Abstract
Objective:
In non-steroidal anti-inflammatory drug (NSAID) users, chronic occult blood loss may lead to decreases in hemoglobin, which may lead to increased healthcare expenditures. This study, therefore, sought to quantify healthcare resource utilization of ≥2 g/dL hemoglobin decrease in osteoarthritis patients.
Methods:
Using a large US managed care database, osteoarthritis patients aged ≥18 years who had exposure to ≥90 days of non-selective or selective COX-2 NSAID use, a hemoglobin value within 6 months before index NSAID, and at least one hemoglobin value 24 months after were evaluated. Resource utilization was evaluated in those with ≥2 g/dL hemoglobin drop vs patients with ≤0.5 g/dL hemoglobin drop (control).
Results:
Of 1800 NSAID users meeting inclusion criteria, 228 patients [mean (SD) = 59.8 (9.3) years] had ≥2 g/dL hemoglobin drop vs 1572 controls [mean (SD) = 58.3 (8.0) years]. Despite relatively low absolute rates, endoscopic procedures were more commonly observed in the ≥2 g/dL hemoglobin drop group [endoscopy: 37/228 (16.2%) vs 65/1572 (4.1%); adjusted odds ratio (AOR) 3.5, (95% confidence interval [CI] = 2.1–6.0); colonoscopy: 36/228 (15.8%) vs 137/1572 (8.7%); AOR 2.0 (95% CI 1.2–3.2)]. During the 12-month follow-up, patients with ≥2 g/dL hemoglobin drop utilized significantly more healthcare resources [adjusted relative risk (95% CI) for hospitalization, 2.1 (1.5–2.9); outpatient visits, 1.4 (1.3–1.5); physician visits, 1.3 (1.1–1.4)] and charges (total adjusted charges $47,766 vs $23,342) across major categories of healthcare services.
Limitations:
This was a retrospective analysis with baseline demographic differences. The source or cause of the hemoglobin drops could not be verified; and it is assumed that they are related to occult gastrointestinal loss. Differences with healthcare utilization and charges were not linked to hemoglobin-associated complications.
Conclusion:
In patients exposed to NSAIDs, those with significant hemoglobin drops experienced higher subsequent healthcare utilization and charges than controls who did not have a significant hemoglobin drop.
Introduction
Osteoarthritis (OA) is a chronic painful condition that can impact an individual’s physical function and health-related quality-of-lifeCitation1. Based on various societal and expert panel guidelines, non-steroidal anti-inflammatory drugs (NSAIDs) are a mainstay of clinical care and are commonly used for the relief of mild and moderate pain and treatment of the signs and symptoms associated with arthritisCitation2–5.
Based, in part, on their inhibition of cyclooxygenase enzyme catalysis (COX-1 and COX-2) in the gastrointestinal (GI) mucosa, use of NSAIDs is associated with an increased risk of upper and lower GI adverse outcomesCitation6–9. Multiple studies and clinical trials demonstrate that sustained inhibition of COX by NSAIDs is associated with an increased propensity to develop mucosal injury in the small intestineCitation10–13, and in prospectively designed capsule endoscopy studies it has been demonstrated that there are higher rates of small bowel mucosal breaks/ulcers observed in patients receiving NSAIDsCitation14–17.
Moreover, even in the absence of acute clinical GI bleeding events, it has been suggested that this clinically silent mucosal injury in the small bowel may result in chronic occult blood loss, potentially leading to decreases in hemoglobin with resultant anemia and iron deficiencyCitation6,Citation8,Citation18–22. Prospective, large-scale, multi-center, long-term clinical outcomes trials have demonstrated that exposure to NSAIDs has been associated with decreases in hemoglobin valuesCitation6,Citation18,Citation21–24. These trials have often used a pre-determined end-point of a ≥2 g/dL decrease in hemoglobin because this decline is thought to be clinically significantCitation25–27.
As we have previously described in the Celecoxib Long-term Arthritis Safety Study (CLASS)Citation22 and in the Celecoxib vs Omeprazole and Diclofenac for At-Risk Osteoarthritis and Rheumatoid Arthritis Patients (CONDOR) trialCitation6, a ≥2 g/dL decrease in hemoglobin is not uncommon. We have reported that, at 6 months, 3.4% and 5.7% of arthritis patients treated with diclofenac, in the CLASS and CONDOR trials, respectively, demonstrated such a declineCitation19.
Despite the widespread use of this endorsed end-point (outcome), the clinical impact and relevance associated with a decrease of ≥2 g/dL in hemoglobin appears to be under-studied. Unlike overt bleeding, a ≥2 g/dL decrease in hemoglobin with or without the development of anemia may not necessarily lead to an emergent hospital admission. However, such a decline in hemoglobin may prompt further clinical investigation, resource utilization, and early termination of treatment.
Regardless of the recognition that decreases in hemoglobin are associated with the chronic use of NSAIDs, the impact on healthcare utilization and associated charges in such patients has not been investigated. Therefore, we hypothesized that in NSAID-treated OA patients, a ≥2 g/dL drop in hemoglobin, with or without the development of anemia, would be associated with increased resource utilization and charges compared with patients also taking NSAIDs who had stable hemoglobin (hemoglobin changes ≤0.5 g/dL during the 24 months after NSAID treatment started).
Patients and methods
Data source
This retrospective analysis was conducted using patients selected from OptumInsight’s Life Sciences Research Database, a database affiliated with a large US managed care plan. This database provides integrated enrollment as well as medical and prescription claims data, augmented with laboratory test results data for ∼15 million members.
The majority of health plans covered in the database are managed by commercial companies (∼97%) and ∼3% are managed by Medicaid or Medicare. The database includes de-identified records of administrative data (e.g., gender, age, dates of eligibility), pharmacy claims data (e.g., drug dispensed, drug strength, dollar amounts), physician and facility claims data (e.g., procedures, diagnosis, admission and discharge dates, and dollar amounts), and laboratory test results data. We studied data spanning 2002–2009 in patients with OA.
Patient selection
Patients were eligible for inclusion in the study if they were aged ≥18 years, undergoing NSAID treatment (non-selective NSAIDs or selective COX-2 NSAIDs) between 2002–2009, and carried the diagnosis of OA preceding treatment with the index NSAID. Patients with OA were defined as those with ICD-9-CM (International Classification of Disease, 9th Revision, Clinical Modification) codes of 715.XX in any diagnosis field in the claims data.
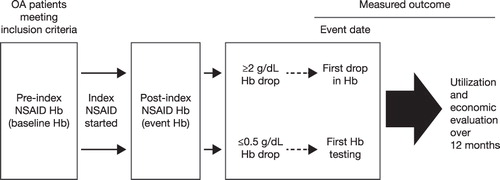
From this list of OA patients, patients on ≥90 days of NSAID treatment, ≥80% NSAID coverage at any dose during the first 90 days of NSAID treatment (based on the proportion of days covered by NSAIDs during this period), and who had at least one hemoglobin value at or during the 6 months before the index NSAID start date (pre-index; baseline hemoglobin value) and at least one hemoglobin value during the 24 months after the index NSAID start date (follow-up hemoglobin value) were identified. When two or more hemoglobin values were obtained during the pre-index period, the one closest to the index NSAID start date was considered to be the baseline hemoglobin value ().
The NSAID-treated OA patients with both baseline and follow-up hemoglobin values were divided into two groups: those with a ≥2 g/dL hemoglobin drop and those with any hemoglobin change ≤0.5 g/dL. Patients with a ≥2 g/dL hemoglobin drop were identified as those who had a hemoglobin decrease of ≥2 g/dL from baseline during the subsequent 24 months after the index NSAID start date. The first occurrence of a ≥2 g/dL hemoglobin drop was considered to be the event date. Patient controls included patients who had any hemoglobin change that did not exceed a decrease of ≤0.5 g/dL during the 24 months after the index NSAID start date. The first hemoglobin follow-up date after baseline for a patient with ≤0.5 g/dL hemoglobin drop was considered the event date.
Patients were excluded if they were not continuously enrolled for a period of 6 months before and 1 year after the event date, and if the time between the baseline hemoglobin and the occurrence of the 2 g/dL drop was <30 days.
Demographic data were collected to describe treatment groups based on age, gender, comorbidities, and medication use. The choice of comorbidities was based on the availability and frequency within the OA population we studied. Additionally, many of the comorbidities are also included in the Charlson comorbidity index. Comorbidities considered included the following conditions and ICD-9-CM codes: myocardial infarction (ICD-9-CM codes 410–410.9), congestive heart failure (428–428.9), peripheral vascular disease (443.9, 441–441.9, 785.4, and V43.4), cerebrovascular disease (430–438), dementia (290–290.9), chronic pulmonary disease (490–496, 500–505, and 506.4), rheumatologic disease (710.0, 710.1, 710.4, 714.0–714.2, 714.81, and 725), peptic ulcer disease (531–534.9), mild liver disease (571.2, 571.5, 571.6, and 571.4–571.49), diabetes (250–250.3 and 250.7), diabetes with chronic complications (250.4–250.6), hemiplegia or paraplegia (344.1 and 342–342.9), renal disease (582–582.9, 583–583.7, 585, 586, and 588–588.9), malignancy (140–172.9, 174–195.8, and 200–208.9), moderate or severe liver disease (572.2–572.8 and 456.0–456.21), metastatic solid tumor (196–199.1), AIDS (042–044.9), ischemic heart disease (410 and 411, exclude 4111 and 414), angina (4111, 413), stroke (430–438), and peripheral vascular disease (4438, 44389, and 4339). Patients could be classified under more than one comorbidity category.
Outcome measures
Healthcare utilization
Healthcare utilization refers to all claims made for medication and services covered by the healthcare plan including office and outpatient visits, emergency department (ED) visits, hospitalizations, prescription medications, procedures, and laboratory services.
The following endoscopic procedures were specifically investigated: colonoscopy (ICD-9-CM code 4523; CPT-4 codes [Current Procedural Terminology, 4th Edition] 45378–45380, 45382, 45384, and 45385), upper GI endoscopy (ICD-9-CM codes 4222, 4223, 4516, and 4513; CPT-4 codes 43200, 43202, 43215–43217, 43220, 43227, 43234, 43235, 43239, 43241, 43246, 43247, 43250, 43251, and 43255), sigmoidoscopy (ICD-9-CM code 4524; CPT-4 codes 45330–45334 and 45338), capsule endoscopy (CPT-4 codes 91110 and 91111), small bowel endoscopy (ICD-9-CM codes 4511 and 4512; CPT-4 codes 44360, 44361, 44363–44366, 44369, 44370, 44372, 44373, and 44376–44379), and anoscopy (ICD-9-CM code 4921; CPT-4 codes 46600–46615). In addition, blood transfusion (ICD-9-CM code 9900, 9902, and 9903; CPT-4 code 36430) was also investigated.
All endoscopic procedures and blood transfusions were followed up 6 months after the event date, while office and outpatient visits, ED visits, hospitalizations, prescription medications, and laboratory procedures were investigated 12 months after the event date.
Economic analysis
Healthcare claim charges include all charges associated with the use of the above-cited procedures, medications, and services. The charges associated with the ICD-9-CM and CPT-4 codes were aggregated and assessed.
Data analysis
Baseline demographics and characteristics were summarized and compared between groups (≥2 g/dL hemoglobin drop vs ≤0.5 g/dL hemoglobin drop) using descriptive statistics (t-test for comparison between group means; χ2 test for comparison between group proportions).
The use of endoscopic procedures for OA patients experiencing a ≥2 g/dL hemoglobin drop were compared with the patient controls 6 months after the event date using simple and multivariate logistic regression models, which were used to quantify the odds of experiencing an event in one group relative to another. In the multiple logistic regression modelsCitation28, the use of endoscopic procedures between groups was adjusted for baseline factors. Baseline factors considered included demographics (age at event, event year, and gender), comorbidities (Charlson comorbidity index, ischemic heart disease, angina, stroke, and peripheral vascular disease), utilizations (ED visits, hospitalization, number of office visits, and number of outpatient visits), and use of medications (warfarin, other anticoagulant or antiplatelet medications, steroids, and proton pump inhibitors) during the 6 months before the event date.
Healthcare utilization data was compared between OA patients with a ≥2 g/dL drop in hemoglobin and patients with a ≤0.5 g/dL hemoglobin drop using a general linear model with a negative binomial response distribution having a log link function to estimate mean and 95% confidence intervals (CIs)Citation28. The economic impact based on charges was compared among OA patients with a ≥2 g/dL hemoglobin drop and patients with a ≤0.5 g/dL hemoglobin drop using a general linear model with a gamma response distribution having a log link function to estimate mean and 95% CIsCitation28. Models were adjusted for the same set of baseline factors considered for multivariate logistic regression models. These generalized linear models provided adjusted (for the baseline factors) and unadjusted relative risks to compare the outcome between the two groups.
All analyses were performed in SAS (SAS Institute Inc., Cary, NC)Citation29.
Results
Patient characteristics
A total of 1800 NSAID users met our inclusion/exclusion criteria, of which 228 patients with a hemoglobin drop of ≥2 g/dL were compared with 1572 patients with a ≤0.5 g/dL drop in hemoglobin (). Patients with a ≥2 g/dL drop in hemoglobin had a slightly higher mean age (SD) than patients with a ≤0.5 g/dL drop in hemoglobin [59.8 (9.3) years vs 58.3 (8.0) years; p = 0.0258] ().
Figure 2. Patient selection process. COX-2, cyclooxygenase-2; NSAID, non-steroidal anti-inflammatory drug; OA, osteoarthritis.
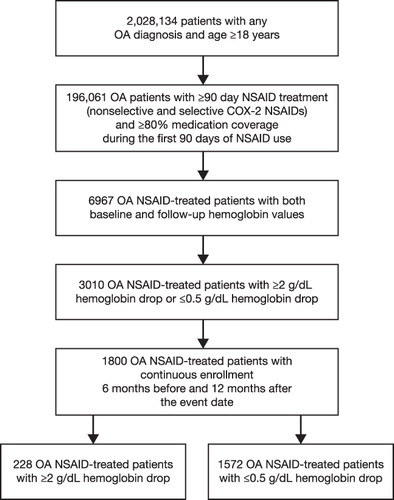
Table 1. Demographic and clinical characteristics of osteoarthritis patients with ≥2 g/dL hemoglobin drop 6 months before the event date.*
The mean (SD) baseline hemoglobin values were similar in patients with a ≥2 g/dL hemoglobin drop and in patients with a ≤0.5 g/dL hemoglobin drop [14.0 (1.5) g/dL vs 14.0 (1.2) g/dL; p = 0.6413] (). Patients with a ≥2 g/dL hemoglobin drop had a statistically significant longer follow-up time from the index NSAID date to the event date than patients with a ≤0.5 g/dL hemoglobin drop [mean (SD) = 332.6 (198.2) vs 284.8 (183.7); p = 0.0003].
Table 2. Hemoglobin characteristics of osteoarthritis patients with ≥2 g/dL hemoglobin drop.
During the 6 months before the event date, patients with a hemoglobin drop of ≥2 g/dL had a higher Charlson comorbidity index [1.4 (1.9) vs 0.5 (0.9); p < 0.0001], had more comorbidities, greater health resource utilization such as hospitalization (38.2 vs 3.9%; p < 0.0001), and medication utilization, including the use of proton pump inhibitors (36.8 vs 22.8%; p < 0.0001), than control patients ().
Use of endoscopic procedures in OA patients with a ≥2 g/dL hemoglobin decrease
The absolute rates of GI-specific endoscopic procedures performed during 6 months after the event date are shown in , comparing patients with a ≥2 g/dL drop in hemoglobin with controls. For the more commonly performed endoscopic procedures, significantly more procedures were performed in patients with hemoglobin changes meeting the pre-defined criteria of a ≥2 g/dL hemoglobin drop than patients with a ≤0.5 g/dL drop in hemoglobin. Unadjusted and adjusted odds ratios (ORs) further clarify the likelihood for higher rates of utilization in the ≥2 g/dL hemoglobin drop group.
Table 3. Comparison of GI-specific healthcare resource utilization between patients with ≥2 g/dL hemoglobin drop and patients with ≤0.5 g/dL hemoglobin drop 6 months after event date.*
Using World Health Organization (WHO)-defined criteria of anemia where laboratory hemoglobin values of ≤12 g/dL for women and ≤13 g/dL for men were considered as the lower limit of normalCitation30, patients with a ≥2 g/dL drop in hemoglobin were more likely to have event values consistent with anemia (76.8 vs 7.1%; p < 0.0001). Patients with a ≥2 g/dL drop in hemoglobin also underwent more blood transfusions than patients with a ≤0.5 g/dL drop in hemoglobin [1.3 vs 0.2%; OR (95% CI) = 7.0 (1.4–34.8)].
Despite the occurrence of a ≥2 g/dL hemoglobin drop, and the development of anemia in a significant number of patients, it is interesting to note that the rate of endoscopic procedures was relatively low within 6 months after the event date.
Healthcare utilization in OA patients with a ≥2 g/dL hemoglobin decrease
shows the comparison of healthcare utilizations (ED visits, office visits, outpatient visits, surgical procedures, hospitalizations, medications, and laboratory procedures) between patients with a ≥2 g/dL drop in hemoglobin and patients with a ≤0.5 g/dL drop in hemoglobin 12 months after the event date.
Table 4. Comparison of healthcare resource utilization between patients with ≥2 g/dL hemoglobin drop and patients with ≤0.5 g/dL hemoglobin drop 12 months after event date.*
During the 12 months after the event date, patients with a ≥2 g/dL hemoglobin drop utilized significantly more healthcare resources across the major resource categories than patients with a ≤0.5 g/dL drop in hemoglobin after adjusting for potential risk factors (p < 0.0001; ). For example, during the 12-month follow-up, patients with a ≥2 g/dL hemoglobin drop utilized significantly more healthcare resources across major categories of health services [adjusted relative risk (95% CI): hospitalization = 2.1 (1.5–2.9); outpatient visits = 1.4 (1.3–1.5); physician visits = 1.3 (1.1–1.4)].
Economic impacts of a decrease in hemoglobin of ≥2 g/dL in OA patients
shows the comparison of the calculated healthcare charges between patients with a ≥2 g/dL drop in hemoglobin and patients with a ≤0.5 g/dL drop in hemoglobin 12 months after the event date. During the 12 months after the event date, patients with a ≥2 g/dL hemoglobin drop incurred significantly more healthcare charges than patients with a ≤0.5 g/dL drop in hemoglobin after adjusting for potential risk factors ().
Table 5. Comparison of healthcare charges between patients with ≥2 g/dL hemoglobin drop and patients with ≤0.5 g/dL hemoglobin drop 12 months after event date.*
The main mean adjusted healthcare charges incurred by patients with a ≥2 g/dL decrease in hemoglobin and patients with a ≤0.5 g/dL drop in hemoglobin was outpatient charges ($22,390 vs $13,448), and the mean adjusted 12-month total charges for patients with a ≥2 g/dL drop in hemoglobin was ∼$20,000 more than for patients with a ≤0.5 g/dL drop in hemoglobin ($47,766 vs $23,342; adjusted relative risk [95% CI] = 2.1 [1.8–2.4]).
Discussion
In previous prospective, international, multi-center clinical outcomes trials, decreases in hemoglobin of ≥2 g/dL were reported in up to 5.7% in patients receiving NSAIDs over 6 monthsCitation19. While a ≥2 g/dL drop in hemoglobin is used as a measure in clinical trials, the clinical relevance and economic consequence of this decrease in hemoglobin is not well understood. Our analysis clearly demonstrates that these patients with a decrease in hemoglobin of ≥2 g/dL incurred significantly higher healthcare utilization rates and associated charges when compared with patients with a ≤0.5 g/dL drop in hemoglobin. As such, our data support the relevance and use of this pre-defined end-point for larger outcomes trials evaluating the impact associated with long-term use of NSAIDs.
The clinical relevance of decreases or low hemoglobin values has been studied by several authors. As reported by Sands et al.Citation31, arthritis patients who had a decrease in hemoglobin of ≥2 g/dL had heightened risk for clinically relevant outcomes such as coronary artery disease, myocardial infarction, pneumonia, and the need to withdraw from clinical trials for various medical conditions. In a different population, a post hoc analysis of pooled data from 14 prospective randomized controlled trials measuring both patient-reported outcomes and efficacy, patients with a decrease in hemoglobin of ≥2 g/dL did not report clinically meaningful improvements in physical function in contrast with those without changes in hemoglobinCitation1.
In other studies, and especially among the elderly, lower levels of hemoglobin or a decrease in hemoglobin have reproducibly demonstrated lower functionality and vitalityCitation32, health utilityCitation33, and quality-of-lifeCitation34. Not unexpectedly, anemia was found to be associated with an increased risk of mortalityCitation34,Citation35, more hospitalizations and more days spent in hospitalCitation34,Citation35, as well as the recurrent risk of falls and fracturesCitation34,Citation36. Anemia in the elderly is also associated with complications such as cardiovascular disease, cognitive dysfunction, longer hospitalizations, and reduced bone densityCitation34. Together, these studies highlight the medical relevance of lower levels of hemoglobin, particularly in the elderly.
Our data extend the findings of the above-mentioned observations in that this is the first study to directly evaluate whether greater health expenditures are being made in patients with a ≥2 g/dL drop in hemoglobin. This analysis demonstrates that GI endoscopic procedures occurred more commonly in patients with a ≥2 g/dL drop in hemoglobin compared with our control population. While this finding is expected, we were surprised by the low absolute rate of procedures in this patient group. The relatively low rate of procedures is surprising not only because of the magnitude of drop in hemoglobin, but by the fact that 175 out of 228 (76.8%) patients in the hemoglobin drop group reached a level considered to be anemic.
We believe that the low rates of procedures cannot be explained by the limited follow-up time frame. shows the procedure data for 6 months after the event date. When the analysis was expanded to 12 months after the event date, the rate of GI procedures did not significantly increase (data not shown). Given the increase in medication use—including proton pump inhibitor use—after the hemoglobin drop, one might conjecture that physicians expect the primary treatment of NSAID-associated complications to be directed at the well-recognized upper gastropathy, as opposed to performing a more comprehensive evaluation of the entire GI tract for NSAID-related and -non-related pathologies.
Regardless of the level of aggressiveness of the GI-specific evaluation, our 12-month follow-up data suggest significantly higher utilization of hospital and clinical resources, medications, and procedures in the group of patients with a hemoglobin decrease; however, we would like to highlight this as a significant limitation of our analysis. The difference in resource utilization between the two groups we identified cannot be explained solely by resource utilization associated with NSAID use or specific GI complications. In fact, given the differences in baseline between the two groups, and even after adjusting for baseline differences in demographic and clinical characteristics, we believe that there are GI-related and non-GI-related clinical scenarios leading to differences in our study cohorts and the heightened use of healthcare resources in the presence of a significant decrease in hemoglobin. Moreover, we recognize that this limitation is further magnified by the fact that we did not specifically evaluate clinical events; nor did we link increased utilizations to specific diagnoses, which would be an important area for future research.
This study has several additional limitations. First, this was a retrospective analysis and there are baseline differences in our two populations. Patients with a ≥2 g/dL decrease in hemoglobin entered our analysis with greater Charlson comorbidity scores and higher baseline healthcare utilization, indicating worse health status. We adjusted for known differences in baseline healthcare utilization, comorbidity, and demographic factors, as shown in , and thereby made the two groups equivalent for these factors. While the adjustment measurably reduced group differences, it is still possible that the difference between the two arms of the study may have been driven in part by factors neither identified nor included in our adjusted analyses. Further mitigating the impact of this limitation, though, is the fact that the hemoglobin values at baseline were not different between the two groups.
Second, we were unable to verify the source or cause of the hemoglobin drops and it is only an assumption that these changes in hemoglobin are related to possible occult GI loss. Moreover, we are unable to directly link or evaluate the association of the hemoglobin drops to the use of NSAIDs. As the drop in hemoglobin is assumed to be a gradual event, there is no specific diagnosis or event that will allow us to develop attribution. Even in prospective randomized trials, identifying a specific diagnosis or attribution to the use of NSAIDs specifically for the presumed blood loss poses great difficultyCitation6. However, despite this, the decrease in hemoglobin regardless of causality remains the driving difference between the two groups.
Third, the differences associated with healthcare resource utilization and related healthcare charges were studied in aggregate and were not linked to possible anemia/low hemoglobin-associated complications that one might expect such as falls, fractures, and cardiovascular eventsCitation34,Citation36.
Our fourth consideration of the limitations is the potential confounding effect of aspirin. Given the limitations of our dataset, over-the-counter use of aspirin for primary or secondary prophylaxis was not captured in our analysis. This would have been an important factor to include in the adjustment of the data. However, as previously reported, adjusting the impact of aspirin may in part be mitigated by the use of cardiovascular diagnoses as a surrogate markerCitation37, as we did in our study.
A final consideration of the limitations is the potential confounding effect of over-the-counter medication use. Due to the lack of information in the current claims database, it is impossible to evaluate, quantify, or incorporate over-the-counter drug use.
Conclusions
Despite the acknowledged limitations of this retrospective analysis, the results suggest that a ≥2 g/dL decrease in hemoglobin represents a significant medical burden associated with disproportionally increased healthcare utilization and related healthcare charges. This study further supports the meaningful use of this end-point in large-scale prospective, randomized clinical trials evaluating the impact associated with long-term use of NSAIDs.
Transparency
Declaration of funding
The study was funded in full by Pfizer Inc.
Declaration of financial/other relationships
JLG has served as a speaker for Pfizer, AstraZeneca, and Takeda, as a consultant for Pfizer, AstraZeneca, Takeda, Logical Therapeutics, Novartis, Astellas, GlaxoSmithKline, Proctor and Gamble, and Pozen, and has received research funding from Pfizer, AstraZeneca, Logical Therapeutics, Pozen, and Sucampo. XL, JCC, and GHS are employees of Pfizer Inc and all own stocks and shares in Pfizer Inc. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgments
Writing support was provided by C. Campbell, PhD, of PAREXEL, and was funded by Pfizer Inc.
References
- Strand V, Cryer B, Luo X, et al. Effect of blood loss on physical function in arthritis patients: a pooled analysis. Health Outcomes Res Med 2011;2:e27-e38
- Scheiman JM, Hindley CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther 2010;32:667-77
- Hochberg MC, Altman RD, Toupin April K, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465-74
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis 2003;62:1145-55
- Zhang W, Doherty M, Arden N, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2005;64:669-81
- Chan FK, Lanas A, Scheiman J, et al. Celecoxib versus omeprazole and diclofenac in patients with osteoarthritis and rheumatoid arthritis (CONDOR): a randomised trial. Lancet 2010;376:173-9
- Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994;343:769-72
- Laine L, Connors LG, Reicin A, et al. Serious lower gastrointestinal clinical events with nonselective NSAID or coxib use. Gastroenterology 2003;124:288-92
- Chan FK, Cryer B, Goldstein JL, et al. A novel composite endpoint to evaluate the gastrointestinal (GI) effects of nonsteroidal antiinflammatory drugs through the entire GI tract. J Rheumatol 2010;37:167-74
- Bjarnason I, Zanelli G, Smith T, et al. Nonsteroidal antiinflammatory drug-induced intestinal inflammation in humans. Gastroenterology 1987;93:480-9
- Allison MC, Howatson AG, Torrance CJ, et al. Gastrointestinal damage associated with the use of nonsteroidal antiinflammatory drugs. N Engl J Med 1992;327:749-54
- Bjarnason I, Hayllar J, MacPherson AJ, et al. Side effects of nonsteroidal anti-inflammatory drugs on the small and large intestine in humans. Gastroenterology 1993;104:1832-47
- Ng SC, Chan FK. NSAID-induced gastrointestinal and cardiovascular injury. Curr Opin Gastroenterol 2010;26:611-7
- Goldstein JL, Eisen GM, Lewis B, et al. Video capsule endoscopy to prospectively assess small bowel injury with celecoxib, naproxen plus omeprazole, and placebo. Clin Gastroenterol Hepatol 2005;3:133-41
- Goldstein JL, Eisen GM, Lewis B, et al. Small bowel mucosal injury is reduced in healthy subjects treated with celecoxib compared with ibuprofen plus omeprazole, as assessed by video capsule endoscopy. Aliment Pharmacol Ther 2007;25:1211-22
- Graham DY, Opekun AR, Willingham FF, et al. Visible small-intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol 2005;3:55-9
- Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology 2005;128:1172-8
- Bombardier C, Laine L, Reicin A, et al. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000;343:1520-8
- Goldstein JL, Chan FK, Lanas A, et al. Haemoglobin decreases in NSAID users over time: an analysis of two large outcome trials. Aliment Pharmacol Ther 2011;34:808-16
- Lanas A, Sopena F. Nonsteroidal anti-inflammatory drugs and lower gastrointestinal complications. Gastroenterol Clin North Am 2009;38:333-52
- Schnitzer TJ, Burmester GR, Mysler E, et al. Comparison of lumiracoxib with naproxen and ibuprofen in the Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET), reduction in ulcer complications: randomised controlled trial. Lancet 2004;364:665-74
- Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000;284:1247-55
- Cannon CP, Curtis SP, FitzGerald GA, et al. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 2006;368:1771-81
- Singh G, Fort JG, Goldstein JL, et al. Celecoxib versus naproxen and diclofenac in osteoarthritis patients: SUCCESS-I Study. Am J Med 2006;119:255-66
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011;365:981-92
- Schulman S, Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 2005;3:692-4
- Food and Drug Administration. Advisory Committee Briefing Document. February 7, 2000. Medical Officer's Gastroenterology Advisory Committee Briefing Document Division of Anti-Inflammatory, Analgesic and Ophthalmologic Drug Products: HFD-550. FDA Website. http://www.fda.gov/ohrms/dockets/ac/01/briefing/3677b1_05_gi.pdf. Accessed March 13, 2012
- McCullagh P, Nelder JA. Generalized linear models. 2nd ed. London, UK: Chapman and Hall, 1989
- SAS Institute Inc. SAS/STAT® user's guide. Cary, NC: SAS Institute Inc, 2008
- Blanc B, Finch CA, Hallberg L, et al. Nutritional anaemias. Report of a WHO scientific group. Geneva: World Health Organization, 1968
- Sands GH, Shell B, Zhang R. Adverse events in patients with blood loss: a pooled analysis of 51 clinical studies from the celecoxib clinical trials database. Open Rheumatol J 2012;6:44-9
- Eaton CB, Hochberg MC, Assaf A, et al. The cross-sectional relationship of hemoglobin levels and functional outcomes in women with self-reported osteoarthritis: results from the Women's Health Initiative. Semin Arthritis Rheum 2011;41:406-14
- Harrow BS, Eaton CB, Roberts MB, et al. Health utilities associated with hemoglobin levels and blood loss in postmenopausal women: the Women's Health Initiative. Value Health 2011;14:555-63
- Eisenstaedt R, Penninx BW, Woodman RC. Anemia in the elderly: current understanding and emerging concepts. Blood Rev 2006;20:213-26
- Penninx BW, Pahor M, Woodman RC, et al. Anemia in old age is associated with increased mortality and hospitalization. J Gerontol A Biol Sci Med Sci 2006;61:474-9
- Penninx BW, Pluijm SM, Lips P, et al. Late-life anemia is associated with increased risk of recurrent falls. J Am Geriatr Soc 2005;53:2106-11
- Goldstein JL, Howard KB, Walton SM, et al. Impact of adherence to concomitant gastroprotective therapy on nonsteroidal-related gastroduodenal ulcer complications. Clin Gastroenterol Hepatol 2006;4:1337-45