenarios, i.e., if mean costs for complications per PBx are either €0 (Scenario 1), €100 (Scenario 2), or €280 (Scenario 3).
As the mean cost for treating complications per PBx for France is not known, the budget impact was calculated for different scenarios: (1) no costs for complications (€0), (2) costs below the estimated cost in the US of €280, i.e., €100, and (3) costs comparable to that estimated in the US (€280 per PBx). When considering repeat PBx, it was observed that, in the unlikely situation of no costs incurred for complications, systematic PCA3 testing would be budget-neutral compared with the current practice. In this scenario, the additional costs for PCA3 testing would be neutralized by a reduction in costs for PBx. If the costs for managing complications of PBx were estimated at €100 and €280, which are more realistic figures, PCA3 testing would even reduce the costs by €1.7 million and €5 million, respectively. In these scenarios, the reduction in costs for PBx and for managing associated complications would exceed the costs of systematic PCA3 testing.
Discussion
The outcome of the current study suggested that implementing PCA3 testing into the routine diagnosis of men suspicious of PCa in France would reduce the number of PBx as well as the costs incurred by the healthcare system. Several previous studies have supported the relevance of the PCA3 assay for guiding decisions on PBx in men suspicious of having PCaCitation13–16. There is also growing evidence that it can aid in distinguishing men with significant PCa from those in whom active surveillance can be an optionCitation13,Citation16,Citation17,Citation27–29.
One patient out of five of the cohort that was included in the current study underwent repeat PBx. In complement to the current best clinical judgement based on life expectancy, DRE outcome, PSA, prostate volume, and number of prior negative PBx, PCA3 testing could have avoided 37% of the repeat PBx. This finding is remarkable considering the sample’s relatively high-risk profile. For France, this would translate into an annual reduction of 18,345 PBx. Controlling the number of unnecessary PBx would be instrumental in reducing anxiety, discomfort, and pain induced by PBx, and the relatively high risk of complications, including hospitalization and urosepsis, associated with PBxCitation8–12.
Of note, the present cohort presented with a high-risk profile, as shown by a higher proportion of suspicious DRE and positive (initial) PBx than in the published European PCA3 studiesCitation13,Citation16. The positive DRE rate was 32% vs 19% in both European PCA3 studies; the positive initial PBx rate was 52% vs 40% in the study by De La Taille et al.Citation16 The effect of introducing PCA3 testing would be of a larger magnitude in a population with a lower risk profile, as was shown for 1024 men included in the REDUCE (REduction by DUtasteride of prostate Cancer Events) study. A previous analysis revealed that PCA3 implementation could have avoided as many as 64% of repeat PBx in this populationCitation30.
Adoption of the PCA3 test is not anticipated to impact financially the healthcare system as the costs for PCA3 testing would be balanced by the reduction in the number of PBx and of the related complications. With the hypothesis that the mean cost of complications would be similar to that reported in the US (280€) it could even lead to savings of €5 million/year. It should be noted that assuming no costs for complications is hardly valid, as studies have shown that 17% of PBx are associated with at least one complication and that 4% of men with a negative PBx are hospitalized because of complications related to PBxCitation8,Citation10.
A limitation of the current study is the theoretical nature of the analysis. The RAM model combines scientific evidence with expert opinion to assess the appropriateness of performing a PBx. However, randomized prospective clinical trials are needed to confirm the current findings. Another limitation is that PCA3 scores were only available for a minority of men included in the French sample. Therefore, the PCA3 distribution used in the analysis was derived from European initial and repeat PBx studiesCitation13,Citation16,Citation19. This projection can be justified by the fact that PCA3 scores do not depend of other clinical variables such as prostate volume, age, or PSA. Moreover, the PCA3 distribution in the French repeat PBx sub-group was in line with that in the European PCA3 repeat PBx studyCitation13 ().
Table 4. Distribution of PCA3 scores in European initial and repeat prostate biopsy (PBx) studiesCitation13,Citation16 and in the French repeat PBx sub-group.
In clinical practice, the decision to proceed to PBx is a compromise between excess (unnecessary) PBx and missing clinically significant cancers. The risk of missing clinically significant cancers could not be calculated in the current analysis as the exact PCA3 scores were not known for each subject. However, in the above-discussed analysis of the REDUCE population PCA3 scores and PBx outcome were both available for each subjectCitation30. There appeared to be a strong relationship between expert recommendations and actual PBx outcome (negative or positive PBx, low- or high-grade PCa), particularly when PCA3 was included in the model. Among the men for whom a PBx was considered inappropriate when taking into account PCA3 (n = 656), 90% had a negative PBx. Only eight of these men (1%) had high-grade (Gleason sum ≥7) cancer, compared with 14 men (5%) if PCA3 was not considered. This indicates that the reduction in the number of PBx associated with the adoption of the PCA3 score in clinical practice does not seem to increase the risk of missing high-grade PCa.
Conclusions
The results of the current budget impact study strongly suggest that adopting PCA3 testing into the current care model of men suspected of PCa in France would reduce the proportion of repeat PBx and the costs associated with PBx.
Transparency
Declaration of funding
Gen-Probe Inc funded the study and manuscript writing but did not participate in data acquisition, analysis, interpretation, and preparation of the manuscript.
Declaration of financial/other relationships
Louis Smets and Herman Stoevelaar received financial support from Gen-Probe for elaboration of the study design and data analysis. Bernard Malavaud, Olivier Cussenot, Nicolas Mottet, François Rozet, and Alain Ruffion have no conflicts of interest. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors are grateful to Ismar Healthcare for their assistance in the design of the study, the analysis of the data, and the writing of the manuscript.
References
- Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 2010;46:765-81
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter. N Engl J Med 2004;350:2239-46
- Postma R, Schröder FH. Screening for prostate cancer. Eur J Cancer 2005;41:825-33
- Welch HG, Fisher ES, Gottlieb DJ, et al. Detection of prostate cancer via biopsy in the Medicare-SEER population during the PSA era. J Natl Cancer Inst 2007;99:1395-400
- Fowler JE, Jr Bigler SA, Miles D, et al. Predictors of first repeat biopsy cancer detection with suspected local stage prostate cancer. J Urol 2000;163:813-8
- Heidenreich A, Aus G, Bolla M, et al. EAU Guidelines on prostate cancer. Eur Urol 2008;53:68-80
- Djavan B, Zlotta A, Remzi M, et al. Optimal predictors of prostate cancer on repeat prostate biopsy: a prospective study of 1,051 men. J Urol 2000;163:1144-8
- Nam RK, Saskin R, Lee Y, et al. Increasing hospital admission rates for urological complications after transrectal ultrasound guided prostate biopsy. J Urol 2010;183:963-8
- Nickel JC, Furuta A, Chancellor MB, et al. Best of the AUA Annual Meeting. highlights from the 2010 American Urological Association Meeting, May 29-June 3, 2010, San Francisco, CA. Rev Urol 2010;12:e134–46
- Roberts RO, Bergstralh EJ, Besse JA, et al. Trends and risk factors for prostate biopsy complications in the pre-PSA and PSA eras, 1980 to 1997. Urology 2002;59:79-84
- Seitz C, Palermo S, Djavan B. Prostate biopsy. Minerva Urol Nefrol 2003;55:205-18
- Autorino R, De Sio M, Di Lorenzo G, et al. How to decrease pain during transrectal ultrasound guided prostate biopsy: a look at the literature. J Urol 2005;174:209-17
- Haese A, de la Taille A, Van Poppel H, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008;54:1081-8
- Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007;69:532-5
- Deras IL, Aubin SM, Blase A, et al. PCA3: a molecular urine assay for predicting prostate biopsy outcome. J Urol 2008;179:1587-92
- de la Taille A, Irani J, Graefen M, et al. Clinical evaluation of the PCA3 assay in guiding initial biopsy decisions. J Urol 2011;185:2119-25
- Aubin SMJ, Reid J, Sarno MJ, et al. PCA3 molecular urine test for predicting repeat prostate biopsy outcome in populations at risk: validation in the placebo arm of the dutasteride REDUCE trial. J Urol 2010;184:1947-52
- Tombal B, Ameye F, de la Taille A, et al. Biopsy and treatment decisions in the initial management of prostate cancer and the role of PCA3; a systematic analysis of expert opinion. World J Urol 2012;30:251-6
- Van Poppel H, Haese A, Graefen M, et al. The relationship between Prostate CAncer gene 3 (PCA3) and prostate cancer significance. BJU Int 2012;109:360-6
- Rébillard X, Malavaud B, Rischmann P. Étude scientifique sur le dépistage individuel et le traitement précoce du cancer de la prostate en France. Marché 2007-OPEPES-01. Paris: Association Française d’Urologie. http://www.atoute.org/n/article118.html. Accessed November 2011
- Fujita K, Landis P, McNeil BK, et al. Serial prostate biopsies are associated with an increased risk of erectile dysfunction in men with prostate cancer on active surveillance. J Urol 2009;182:2664-9
- Simsir A, Kismali E, Mammadov R, et al. Is it possible to predict sepsis, the most serious complication in prostate biopsy? Urol Int 2010;84:395-9
- Grover SA, Zowall H, Coupal L, et al. Prostate cancer: 12. The economic burden. CMAJ 1999;160:685-90
- Sun P, Seftel A, Swindle R, et al. The costs of caring for erectile dysfunction in a managed care setting: evidence from a large national claims database. J Urol 2005;174:1948-52
- Edbrooke DL, Hibbert CL, Kingsley JM, et al. The patient-related costs of care for sepsis patients in a United Kingdom adult general intensive care unit. Crit Care Med 1999;27:1760-7
- US PCA3 Budget Impact Study. Data on hand at Hologic/Gen-Probe
- Nakanishi H, Groskopf J, Fritsche HA, et al. PCA3 molecular urine assay correlates with prostate cancer tumor volume: implication in selecting candidates for active surveillance. J Urol 2008;179:1804-9
- Ploussard G, Durand X, Xylinas E, et al. Prostate cancer antigen 3 score accurately predicts tumour volume and might help in selecting prostate cancer patients for active surveillance. Eur Urol 2011;59:422-9
- Auprich M, Chun FKH, Ward JF, et al. Critical assessment of preoperative urinary prostate cancer antigen 3 on the accuracy of prostate cancer staging. Eur Urol 2011;59:96-105
- Tombal B, Andriole GL, Smets L, et al. PCA3 can reduce repeat prostate biopsies with maintained sensitivity of detecting high-grade cancer: application of an expert recommendations model to the placebo cohort of the REDUCE study. 27th Annual European Association of Urology (EAU) Congress, Milan, 2012; Abs. 262. www.uroweb.org/events/abstracts-online/?AID=36287. Accessed March 2012