Abstract
Objective:
The only effective treatment for severe aortic stenosis (AS) is valve replacement. However, many patients with co-existing conditions are ineligible for surgical valve replacement, historically leaving medical management (MM) as the only option which has a poor prognosis. Transcatheter Aortic Valve Replacement (TAVR) is a less invasive replacement method. The objective was to estimate cost-effectiveness of TAVR via transfemoral access vs MM in surgically inoperable patients with severe AS from the Canadian public healthcare system perspective.
Methods:
A cost-effectiveness analysis of TAVR vs MM was conducted using a deterministic decision analytic model over a 3-year time horizon. The PARTNER randomized controlled trial results were used to estimate survival, utilities, and some resource utilization. Costs included the valve replacement procedure, complications, hospitalization, outpatient visits/tests, and home/nursing care. Resources were valued (2009 Canadian dollars) using costs from the Ontario Case Costing Initiative (OCCI), Ontario Ministry of Health and Long-Term Care and Ontario Drug Benefits Formulary, or were estimated using relative costs from a French economic evaluation or clinical experts. Costs and outcomes were discounted 5% annually. The effect of uncertainty in model parameters was explored in deterministic and probabilistic sensitivity analysis.
Results:
The incremental cost-effectiveness ratio (ICER) was $32,170 per quality-adjusted life year (QALY) gained for TAVR vs MM. When the time horizon was shortened to 24 and 12 months, the ICER increased to $52,848 and $157,429, respectively. All other sensitivity analysis returned an ICER of less than $50,000/QALY gained.
Limitations:
A limitation was lack of availability of Canadian-specific resource and cost data for all resources, leaving one to rely on clinical experts and data from France to inform certain parameters.
Conclusions:
Based on the results of this analysis, it can be concluded that TAVR is cost-effective compared to MM for the treatment of severe AS in surgically inoperable patients.
Introduction
Aortic stenosis (AS) occurs when the aortic valve of the heart becomes narrowed, resulting in a reduction in blood flow out of the heart. As a consequence, the heart needs to contract harder to pump blood into the aorta, which eventually leads to debilitating symptoms such as chest pain, shortness of breath, fatigue and heart failure. Severe AS can be effectively treated by replacing the stenotic valve with a prosthetic valve through conventional surgery requiring the chest to be opened. In 2005, it was estimated that 4000–4500 heart valve replacements were performed in CanadaCitation1. Many AS patients, however, are considered ineligible for conventional valve replacement surgery due to excessively high surgical risk or other conditions prohibiting surgery (e.g., frailty, porcelain aorta, and prior chest wall radiation). The prognosis for patients with severe AS who do not receive a new valve is poorCitation2. Medically managed patients have a short life expectancy: survival after onset of severe AS symptoms has been estimated at 50% at 2 years and 20% at 5 yearsCitation2. These patients may benefit from a less invasive valve replacement procedureCitation1.
Transcatheter Aortic Valve Replacement (TAVR) is less invasive than conventional valve replacement as the chest does not need to be opened, and there is no need for cardiopulmonary bypass, potentially allowing patients with prohibitive surgical risk to have their valves replaced. The recent Placement of AoRTic TraNscathetER Valve (PARTNER) clinical trial randomized patients who were ineligible for conventional surgery to receive either medical management (MM) or TAVR via transfemoral access, and found that patients who received TAVR had significantly greater survival and improved health-related quality-of-life compared to patients who received MMCitation3,Citation4.
The objective of this study was to evaluate the cost-effectiveness of TAVR delivered vs MM in patients who are ineligible for conventional surgery from a Canadian healthcare system perspective, using clinical data from the PARTNER randomized trial and Canadian-specific resource use and costs.
Methods
Economic model
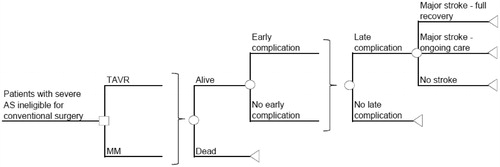
The cost-effectiveness model is a deterministic decision analytic model using a cost-utility framework with two treatment arms: TAVR via transfemoral access and MM (). It was developed as a global model to be adapted to any country, with France being the first. The health economic modeled patient has severe symptomatic AS and would be considered ineligible for conventional valve replacement surgery, as per the PARTNER trial inclusion/exclusion criteriaCitation3. Treatment outcomes were measured by quality-adjusted life years (QALYs, survival weighted by health utility), as reported in the PARTNER Trial by EuroQOL (EQ5D) health status instrumentCitation4.
Figure 1. Model decision tree structure. TAVR, transcatheter aortic valve replacement; MM, medical management; Early complication = 30 days post-surgery; Late complication = 1–3 years post-surgery; Complications = major access site/vascular complication (early only); valve thromboembolism; major paravalvular leak (early only); pacemaker implantation; endocarditis; reoperation; myocardial infarction; renal failure; arrhythmia/afibrillation (early only); balloon aortic valvuloplasty; hospital readmission; aortic valve replacement (MM only); death.
All relevant healthcare costs were considered in the model including those associated with the TAVR procedure (complications, hospitalization, rehabilitation), follow-up inpatient costs (complications, hospitalizations), and follow-up outpatient and nursing home costs (outpatient visits and tests, home and nursing care). The model calculated the incremental cost per QALY gained for TAVR vs MM. To adapt the model to the Canadian setting, we adopted a public payer perspectiveCitation5, specifically the Ontario Ministry of Health and Long-Term Care (MoHLTC) which was used as a proxy for Canada.
Due to the relatively short 2–3 year life expectancy of medically managed patients with severe symptomatic ASCitation2, a time horizon of 3 years was selected in order to capture all meaningful differences in costs and outcomes between TAVR and MMCitation5. The model was structured such that cumulative clinical outcomes for distinct time periods were assessed: 30 days, 6 months, 12 months, 24 months, and 36 months. The base case analysis incorporated costs and outcomes accumulated to 36 months. Costs and outcomes after the first year of the evaluation were discounted at a rate of 5% per annum as recommended by the Canadian Agency for Drugs and Technologies in Health (CADTH)Citation5.
Measurement of treatment efficacy
Mortality rates and health utility data for both treatment strategies were extracted from the results of the PARTNER trialCitation3,Citation6. In this study, a total of 358 patients with AS who were not considered to be suitable candidates for surgery were randomized to TAVR or MM. The groups were relatively well balanced at baseline in terms of mean age (83.1 years TAVR; 83.2 years MM), male gender (82% TAVR; 84% MM), Society of Thoracic Surgeons (STS) score (11.2 TAVR; 12.1 MM), logistic EuroSCORE (26.4 TAVR; 30.4 MM), New York Heart Association (NYHA) classification (92.2% class III or IV for TAVR; 93.9% for MM), as well as other risk factors (complete details available in trial publicationCitation3).
At one year following randomization, the rate of death from any cause was found to be 30.7% in the TAVR group and 50.7% in the MM group (p < 0.001)6. By two years, the rate of death was 43.3% among patients in the TAVR group as compared with 68.0% among patients in the MM group (p < 0.001)Citation6. The 36-month mortality rate for use in the model was estimated by extrapolation of the 1, 6, 12, and 24 month survival data from the trialCitation3,Citation6 using the exponential trend line function in Microsoft Excel 2010 ().
Table 1. Survival data used in the model.
The PARTNER trial used the EQ-5D tool to measure health utilities. Mean baseline EQ-5D utility scores were 0.59 in the TAVR group and 0.57 in the MM group. At 12 months, the mean utility scores increased to 0.72 in the TAVR group and to 0.62 in the MM group (p < 0.05)Citation7. Follow-up data were available for 12 months, and it was assumed the utilities with each treatment group were maintained for the 3-year time horizon for those patients that survived.
Estimation of resource use and costs
All costs were expressed in 2009 Canadian dollars and, as necessary, costs were inflated to 2009 dollars using the Statistics Canada Consumer Price Index for health and personal careCitation8. To the extent possible, resource use and costs data were obtained from Canadian sources, primarily from the Ontario Health Insurance Plan (OHIP) fee schedule and the Ontario Case Costing Initiative (OCCI) database, which contains case-level costs from up to 37 participating hospitals in OntarioCitation9.
For some parameters where Canadian-specific costs were not available in the literature or from other publicly available sources that were specific to our target population, data from the French analysis were adapted (). In these instances, known costs of three complications (renal dialysis, pacemaker implantation, and endocarditis) were used to calculate an overall relative healthcare cost relationship between Canada and France. These costs were considered comparable as they came from similar public cost databases in each country (OCCI in Canada, Échelle Nationale de Coût in France). The relative weight of ∼1.25:1 (Canada: France) was used as the adjustment factor. Canadian physicians with TAVR experience and co-authors of the Canadian special access trialCitation10 also provided input on costs when data was not available from other sources. The effect of uncertainty in these estimates was explored in sensitivity analyses.
Table 2. TAVR procedure-related costs and resource use.
Table 3. Incremental hospital costs and probabilities of complications following TAVR.
Table 4. Costs and probabilities of long-term complications.
Valve replacement procedure costs, including hospitalization
The costs associated with the TAVR procedure included cost of the device, personnel, other materials, hospital stay (uncomplicated), and the incremental hospital cost of complications (). Canadian clinical advisors described current billing practices, and corresponding physician fees were obtained from OHIP billing codesCitation11. It should be noted that it is possible that billing practices among physicians for the TAVR procedure may vary given that a specific code has yet to be added to the Ontario billing schedule for this procedure. It was estimated that the total length of stay (LOS) for an uncomplicated case was 4 days, comprised of 1 day in the intensive care unit (ICU) and 3 days in a general ward; this was based on LOS data provided from the Canadian special access study investigatorsCitation10. It was conservatively estimated that approximately a quarter of patients would require 10 additional days in hospital for post-operative cardiac rehabilitation at the same cost per day as for the general ward (the 10 days estimate was based on the standard deviation for length of stay for standard aortic valve replacement for patients over 70 years of age from the OCCI database, 2008–2009). The costs for staff on hospital salary, other materials, medications, contrast media, blood transfusions, ICU cost per day and general ward cost per day were based on relative costs from the French analysis.
Post-operative complication costs were also considered (). The expected probabilities at which these complications occurred were based on the number of events that occurred within 30 days of the valve procedure in the PARTNER trialCitation3 and were assumed to occur while the patient was already in hospital. The incremental costs of these complications added to the index hospitalization were based on relative costs from the French analysis.
Follow-up inpatient costs
Costs of complications that occurred after discharge from hospital following the TAVR procedure, or at any time point for MM patients, were also included in the analysis (). The probabilities for treatment-specific complications through 24 months were taken from the PARTNER randomized trialCitation6. Complication rates at 36 months were held constant. With the exception of balloon aortic valvuloplasty (BAV), costs for complications were obtained from the OCCI database; for all events except endocarditis, costs were restricted to patients in the database over 70 years of ageCitation9. It was assumed that patients would receive one doctor visit per day during hospitalization. When selecting appropriate International Classification of Diseases (ICD) and case mix grouper (CMG) codes, general categories were used. It can be seen in that the follow-up complication costs were higher for MM patients than TAVR patients (these costs do not include the procedure cost, which was described in the previous section); the main cost drivers for MM patients were balloon aortic valvuloplasty, AVR, and pacemaker implantation.
In addition to the treatment-specific complications, costs of general re-hospitalizations were also included in our analysis. In contrast to specific complications, these hospitalizations were estimated to occur throughout the entire three year time horizon. While the primary diagnosis of patients in the model is AS, clinical advisor input suggested that the coding for such a patient is more likely to be done on the basis of their symptoms (likely most similar to heart failure) rather than their primary diagnosis (i.e., AS). Therefore, the OCCI cost for heart failure patients over the age of 70 years who did not undergo any procedures during their stay was used ($6664)Citation9. The probability of hospitalization was based on PARTNER data, with the 12-month data extrapolated to 36 monthsCitation3; over the 3-year time horizon, surviving patients were estimated to have been re-hospitalized 2.3-times (TAVR), and 4.1-times (MM), resulting in a cost of $11,017 for a TAVR patient and $17,762 for a MM patient. These costs were only accrued by surviving patients.
Follow-up outpatient and Nursing Home costs
Follow-up outpatient costs included in the model were monitoring, nursing, and home care (). The monitoring frequency of each test and number of physicians’ visits within a year were obtained from clinical advisor input. The unit costs for each of the monitoring tests and physician visits were obtained from the Ontario MoHLTC Schedule of BenefitsCitation11. It was estimated that more MM patients would require home care than TAVR patients (home care consisting of three 1-h visits per week for elderly people who are ambulatory, continent, and can bathe independently). The hourly cost of home care was obtained from a provider in the Greater Toronto Area (personal communication with ComCare, 2010). Monitoring costs were similar for both groups, but nursing and home care was estimated to be more expensive for MM patients.
Table 5. Follow-up outpatient costs: monitoring, nursing, and home care.
The annual probability nursing home care is required has been published for BelgiumCitation12 and FranceCitation13, but literature specific to Canada was not available. As practices regarding admission to long-term care and general cultural norms around caring for aging relatives may be quite different between countries, it was decided to use the Canadian clinical advisors estimates for the model. The MoHLTC provides funding for long-term care homes. Residents are charged a co-payment. The co-payment amount is set by the MoHLTC. The co-payment amount is slightly less for short-term stays than for permanent stays. The relevant costs are presented in .
Medication costs
Costs of medications were not included in the analysis due to lack of appropriate data. It is likely that MM patients have higher medication costs than TAVR patients; however, since medication costs are relatively minor it was expected they would have a minimal effect on the overall conclusions of our analysis.
Analysis
The base case incremental cost-effectiveness ratio (ICER) was calculated by dividing the difference in total cost between the two interventions by the difference in total QALYs gained over the analysis time horizon. To assess the impact of assumptions in key model parameters, a series of deterministic sensitivity analyses were performed. Parameters varied in these analyses included mortality rates for TAVR and MM (±20%), MM utility values (±20%), 30-day TAVR complication rates (varied by Canadian Special Access trial ratesCitation10), cost of re-hospitalization (±50%), TAVR stroke rates (±20%), cost of complications (±50%), MM nursing home rate (±50%), time horizon (12 and 24 months), and TAVR procedure costs (±20%). Further, a probabilistic sensitivity analysis (PSA) was conducted on the base case scenario to assess parameter uncertainty by sampling 1000 iterations of the cost and clinical outcome variables and value ranges tested in the deterministic sensitivity analyses.
Results
Base case analysis
The results for the base case cost-effectiveness analysis are shown in . Over three years, TAVR resulted in 1.325 QALYs per patient, while MM resulted in 0.837 QALYs per patient, an incremental increase of 0.49 QALYs per patient treated with TAVR. At a total cost of $58,357, TAVR was more costly than MM (total cost $42,670) by $15,687. The resultant ICER was $32,170 per QALY gained per patient treated with TAVR vs MM.
Table 6. Base case cost-effectiveness results: TAVR vs MM.
Sensitivity analyses
displays the results of our deterministic sensitivity analyses. The model was most sensitive to the time horizon selected, with the ICER increasing from its base case value of $32,170 per QALY gained for TAVR compared to MM (36 month time horizon) to $52,848 and $157,429, at 24 and 12 month horizons, respectively. The model was also moderately sensitive to the costs of complications, the MM utility, and cost of TAVR procedure with ICERs for TAVR vs MM ranging from $16,197 per QALY gained to $48,983 per QALY gained. With the exception of time horizon, there was no sensitivity analysis where the ICER exceeded $50,000 per QALY gained.
Table 7. Variables and ranges used in deterministic sensitivity analyses with results.
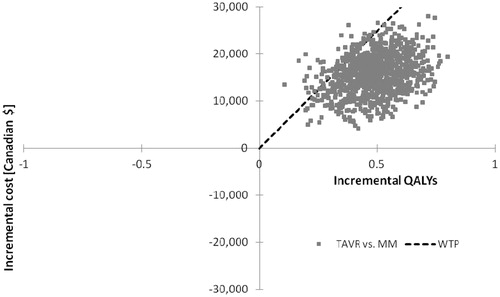
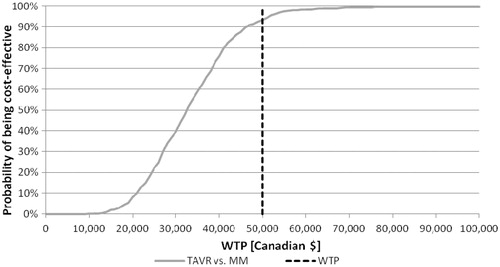
The individual results of 1000 probabilistic iterations showed 93.2% of all results fell below the $50,000 per incremental cost per QALY gained willingness-to-pay threshold (). Similarly, the cost-effectiveness acceptability curve of TAVR vs MM indicates nearly all iterations resulted in ICERs between $20,000–$50,000 per quality-adjusted life year gained ().
Discussion
This analysis determined that, in Canada, TAVR would be considered cost-effective compared to MM in surgically inoperable patients with severe AS at generally accepted cost-effectiveness thresholds (ICER $32,170). Some jurisdictions set an acceptable cost per QALY threshold; if the additional cost per QALY gained is below the threshold, it is deemed to be cost-effective. Canadian decision-making agencies have not set an explicit cost per QALY threshold, although generally interventions in the range of CDN$20,000–$100,000 are considered to provide reasonable value for moneyCitation14.
A strength of this model is that the key clinical outcomes were based on the PARTNER randomized controlled trial which are consistent with previous observational studies that have shown that TAVR patients experience better health outcomes than patients who are medically managedCitation15,Citation16. Encouragingly, the findings of the PARTNER trial are generally in agreement with the Canadian experience with TAVR to dateCitation10.
US, UK, and Belgian economic evaluations of TAVR also based on the PARTNER Cohort B trial data have been publishedCitation7,Citation17,Citation18. Similar findings to the current model were reported. Both the US and UK analyses found that TAVR represents good value for money at standard cost-effectiveness acceptability thresholds in surgically inoperable patients with severe AS, while the ICER reported in the Belgian analysis was slightly less favourable (base case ∼45,000 Euros/QALY gained) but below the 50,000 Euros/QALY cost-effectiveness threshold. In the analysis from the UK National Health System (NHS) perspective, the ICER was £16,000/QALY gained over a 10-year time horizonCitation18. Under a 2-year time horizon, the ICER was below £30,000, generally considered the cost-effectiveness threshold in the UK, and similar to the 24-month ICER generated by the current modelCitation18. We did not extend the time horizon of our analysis past our base case value of 36 months, although, based on the results of the longer-term UK analysis as well as the trend observed in our results when moving from a time horizon of 12 months to 24 months to 36 months, we would expect the ICER to continue to become more favourable as the time horizon extends.
In Canada, an institutional health technology assessment (HTA) of TAVR at McGill University Medical Centre has been conducted, using data from their experience treating 11 TAVR patientsCitation19. They estimated that each TAVR procedure costs ∼$26,000 (with the valve itself costing $20,000), and that this cost would be offset by a reduction in resource use due to untreated AS of ∼$2000 per patient (due to a decrease in frequency of hospitalizations and length of stay), leading to an overall cost to the hospital of $24,000 per TAVR case. Health outcomes were not available against which to weigh this additional cost; however, the authors acknowledged that the prognosis for patients with severe AS who receive no treatment is extremely poorCitation19.
It is reasonable to assume that, with time, as physicians become more familiar with the TAVR procedure, associated outcomes may further improve. Data demonstrate that TAVR mortality rates have decreased over time as clinicians have gained experienceCitation20,Citation21. Moreover, the ongoing development of new generations of delivery systems (e.g., smaller size delivery catheters) and newer generation aortic valves are likely to further improve procedure-related morbidity and mortality. Particularly, it is thought that rates of vascular complications may improve over time.
A limitation of this analysis was the lack of available Canadian-specific cost data for some variables. As TAVR is still relatively new, procedure costs and follow-up data are limited in Canada. The Ontario physician fee schedule currently does not have a specific code for TAVR, while the fee in British Columbia is $1125Citation22. Resource use for cost items such as nursing home and outpatient care have not been studied in Canada, and model estimates were, therefore, based on expert opinion. It was found, however, in our sensitivity analyses that varying the costs of the TAVR procedure and complications did not alter the overall conclusions of our analysis.
Another limitation of the current model is that it did not consider the costs of evaluating and imaging patients to assess eligibility for TAVR since our analysis was specific to patients already identified as being eligible for treatment. The McGill HTA found that, of the first 73 patients deemed eligible for the procedure, only nine had appropriate femoral vasculatureCitation19. In a study of 85 patients considered for TAVR, only 38 (45%) were deemed eligible based on echocardiographic, angiographic, or clinical criteriaCitation23. Similarly, another study reported that, of 98 patients with severe symptomatic AS judged inoperable by open surgery, 45 (46%) met the criteria for TAVRCitation24. The costs associated with processing patients who are ultimately ineligible for TAVR were not included.
Conclusions
It is well acknowledged that elderly patients with severe AS have a very poor prognosis. Any intervention, therefore, that improves patient outcomes is likely to appear clinically attractive. In this analysis, transcatheter aortic valve replacement via transfemoral access in inoperable severe AS patients was found to be cost-effective compared to medical management in patients not suitable for surgical valve replacement. Although the current model was limited by available cost and resource use data, the findings were robust when tested in sensitivity analyses.
Transparency
Declaration of funding
This study was funded by Edwards LifeSciences. Co-authors from Edwards LifeSciences reviewed and provided comment on the draft versions of the manuscript and also approved the final version.
Declaration of financial/other relationships
RH-H is an employee of OptumInsight, an independent contract research organization. OptumInsight was funded by Edwards LifeSciences to adapt the model, conduct analyses, and provide editorial support for the manuscript. RH-H did not receive individual payment from Edwards LifeSciences for her involvement in this study. CF, JC, and JW received funding from Edwards LifeSciences to advise on the clinical aspects of this project. AT is an employee of Edwards LifeSciences. KB is an employee of Outcomes International. Outcomes International is an independent contract research organization and was funded by Edwards LifeSciences to design and develop the model. KB did not receive individual payment from Edwards LifeSciences for his involvement in this study
Acknowledgments
We would like to thank Shawn Barry and Jennifer Haig of OptumInsight, and Brian Heyland of Edwards LifeSciences for their guidance and support in the design and conduct of this analysis.
References
- Canadian Coordinating Office for Health Technology Assessment (CCOHTA). Emerging technologies list: percutaneous heart valve replacement. Ottawa: Government of Canada, 2005. Report No.: 28.
- Lester SJ, Heilbron B, Gin K, et al. The natural history and rate of progression of aortic stenosis. Chest 1998;113:1109-14
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-607
- Reynolds MR, Magnuson EA, Lei Y, et al. Health-related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 2011;124:1964-72
- Canadian Agency for Drugs and Technologies in Health. Guidelines for the economic evaluation of health technologies: Canada. 3rd edn. Ottawa: Government of Canada, 2006.
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696-704
- Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis. Results from the Placement of Aortic Transcatheter Valves (PARTNER) trial (Cohort B). Circulation 2012;125:1102-9
- Statistics Canada. The Consumer Price Index. Ottawa: Government of Canada, 2010. Report No.: 62-001-X, vol. 89, no. 3.
- Ontario Case Costing Initiative. Ontario guide to case costing. Province of Ontario, Canada: Ministry of Health and Long-Term Care, 2010.
- Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080-90
- Ontario Ministry of Health and Long-Term Care. Schedule of Benefits: Physician Services under the Health Insurance Act. Ontario: 2010. http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html. Accessed July 1, 2008.
- Kolh P, Kerzmann A, Honore C, et al. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg 2007;31:600-6
- Maillet JM, Somme D, Hennel E, et al. Frailty after aortic valve replacement (AVR) in octogenarians. Arch Gerontol Geriatr 2009;48:391-6
- Laupacis A, Feeny D, Detsky AS, et al. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ 1992;146:473-81
- Dewey TM, Brown DL, Das TS, et al. High-risk patients referred for transcatheter aortic valve implantation: management and outcomes. Ann Thorac Surg 2008;86:1450-6
- Descoutures F, Himbert D, Lepage L, et al. Contemporary surgical or percutaneous management of severe aortic stenosis in the elderly. Eur Heart J 2008;29:1410-7
- Neyt M, Van Brabandt H, Devriese S, et al. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2012;2:e001032
- Watt M, Mealing S, Eaton J, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart 2012;98:370-6
- McGregor M, Esfandiari S. Transcatheter Aortic Valve Implantation (TAVI) at the MUHC: a Health Technology Assessment. Montreal: McGill University Health Centre, 2009. Report No.: 45
- Thomas M, Schymik G, Walther T, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards Sapien Valve. Circulation 2010;122:62-9
- Wendt D, Eggebrecht H, Kahlert P, et al. [Experience and learning curve with transapical aortic valve implantation]. Herz 2009;34:388-97
- British Columbia Ministry of Health, Medical Services Commission. Physician payment schedule, cardiology. British Columbia: Ministry of Health, 2011
- Rajani R, Buxton W, Haworth P, et al. Prognostic benefit of transcatheter aortic valve implantation compared with medical therapy in patients with inoperable aortic stenosis. Catheter Cardiovasc Interv 2010;75:112-6
- Saia F, Marrozzini C, Dall'ara G, et al. How many patients with severe symptomatic aortic stenosis excluded for cardiac surgery are eligible for transcatheter heart valve implantation? J Cardiovasc Med (Hagerstown) 2010;11:727-32