Abstract
Objective:
This study compared healthcare resource usage and costs before and after initiating LAI antipsychotics among Medicaid-insured schizophrenia patients.
Methods:
Schizophrenia patients ≥13 years of age initiating LAI antipsychotics were identified from the Thomson Reuters MarketScan® Research Medicaid database between 7/1/2005 and 6/30/2010. Patients were required to have 6 months of continuous medical/prescription drug coverage prior to LAI initiation (baseline period) and during a variable follow-up period. Annualized healthcare resource usage and costs for the baseline and follow-up periods were determined and compared.
Results:
Among 5694 eligible patients, 55% were male and 45% were female, and the majority of the population was between the ages of 18–55 (86%). The study population had low general comorbidity, as assessed by the Charlson Comorbidity Index (CCI). Diabetes (17%) and chronic pulmonary disease (14%) were the most prevalent comorbidities. In comparison to the baseline period, during the follow-up period (mean duration = 25.7 months) the mean number of hospitalizations, all cause (1.52 ± 2.41 vs 0.70 ± 1.61, p < 0.001) and schizophrenia-related (1.21 ± 2.04 vs 0.57 ± 1.41, p < 0.001) declined as well as hospital lengths of stay (all cause: 14.77 ± 28.61 vs 5.75 ± 16.26 days, p < 0.001; schizophrenia-related: 12.39 ± 25.86 vs 4.67 ± 13.54 days, p < 0.001). As a result, annualized hospital payments were much lower (all cause: $16,249 ± $36,404 vs $7380 ± $21,087, p < 0.001; schizophrenia-related: $13,388 ± $31,614 vs $5645 ± $15,767, p < 0.001).
Limitations:
This study attempted to minimize the impact of differences in patient characteristics by having patients serve as their own controls in the before vs after comparison, however one still may not be able to account for all confounders in this non-randomized study population.
Conclusion:
For patients with schizophrenia who initiate LAI antipsychotic therapy, there is an improvement in disease management based on fewer hospitalizations for relapses, which is also associated with a marked reduction in healthcare costs.
Introduction
In the US, ∼2 million individuals have schizophrenia, a chronic, disabling disease with large economic, healthcare, and societal burdensCitation1. Approximately 25% of hospital bed days in the US are for the care of patients with schizophrenia, significantly contributing to it being the second most costly-to-treat illness, the first being Alzheimer’s diseaseCitation1. One of the greatest challenges for treating patients with schizophrenia is maintaining the continuity of antipsychotic therapy. Adherence to antipsychotic therapy can considerably improve symptom severity, reducing the risk of relapse and hospitalizationCitation2. However, non-adherence to antipsychotic therapy is widespread, with a meta-analysis of 10 studies reporting a mean non-adherence rate of 41.2%Citation3. Partial non-adherence is even more prevalent, affecting nine out of 10 patients with schizophreniaCitation4.
Long-acting injectable (LAI) formulations of antipsychotics are another option for treating patients with schizophrenia who are taking antipsychotic therapy. Patients treated with LAI antipsychotic medications must visit clinics to receive treatment every 2–6 weeks. The benefits of LAI antipsychotic therapy are that it eliminates the daily need for taking oral antipsychotics and provides a tool to monitor antipsychotic administration and an opportunity for intervention, and thereby supports the therapeutic alliance between patients/caregivers and healthcare professionalsCitation5. Some studies have shown that schizophrenia relapses, hospitalizations, and inpatient care costs decline after patients begin treatment with LAI antipsychoticsCitation6–8; however, these results have contrasted with others in which there was increased or no change in healthcare resource usage after switching to LAI antipsychoticsCitation9,Citation10.
LAI antipsychotic therapy is most frequently prescribed to patients with schizophrenia who are frequently non-adherent, and have greater illness severity and comorbidity, and results of studies evaluating the association of their usage with patient outcomes are often confounded by patient selection bias. Additionally, the process of switching to other antipsychotic medication is itself associated with increased healthcare resource usage when compared to continuing treatment with the same medicationCitation11. There is a need for further studies examining outcomes of patients with schizophrenia after beginning treatment with LAI antipsychotics to gain a more comprehensive understanding of their impact on disease management. In the US, Medicaid insures the greatest population of patients with severe mental illness; however, patients with schizophrenia insured by Medicaid are less well studied than those insured commercially or by Medicare. This study compared healthcare resource usage and costs and schizophrenia relapse rate before and after initiating LAI antipsychotic medications among US Medicaid insured patients with schizophrenia.
Methods
Study design
This was a retrospective study of Medicaid-insured patients with schizophrenia in which healthcare resource usage and costs and schizophrenia hospitalization rates were compared before and after the initiation of LAI antipsychotic therapy.
Study population
Patients with schizophrenia who initiated the use of LAI antipsychotics were identified from the Thomson Reuters MarketScan® Medicaid research claims database between July 1, 2005 and June 30, 2010. The claims data includes inpatient and outpatient information, fully integrated health and productivity data, and laboratory data, reflecting treatment patterns and costs in routine clinical practice for Medicaid beneficiaries. The database facilitates longitudinal studies by providing integrated standardized data for patients spanning extensive time periods. In compliance with the Health Insurance Portability and Accountability Act of 1996 (HIPAA), the database consists of fully de-identified data sets, with synthetic identifiers applied to patient-level and provider-level data to protect the identities of both the patients and data contributors. Therefore, this study is exempt from Institutional Review Board overview under the Common Rule (45 CFR §46.101(b)(4))Citation12.
The date at which LAI antipsychotic treatment was initiated was defined as the index event with the associated date as the index date. Inclusion in the study population required that patients were ≥13 years of age at the year of index date, that they had at least one inpatient or two outpatient visits on separate dates with a primary or secondary diagnosis of schizophrenia, identified by ICD-9-CM code 295.X prior to the index event (baseline period), and that they had 6 months of continuous medical and prescription drug coverage prior to the index event. After the index event patients were followed for a variable length of time (follow-up period), which ended when the patient disenrolled from Medicaid coverage, or at the end of the study period, whichever was earlier.
Baseline measurements
Demographics, consisting of age, age group, gender, race, health plan type, and index LAI antipsychotic medication and clinical characteristics, consisting of Deyo-Charlson Comorbidity Index (CCI), used to measure the burden of comorbidities among patientsCitation13, frequencies of comorbid conditions, baseline oral antipsychotic usage, and some additional baseline medication usage, were evaluated for schizophrenia patients included in the study population during the 6 month baseline period.
Outcome measurements
Hospital-related resource usage, including the number of hospitalizations and total length of stay (LOS) and outpatient claims (all, office visits, emergency room (ER), community mental health clinic (CMHC), and pharmacy) and associated costs were evaluated during the baseline and follow-up periods and compared to one another. All inpatient and outpatient healthcare resource usage were further stratified into healthcare usage for any reason and that which was schizophrenia-related. All such resource usage and cost measurements were annualized to reflect such measurements on a basis of 12 months.
Statistical analyses
Descriptive statistics were used to evaluate differences in patient demographics and clinical characteristics, with p-values provided by chi-square test and t-test when appropriate. Univariate statistics were also used to evaluate the differences in healthcare resource usage and associated costs before and after initiation of LAI antipsychotic therapy by t-test. Multiple comparison adjustment was not applied. A p-value of 0.05 was used to determine the level of statistical significance. All statistical analyses were carried out using SAS 9.2.
Results
Study population
A total of 5694 schizophrenia patients within the Medicaid study population met the inclusion criteria.
Patient demographics
All evaluated demographics for the Medicaid insured study population are reported in .
Table 1. Baseline demographics of the Medicaid study population.
The majority of patients initiating LAI antipsychotics were between the ages of 18–55 (86.00%) and had comprehensive coverage (66.79%). A greater proportion of patients were male (54.64%) and more were Black (53.13%). Of the study population, 14.47% initiated fluphenazine decanoate, 41.11% haloperidol decanoate, and 44.42% long-acting risperidone.
Patient clinical characteristics
All evaluated clinical characteristics for the Medicaid insured study population are reported in . The majority of patients (67.02%) with schizophrenia had low general comorbidity, as indicated by a CCI score of 0. The most common comorbid conditions were diabetes (16.84%) and chronic pulmonary disease (13.65%). Prior to initiating LAI antipsychotics the most frequent oral antipsychotic prescriptions among the study population were for risperidone (44.63%), quetiapine (25.43%), and haloperidol (21.22%). Among the study population, 46.40% were receiving anticonvulsants, 46.61% took antidepressants, and 31.19% took analgesics/antipyretics.
Table 2. Baseline clinical characteristics of the Medicaid study population.
Healthcare resource usage before and after initiating LAI antipsychotics
contains the mean ± standard deviation number of hospitalizations, LOS, outpatient claims, and their associated total and health plan payments, all cause and schizophrenia-related before and after initiating LAI antipsychotics and their differences. The mean duration of the follow-up period was 25.7 months. In comparison to before initiating LAI antipsychotics, during the follow-up period the mean number of hospitalizations per patient per year for any cause (1.52 ± 2.41 vs 0.70 ± 1.61, p < 0.001) and schizophrenia relapses (1.21 ± 2.04 vs 0.57 ± 1.41, p < 0.001) were less. The mean time spent in the hospital per patient per year was also less during the follow-up period for any cause (14.77 ± 28.61 vs 5.75 ± 16.26 days, p < 0.001) and schizophrenia relapses (12.39 ± 25.86 vs 4.67 ± 13.54 days, p < 0.001). As a result, annualized mean total hospital payments were much lower per patient per year (all cause: $16,249 ± $36,404 vs $7380 ± $21,087, p < 0.001; schizophrenia-related: $13,388 ± $31,614 vs $5645 ± $15,767, p < 0.001) after initiation of LAI antipsychotic therapy.
Table 3. Healthcare resource usage and costs, any cause, and schizophrenia-related for the Medicaid study population before and after initiation of LAI antipsychotics.
During the follow-up period the mean total number of outpatient claims made for any cause was similar to that before beginning treatment with LAI antipsychotics, except that the mean number of ER claims declined by 4.74, as well as ER total and health plan payments, but usage of a CMHC for any cause was greater. The mean total number of schizophrenia-related outpatient claims (44.88 ± 67.13 vs 49.68 ± 66.28, p < 0.001), as well as office visits (8.11 ± 27.99 vs 9.71 ± 28.57, p = 0.003) and CMHC claims (15.22 ± 43.83 vs 19.16 ± 44.68, p < 0.001) increased during the follow-up period, as did their costs, relative to the baseline period; however, schizophrenia-related ER visits declined (3.48 ± 10.99 vs 2.16 ± 7.31, p < 0.001), as did their cost. In comparison to the baseline period, the mean number of pharmacy claims made for any drugs (44.89 ± 49.71 vs 51.11 ± 45.11, p < 0.001) increased, as did overall total pharmacy payments ($4571 ± $5371 vs $6336 ± $5906, p < 0.001) during the follow-up period. The majority of pharmacy claims and associated costs in the follow-up period were attributed to LAI antipsychotic medication.
Differences in total, inpatient, outpatient, and pharmacy payments before vs after initiation of LAI antipsychotics
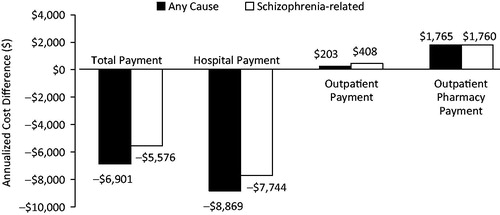
shows the cost differences of total, inpatient, outpatient, and pharmacy payments before vs after the initiation of LAI antipsychotics. In comparison to the baseline period, for any cause mean inpatient payment decreased by $6901 (p < 0.001), mean hospitalization payment decreased by $8869 (p < 0.001), and mean pharmacy payment increased by $1765 (p < 0.001) during the follow-up period. There was a non-significant increase in mean outpatient payment for any cause ($203, p = 0.379). In comparison to the baseline period, schizophrenia-related inpatient payment decreased by $5576 (p < 0.001), hospitalization payment decreased by $7744 (p < 0.001), outpatient payment increased by $408 (p < 0.001), and pharmacy payment increased by $1760 (p < 0.001) during the follow-up period.
Discussion
This study, involving more than 5000 Medicaid insured patients with schizophrenia, shows that after beginning treatment with LAI antipsychotic medications patients have less schizophrenia relapses and are hospitalized less, have a shorter length of stay, and incur less healthcare costs in comparison to before LAI antipsychotic treatment was tried. Also, our results show that the vast majority of healthcare resource usage among Medicaid beneficiaries with schizophrenia is schizophrenia-related. Furthermore, they show that, after beginning treatment with LAI antipsychotics, schizophrenia patients used community mental health centers and visited healthcare providers more often, suggesting that they were more frequently monitored and that there were more opportunities for intervention and to build the therapeutic alliance among patients/caregivers and staff.
Although LAI antipsychotic medications were introduced to routine clinical practice decades ago, they remain under-utilized, especially among Medicaid-insured patients. A US Department of Health and Human Services study conducted on Medicaid beneficiaries with schizophrenia and bipolar disorder, with patient data extracted in 2007, reported that the proportion of Medicaid-insured patients with schizophrenia who were administered LAI antipsychotic medications was only 10%Citation14. This DHHS study also reported that the continuity of medication for those insured by Medicaid was poor in many states and highly variable (High: 72% vs Low: 35%)Citation14.
There is a significant need for improvement of the management of schizophrenia in order to reduce the burden on patients, their families, society, and the healthcare system. Patients with schizophrenia who experience symptom remission have marked improvements in social functioning and quality-of-lifeCitation15. However, symptom remission is rare, with a large US, 3-year prospective, observational study reporting that only 10% of patients with schizophrenia had a sustained favorable outcome as predicted by symptom severity, level of functioning, and use of acute care servicesCitation16. Many studies conducted in the real-world setting have demonstrated that, when patients with schizophrenia are treated with LAI antipsychotics, in particular risperidone, adherence improves, relapses are reduced and healthcare resource use is lessCitation6–8,Citation17. Our study provides further evidence that LAI antipsychotics are a good alternative treatment option for patients with schizophrenia, independent of whether or not they have problems with treatment adherence.
Limitations
While our study yields evidence that usage of LAI antipsychotics improves outcomes of Medicaid beneficiaries with schizophrenia, there are some limitations. First, the LAI antipsychotics evaluated in our study included both typical and atypical LAI antipsychotics, and their efficacy, safety, and outcomes may differ and further study is needed to evaluate outcomes of patients with schizophrenia treated with particular LAI antipsychotics. Also, the usage of CMHCs may have been under-estimated as the designation of a CMHC depends on data submitted by the facility and services provided by CMHC affiliated physicians at other non-CMHC facilities would not have been recorded as associated with CMHC. Additionally, the MarketsScan® Medicaid database consists of claims submitted by healthcare providers to the federal government for reimbursement on behalf of individuals who are covered by Medicaid, and such claims are subject to possible coding errors, coding for the purpose of rule-out rather than actual disease, and under-coding, either by the healthcare provider or due to limitations imposed by the database. It is also difficult to obtain complete medical histories based on Medicaid claims data. Changes in Medicaid eligibility status, disenrollment, and the likelihood of multiple insurance plans for some individuals may have also confounded the results of this study. In addition, the MarketScan® claims Medicaid database is based on a large convenience sample and, as the sample is not random, it may contain biases or fail to generalize well to other populations, particularly those who have alternate healthcare coverage, such as those who are commercially insured. Lastly, patients who have schizophrenia often seek care erratically and across multiple settings, feel alienated and distrustful of the medical community, and are aware of the social stigma of schizophrenia, making it a challenge to study treatment patterns and outcomes among this population. Many of these same characteristics of this patient population in addition to Medicaid funding cutbacks increase the difficulty of providing quality healthcare to persons with schizophrenia covered by Medicaid
Conclusions
Based on the results of this study, Medicaid insured patients with schizophrenia who begin treatment with LAI antipsychotic therapy have better disease management afterward vs before and, as a consequence, Medicaid incurs less cost for their care.
Transparency
Declaration of funding
This research and preparation of this manuscript were supported by Otsuka America Pharmaceutical, Inc. and H. Lundbeck A/S.
Declaration of financial/other relationships
RB is on the speakers bureau for Astra Zeneca, Forest, Novartis, and Sunovion. SO, DZ, and GL are employees of Otsuka America Pharmaceutical, Inc. JL is an employee of Novosys Health, which has received financial funds from Otsuka America Pharmaceutical, Inc. in connection with conducting this study and development of this manuscript. CK is a paid consultant for Otsuka America Pharmaceutical, Inc.
Acknowledgments
We would like to acknowledge Melissa Lingohr-Smith from Novosys Health in the editorial support and review of this manuscript.
References
- New York State. Office of Mental Health. 2005-2009 Statewide Comprehensive Plan for Mental Health Service Services. Chapter 4: basic and clinical research. Albany, New York: New York Sate Office of Mental Health. 2012. http://www.omh.ny.gov/omhweb/statewideplan/2005/chapter4.htm. Accessed August 27, 2012
- West JC, Marcus SC, Wilk J, et al. Use of depot antipsychotic medications for medication nonadherence in schizophrenia. Schizophr Bull 2008;34:995-1001
- Lacro JP, Dunn LB, Dolder CR, et al. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry 2002;63:892-909
- Lambert T, Olivares JM, Peuskens J, et al. Effectiveness of injectable risperidone long-acting therapy for schizophrenia: data from the US, Spain, Australia, and Belgium. Ann Gen Psychiatry 2011;10:10
- Olfson M, Marcus SC, Ascher-Svanum H. Treatment of schizophrenia with long-acting fluphenazine, haloperidol, or risperidone. Schizophr Bull 2007;33:1379-87
- Fuller M, Shermock K, Russo P, et al. Hospitalisation and resource utilisation in patients with schizophrenia following initiation of risperidone long-acting therapy in the Veterans Affairs Healthcare System. J Med Econ 2009;12:317-24
- Niaz OS, Haddad PM. Thirty-five months experience of risperidone long-acting injection in a UK psychiatric service including a mirror-image analysis of in-patient care. Acta Psychiatr Scand 2007;116:36-46
- Peng X, Ascher-Svanum H, Faries D, et al. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res 2011;3:9-14
- Young CL, Taylor DM. Health resource utilization associated with switching to risperidone long-acting injection. Acta Psychiatr Scand 2006;114:14-20
- Taylor D, Fischetti C, Sparshatt A, et al. Risperidone long-acting injection: a 6-year mirror-image study of healthcare resource use. Acta Psychiatr Scand 2009;120:97-101
- Noordsy DL, Phillips GA, Ball DE, et al. Antipsychotic adherence, switching, and health care service utilization among Medicaid recipients with schizophrenia. Patient Prefer Adherence 2010;4:263-71
- US Department of Health and Human Services. Code of Federal Regulations. Human Subjects Research (45 CFR 46), Washington D.C.: US Department of Health and Human Services. 2009
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83
- Brown, JD, Barrett A, Ireys H, et al. Evidence-based practices for Medicaid Beneficiaries with schizophrenia and bipolar disorder. Washington D.C.: U.S. Department of Health and Human Services, 2012. http://aspe.hhs.gov/daltcp/reports/2012/ebpsbd.shtml. Accessed August 28, 2012
- Brissos S, Dias VV, Balanzá-Martinez V, et al. Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr Res 2011;129:133-6
- Cuyun Carter GB, Milton DR, Ascher-Svanum H, et al. Sustained favorable long-term outcome in the treatment of schizophrenia: a 3-year prospective observational study. BMC Psychiatry 2011;11:143
- Macfadden W, DeSouza C, Crivera C, et al. Assessment of effectiveness measures in patients with schizophrenia initiated on risperidone long-acting therapy: the SOURCE study results. BMC Psychiatry 2011;11:167