Abstract
Background:
Many countries have various requirements for local economic analyses to assess the value of a new health technology and/or to secure reimbursement. This study presents a case study of an economic model developed to assess the cost-effectiveness of posaconazole vs standard azole therapy (fluconazole/itraconazole) to prevent invasive fungal infections (IFIs), which was adapted by at least 11 countries.
Methods:
Modeling techniques were used to assess the cost-effectiveness of posaconazole vs fluconazole/itraconazole as IFI prophylaxis in patients with acute myelogenous leukemia or myelodysplastic syndromes and chemotherapy-induced neutropenia. For the core model, the probabilities of experiencing an IFI, IFI-related death, and death from other causes were estimated from clinical trial data. Long-term mortality, drug costs, and IFI treatment costs were obtained from secondary sources. Locally changed parameters were probabilities of long-term death and survival, currency, drug costs, health utility, IFI treatment costs, and discount rate.
Results:
Locally adapted cost-effective modeling studies indicate that prophylaxis with posaconazole, compared with fluconazole/itraconazole, prolongs survival, and, in most countries, is cost-saving. In all countries, the model predicted that prophylaxis with posaconazole would be associated with an increase in life-years, with increases ranging from 0.016–0.1 life-year saved. In all countries, use of the model led to posaconazole being approved by the appropriate reimbursement authority.
Limitations:
The study did not have power to detect differences between posaconazole and fluconazole or itraconazole separately. The risk of death after 100 days was assumed to be equal for those who did and did not develop an IFI, and equal probabilities of IFI-related and other death during the trial period were used for both groups.
Conclusions:
A core economic model was successfully adapted locally by several countries. The model showed that posaconazole was cost-saving or cost-effective vs fluconazole/itraconazole and led to positive reimbursement listings.
Introduction
Rising global healthcare costs mean that evidence of the quality, safety, and efficacy of a drug is no longer sufficient to ensure reimbursement for use in public markets. In a growing number of areas, evidence of cost-effectiveness is also requiredCitation1,Citation2. Individual countries have their own pharmacoeconomic guidelines or requirements dictating how cost-effectiveness studies need to be designed and reported, and many country-specific guidelines have been summarized by the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)Citation3.
National guidelines may insist on the presentation of local data, especially if available clinical trials were undertaken wholly outside the jurisdiction of interest and one or more components of evidence cannot be generalizedCitation2. Mathematical modeling can provide evidence on clinical and economic outcomes in a form that can promote informed decisions about clinical practices and healthcare resource allocationsCitation4. Decision-analytic models also provide a framework within which evidence from a variety of sources can be compiledCitation2. Often a core model is developed early in a product’s life cycle (i.e., when local needs have not been identified). Such core models may then be adapted for local use. For local adaptations, it is important to carefully consider which parameters need to be tailored to the specific healthcare system (e.g., prices and treatment patterns) and which may be generalized (e.g., treatment effect or relative risk reduction)Citation2,Citation5.
Local adaptation of an existing model may allow cost-effectiveness data to be produced in a timely fashion because a lag between the availability of efficacy and cost-effectiveness data could mean that decision-makers are faced with demands to adopt a new drug before adequate health economic data are available, potentially resulting in an inappropriate decision or an unacceptably long delayCitation6.
Global cost-effectiveness models need to be able to deliver a number of components in order for them to be adapted and used locally. Models need to be flexible for input of local data; transparent for clarity of design and results; designed for minimal required adaptation locally; robust to assumptions; and able to be used in health technology assessment, reimbursement, or other decision-making situations. Model developers should conduct studies according to the highest possible standards of quality and communicate results with adequate disclosure of assumptionsCitation4.
The incidence of invasive fungal infection (IFI) has risen dramatically over the past 20 years, and costs associated with treatment are highCitation7,Citation8. Preventing IFI in high-risk patients, such as those with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) who have chemotherapy-induced neutropenia, may therefore reduce overall healthcare costsCitation8,Citation9. Posaconazole is an extended-spectrum triazole with demonstrated efficacy as prophylaxis for and treatment of IFICitation9–12. Posaconazole is approved for use in the US and Europe for the prophylaxis of IFI in immunocompromised patients, and, additionally, in Europe as treatment for refractory IFICitation13,Citation14.
This report presents a case study of how a cost-effectiveness model was developed for posaconazole in a global headquarters and then distributed and adapted by at least 11 countries.
Methods
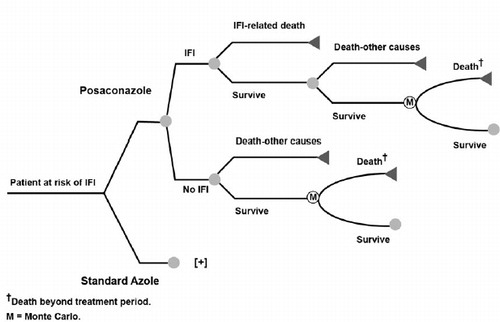
A decision-analytic model was developed by Schering-Plough Corp. (subsequently acquired by Merck Sharp & Dohme Corp., Whitehouse, NJ) and i3 Innovus (now OptumInsight, Waltham, MA) to estimate the cost-effectiveness of antifungal prophylaxis therapy with posaconazole among patients with acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) at high risk for IFI because of chemotherapy-induced neutropenia (). The model was first described in detail in O’Sullivan et al.Citation15 and has subsequently been adapted and used by various third parties. The decision analytic model consisted of an initial decision tree followed by a Markov model with 1-month cycle lengths. In the model, patients were assumed to receive prophylaxis with posaconazole or standard azole therapy (fluconazole or itraconazole), and distributions of patients receiving fluconazole and itraconazole in the comparator group were assumed to be the same as those in a clinical trial of Cornely et al.Citation9 (i.e., 81% fluconazole and 19% itraconazole) conducted in patients with AML or MDS who were considered at high risk of IFI. The duration of antifungal prophylaxis in the model was assumed to equal the mean duration of prophylaxis in the Cornely et al.Citation9 trial. The base case risk for experiencing IFI was assumed to be treatment-specific; probabilities of experiencing an IFI, IFI-related death, and death from other causes during the study period were also estimated with data from the clinical trialCitation9. One-month Markov cycles were used to extrapolate survival to a lifetime horizon. Patients alive at the end of the trial period (100 days) were assumed to have a risk for death specific to underlying disease. contains the original model parameters and data sourcesCitation9,Citation15–18. The probability of IFI with either standard azole therapy (fluconazole/itraconazole) or posaconazole are core parameters that were not changed at the country level when the model was adapted for local use.
Table 1. Global model parameters and data sourcesCitation14,Citation15.
The core model was used to estimate total costs, IFIs avoided, life-years gained, utilities gained, and incremental cost (if applicable) of posaconazole compared with standard azole therapy from a third-party perspective in various currencies. One-way sensitivity analyses and second-order probabilistic Monte Carlo sensitivity analyses were conducted to assess the robustness of model results and the effects of parameter uncertainty on the study findings, particularly as related to treatment efficacy and IFI costs. The sensitivity analyses varied parameters of the model such as risk of IFI, IFI-related death, other death within 100 days, mean duration of prophylaxis, and the cost of IFI treatment. shows the parameters that were adapted locally for each country, including probabilities of death and survival, currency, cost of treating an IFI, and the discount rate (for cost and outcomes)Citation5,Citation15,Citation19–33.
Table 2. Model parameters that were changed locallyCitation11,Citation15–24,Citation29.
The core model and technical report were made freely available to local countries, and in most cases the adaptation and analysis was completed locally. The specific study outcome for this analysis was incremental cost per life-year saved.
Results
summarizes the results of the various locally adapted cost-effective modeling studies, which indicate that prophylaxis with posaconazole, compared with fluconazole or itraconazole, prolongs survival, and, in most countries, is cost-savingCitation5,Citation15,Citation19–27. In Germany, South Korea, and Belgium, additional cost per life-year saved was associated with the use of posaconazole compared with fluconazole or itraconazole; however, the incremental cost was well below the pre-determined cost-effectiveness thresholds for these countries.
Table 3. Cost-effectiveness of posaconazole prophylaxis among high-risk patients with neutropenia.
All reported probabilistic sensitivity analyses (PSA) showed that prophylaxis with posaconazole was likely to be cost-effective at the pre-determined cost-effectiveness threshold for that country ()Citation5,Citation15,Citation19–27. It should be noted that, while the model allowed for PSA to be conducted, countries that were not required to conduct a PSA as part of their local reimbursement process may not have conducted the analysis.
In all countries, the model predicted that prophylaxis with posaconazole would be associated with an increase in life-years, with increases ranging from 0.016–0.1 life-year saved.
shows the outcome of the modeling study in each country from a payer’s perspective; in all countries, posaconazole was approved by the respective reimbursement authorityCitation5,Citation15,Citation19–27. In the first instance, the analyses were presented in health technology assessment reports to the reimbursement authorities; in most countries, data from the locally adjusted model were also published in journals or presented at congresses.
Table 4. Outcome of use of the locally adapted global model.
Discussion
This case study reports how a global cost-effectiveness model was successfully adapted to local requirements by importation of local parameters. The study-specific outcome was incremental cost/life-year saved. This analysis shows that, in most countries, use of posaconazole is cost-savingCitation5,Citation15,Citation19–27. There was an additional cost per life-year saved in Germany, South Korea, and Belgium; however, the incremental cost was well below the pre-determined cost-effectiveness thresholds for these countries. Local use of the core model led to reimbursement of posaconazole by local authorities in all countries.
As noted by O’Sullivan et al.Citation15 in the original publication of the posaconazole cost-effectiveness model, a number of limitations should be noted: the lack of power to detect differences between posaconazole and fluconazole or itraconazole separately; the assumption that risk of death after 100 days was equal for those who did and did not develop IFI; and, in the original model, the use of equal probabilities of IFI-related and other death during the trial period for both prophylaxis groups, even though overall and IFI-related survival benefits for the posaconazole group were demonstrated in Cornely et al.Citation9 When treatment-specific case fatality rates were used as an alternative analysis in O’Sullivan et al.Citation15, however, posaconazole was also observed to be cost-saving. Furthermore, when treatment-related death probabilities were used in various local adaptations of the model ()Citation5,Citation15,Citation19–33, posaconazole cost-saving or cost-effectiveness was consistently demonstrated ()Citation5,Citation15,Citation19–27.
Interestingly, an independent decision-analytic model developed by Collins et al.Citation34 at the University of Michigan Health System showed results similar to those of the global model, in which posaconazole prophylaxis was associated with overall treatment costs lower than treatment costs associated with fluconazole or itraconazole prophylaxis in patients with prolonged neutropenia. A recent independent review by Lyseng-WilliamsonCitation35 has also reviewed the cost-effectiveness analyses of posaconazole in this population, presenting results from this original core model. The review reports findings similar to those of our own research; in spite of some inherent limitations, data from several countries support the use of posaconazole as a dominant or cost-effective antifungal prophylaxis relative to standard oral azole prophylaxis in patients with neutropenia at high risk of developing IFICitation35.
The model has the capacity to run both univariate sensitivity analyses, as well as the more robust probabilistic sensitivity analyses. Results from both types of sensitivity analysis suggest that the model is sensitive to variations in the risk of IFI and the mean duration of therapy with posaconazole. In the base case analysis, the probabilistic sensitivity analysis suggests that posaconazole has a 90% probability of having an incremental cost-effectiveness ratio at or below a $50,000 per life-year saved threshold and a 95% probability of being at or below a $100,000 per life-year saved threshold.
Conclusion
A core economic model was developed and successfully adapted locally by several countries. The model showed that posaconazole was cost-saving or cost-effective vs standard azole therapy (fluconazole and itraconazole) and led to positive reimbursement listings.
Transparency
Declaration of funding
This work was funded by Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA.
Declaration of financial/other relationships
Manishi Prasad is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ. At the time of the study, George Papadopoulos was an employee of Merck Sharp & Dohme Corp. Sheena Hunt is an employee of ApotheCom, a company that received funding from Merck Sharp & Dohme Corp., for help in the publication of this study. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
The authors gratefully acknowledge the support of i3 Innovus, Medford, MA, USA, in the development of the model. The authors would also like to thank the researchers in all the countries who used and adapted this model locally.
References
- Taylor RS, Drummond MF, Salkeld G, et al. Inclusion of cost effectiveness in licensing requirements of new drugs: the fourth hurdle. Br Med J 2004;329:972-5
- Drummond M, Barbieri M, Cook J, et al. Transferability of economic evaluations across jurisdictions: ISPOR Good Research Practices Task Force report. Value Health 2009;12:409-18
- International Society for Pharmacoeconomics and Outcomes Research. Pharmacoeconomic guidelines around the world. International Society for Pharmacoeconomics and Outcomes Research Web Site. Princeton, NJ, 2010. Available at: http://www.ispor.org/peguidelines/index.asp [last accessed 16 March 2010]
- Weinstein MC, O'Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research Practices--Modeling Studies. Value Health 2003;6:9-17
- Welte R, Feenstra T, Jager H, et al. A decision chart for assessing and improving the transferability of economic evaluation results between countries. PharmacoEconomics 2004;22:857-76
- Stoykova B, Drummond M, Barbieri M, et al. The lag between effectiveness and cost-effectiveness evidence of new drugs. Implications for decision-making in health care. Eur J Health Econ 2003;4:313-8
- Richardson MD. Changing patterns and trends in systemic fungal infections. J Antimicrob Chemother 2005;56(1 Suppl):i5-i11
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm 2009;66:1711-7
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348-59
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007;356:335-47
- Walsh TJ, Raad I, Patterson TF, et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 2007;44:2-12
- Raad II, Hachem RY, Herbrecht R, et al. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin Infect Dis 2006;42:1398-403
- Merck & Co., Inc. Noxafil® (posaconazole) oral suspension [prescribing information]. Whitehouse Station, NJ: Merck & Co. Inc., 2012
- Merck Sharp & Dohme Limited. Noxafil® (posaconazole) oral suspension [summary of product characteristics]. Hertfordshire, UK: Merck Sharp & Dohme Limited, 2012
- O'Sullivan AK, Pandya A, Papadopoulos G, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health 2009;12:666-73
- National Cancer Institute. SEER cancer statistics review 1975-2003. National Cancer Institute Web site, Bethesda, MD, p 1–88, 2009. Available at http://seer.cancer.gov/csr/1975_2003/ [last accessed 14 July 2009]
- Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer 2006;106:1099-109
- Statistics Canada. Cancer survival statistics 1992-2000. Statistics Canada Web Site, Ontaria, Canada, 2009. Available at http://cansim2.statcan.gc.ca/cgi-win/cnsmcgi.pgm?Lang=E&AS_Sort=0&ChunkStart=1&ChunkSize=25&ResultTemplate=%2FStu-Etu%2FStu-Etu6&AS_Action=Find&AS_Theme=-1&AS_Date=.&AS_Ser=.&AS_Auth=.&AS_Univ=6&AS_Srch=cancer+survival+statistics&AS_Btn=Search [last accessed 22 October 2009]
- Grau S, de la Camara RR, Sanz MA, et al. Cost-effectiveness of posaconazole versus standard azole treatment (fluconazole or itraconazole) in the prevention of invasive fungal infections among high-risk neutropenic patients in Spain. Poster presented at: 18th European Congress of Clinical Microbiology and Infectious Diseases; April 19-22, 2008; Barcelona, Spain
- Michallet M, Gangneux JP, Lafuma A, et al. Cost effectiveness of posaconazole in the prophylaxis of invasive fungal infections in acute leukaemia patients for the French healthcare system. J Med Econ 2011;14:28-35
- Greiner RA, Meier Y, Papadopoulos G, et al. Cost-effectiveness of posaconazole compared with standard azole therapy for prevention of invasive fungal infections in patients at high risk in Switzerland. Oncology 2010;78:172-80
- Tahami Monfared AA, O’Sullivan AK, Papadopoulos G. Posaconazole versus standard azole therapy in the prophylaxis against invasive fungal infections among high-risk neutropenic patients in Canada: a cost-effectiveness analysis. Poster presented at: 18th European Congress for Clinical Microbiology and Infectious Diseases; April 19-22, 2008; Barcelona, Spain
- Stam WB, O’Sullivan AK, Rijnders B, et al. Economic evaluation of posaconazole versus standard azole prophylaxis in high risk neutropenic patients in the Netherlands. Eur J Haematol 2008;81:467-74
- Kim Y, Lee D, Jun S, et al. Cost-effectiveness analysis of posaconazole for prophylaxis in patients with neutropenia in South Korea. Poster presented at: International Society for Pharmacoeconomics and Outcomes Research 14th Annual International Meeting; May 16-20, 2009; Orlando, FL
- Frost MJ, O’Sullivan AO, Morrissey O, et al. Cost effectiveness of antifungal prophylaxis with posaconazole versus standard azole therapy in the prevention of invasive fungal infections (IFIs) among high risk haematology patients in Australia. Poster presented at: 6th Annual Meeting of the Globalization and Health Technology Assessment International Meeting; June 21-24, 2009; Singapore
- Maertens J, Aoun M, Bron D, et al. Cost-effectiveness of posaconazole (Noxafil®) versus standard azole therapy in the prevention of invasive fungal infections among high-risk neutropenic patients in Belgium. Poster presented at: 10th International Symposium on Febrile Neutropenia; February 8-9, 2008; Brussels, Belgium
- Belousov YB, Kolbin AS, Koroleva OA, et al. Clinical and economic expediency of posaconazole comparing with fluconazole and itraconazole in primary prophylaxis of systemic mycoses in severe neutropenic patients. Clin Microbiol Antimicrob Chemother (Russia) 2009;11:170-82
- Grau Cerrato S, Mateu-De AJ, Soto AJ, et al. [Economic evaluation of voriconazole versus amphotericin B in the treatment of invasive aspergillosis]. Farmacia Hospitalaria 2005;29:5-10
- Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health 2002;5:26-34
- Wenzel R, Del Favero A, Kibbler C, et al. Economic evaluation of voriconazole compared with conventional amphotericin B for the primary treatment of aspergillosis in immunocompromised patients. J Antimicrob Chemother 2005;55:352-61
- Jansen JP, Meis JF, Blijlevens NM, et al. Economic evaluation of voriconazole in the treatment of invasive aspergillosis in the Netherlands. Curr Med Res Opin 2005;21:1535-46
- Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002;34:7-14
- Integrale Kankercentra (Netherlands Integral Cancer Centre). Integraal Kankercentrum Amsterdam (national cancer statistics for the Netherlands). Integrale Kankercentra Web Site. Amsterdam, Netherlands, 2010. Available at http://www.ikcnet.nl/ [last accessed 16 March 2010]
- Collins CD, Ellis JJ, Kaul DR. Comparative cost-effectiveness of posaconazole versus fluconazole or itraconazole prophylaxis in patients with prolonged neutropenia. Am J Health Syst Pharm 2008;65:2237-43
- Lyseng-Williamson KA. Posaconazole: a pharmacoeconomic review of its use in the prophylaxis of invasive fungal disease in immunocompromised hosts. PharmacoEconomics 2011;29:251-68