Abstract
Objective:
Adenosine, dipyridamole, and regadenoson are pharmacologic stress agents used in myocardial perfusion imaging (MPI), to diagnose and monitor coronary artery disease. Clinical studies suggest that regadenoson has pharmacologic properties that simplify the MPI procedure through availability to a wider range of patients and easier administrative requirements. This study assesses the operational advantages and laboratory efficiency associated with the use of regadenoson compared to adenosine and dipyridamole.
Methods:
A web-based survey of 141 nuclear medicine technologists working in US-based cardiovascular imaging laboratories from June–July 2009.
Main outcomes measures:
Descriptive statistics measured the adenosine, dipyridamole, and regadenoson cohorts. Bivariate analyses compared the overall and staff-specific time to conduct an MPI test. The site-specific sub-groups were defined by hospital vs non-hospital setting, hours of operation, number of SPECT cameras, and number of full-time equivalent staff, including nurses, nuclear technologists, physicians, and nurse practitioners/physician assistants.
Results:
The total time to conduct an MPI test was shortest with regadenoson 156 (46) min compared to adenosine and dipyridamole 182 (63) and 191 (61) min, respectively. Time from regadenoson administration to the start of the imaging session, including dose calculation and infusion time, was 14.2 min less than adenosine, and 12.0 min less than dipyridamole. The time to manage adverse events was shortest if it occurred with regadenoson compared to adenosine and dipyridamole, with minor exceptions. Due to the nature of survey implementation, possible recall bias may limit the results. Some differences in procedures times may be attributable to differences in laboratories’ protocols.
Conclusions:
Overall time savings and time savings stratified by operational ability (number of staff, number of SPECT cameras, hours of operation) translate to a more efficient utilization of laboratory resources when using regadenoson compared to adenosine and dipyridamole. Regadenoson is the most efficient pharmacologic stress agent compared to adenosine and dipyridamole.
Introduction
Heart disease remains the leading cause of death for both women and men in the USCitation1–3. Coronary artery disease (CAD) is the most common type of heart disease, accounting for ∼68.3% of all heart disease deaths in 2005Citation4. The lifetime risk of developing CAD after 40 years of age is 32% for women and 49% for menCitation5. As a high-risk disease with broad prevalence, CAD imposes an enormous economic burden on the US healthcare system. In 2009, heart disease was projected to cost more than $304.6 billion, including healthcare services, medications, and lost productivityCitation4. Between 2010 and 2030, total direct medical costs of heart disease are projected to triple to $818 billion (USD 2008)Citation6. The 5- and 10-year cumulative costs for CAD patients are $71.5 billion and $126.6 billion, respectively, compared to CAD-free patients’ 5- and 10-year cumulative costs of $9.2 billion and $16.6 billion, respectivelyCitation7. Thus, early detection and vigilant monitoring of CAD is vital in preventing the progression of heart disease, thereby resulting in potential cost savings to the healthcare system.
Cardiac stress testing is the primary method of identifying clinically significant CAD. Non-invasive imaging technologies, such as myocardial perfusion imaging (MPI), play an important role in the diagnosis and monitoring of CAD in elective and acute settingsCitation8. MPI uses either physical exercise or pharmacologic vasodilator stress to induce maximum myocardial hyperemia. Pharmacologic stress testing is used to evaluate the cause of signs/symptoms, or perceived risk from CAD in patients who cannot exercise sufficiently to perform an adequate diagnostic or prognostic exercise test. Currently, vasodilator stress imaging accounts for ∼50% of all stress MPI testsCitation9.
Adenosine and dipyridamole have historically been two of the most common pharmacologic stress agents used in single-photon emission computed tomography (SPECT) MPI; however, several logistical and clinical issues associated with adenosine and dipyridamole complicates the testing procedure. First, dosage and administration are difficult. Due to their short half-lives (10 s for adenosineCitation10 and 3–12 min for dipyridamoleCitation11), adenosine and dipyridamole are administered as continuous infusions. Adenosine is administered as a continuous peripheral intravenous infusion. The recommend intravenous dose for adults is 140 mcg/kg/min infused for six minutes, for a total dose of 0.84 mg/kgCitation12. Dipridamole is administered according to the weight of the patient. The recommended dose is 0.142 mg/kg/min infused over 4 min. Prior to intravenous administration, the solution should be diluted with at least a 1:2 ratio with sodium chloride injection, and thallium-201 should be injected within 5 min following the 4-min infusion of dipridamoleCitation11. Testing is often delayed due to high risk of dosing errors.
Second, adenosine stimulates adenosine A2a receptors on the arteriolar vascular smooth muscle cells and dipyridamole blocks re-uptake of adenosine into platelets, red blood cells, and endothelial cells, inadvertently leading to increased extracellular concentrations of adenosine receptorsCitation9. For both agents, the non-selective activation process results in frequent chest pain and dyspnea, and a less frequent, but more serious, bronchospasm and high-grade atrioventricular (AV) blockCitation13,Citation14. The clinical utility of adenosine is further limited by patients who experience hypotension as a result of adenosine-induced vasodilation in the peripheral circulation systemCitation15. Due to these complications, the protocols for adenosine and dipyridamole require the co-ordination of stress infusion with radiotracer injection while monitoring for potential side-effectsCitation16.
Third, testing is often delayed due to caffeine consumptionCitation9. Methylxanthines (e.g. caffeine and theophylline) are non-specific adenosine receptor antagonists that interfere with the vasodilation activity of pharmacologic stress agents. Caffeine and other theophylline-like drugs, such as aminophylline, inhibit the effects of adenosine and dipyridamole. Patients receiving these agents should not consume caffeine for 24–48 h before testing and should be safely withdrawn from β-blocker treatment before the study, if possibleCitation17,Citation18. The effect is not as severe with regadenoson, and patients are asked to avoid caffeine only 12 h prior to testingCitation15.
Regadenoson, a newer adenosine derivative, has enhanced pharmacologic properties that simplify the MPI procedure through availability to a wider range of patients and easier administrative requirements. Regadenoson binds to the A 2A receptor that opens the ATP-dependent potassium channels leading to hyperpolarization in the coronary vascular smooth muscleCitation19. It produces maximal hyperemia and maintains it for an optimal duration that is practical for an MPI test. Regadenoson is administered as a rapid, single, fixed-dose intravenous injection of 5 mL, followed immediately with a 5 mL saline flushCitation15. The administration is independent of patient weight, is as efficacious as adenosine regardless of weight, and is efficacious in detecting ischemia regardless of body mass index, age, gender, and diabetesCitation20,Citation21. This results in less time spent calculating the dosage and monitoring side-effects during the testing procedure. While this study cannot generate a hypothesis on the magnitude of hyperemic response of the pharmacologic agents, this study can confirm preliminary clinical data that suggested regadenoson offers adequate hyperemic response with more quickly managed adverse events (AE) compared to adenosine and dipyridamoleCitation20,Citation21. In pre-clinical studies of regadenoson, AV conduction abnormalities and bronchospasm were not observedCitation22–24 and only mild effects were observed on blood pressureCitation21,Citation25–27. Regadenoson patients also experienced a significantly lower mean summed score of chest pain, dyspnea, and flushingCitation20,Citation21. Administering regadenoson has potentially beneficial clinical and cost implications; with rapid infusion, there is no need for the constant infusion associated with adenosine or dipyridamole or the associated infusion pumps, microbore tubing, and large-volume syringesCitation28.
Given the importance of and reliance upon MPI as a diagnostic and prognostic tool for CAD, the primary study objective was to evaluate the difference in time, laboratory characteristics, and staffing requirements while conducting an MPI test, including time to manage AEs, with regadenoson compared to adenosine and dipyridamole. The secondary study objective was to explore the association between the laboratory characteristics and the number of weekly MPIs that are administered to determine operational advantages of regadenoson, adenosine, or dipyridamole. Additional objectives included examining operational patterns (i.e., test cancellations and re-scheduling patterns), potential for additional tests per week, and staff resource requirements, such as time spent preparing or assisting the patient, as related to an ideal stress agent.
Methods
A standard web-based survey was developed to assess operational tasks and staffing requirements for conducting MPI tests using adenosine, dipyridamole, and regadenoson. The survey was administered by Directions Research, Inc. from June to July 2009. Eligible participants included nuclear medicine technologists who were working in a US-based cardiovascular imaging laboratory and involved in MPI.
Study sample and data sources
The targeted sample size was 120–150 respondents, and eligible participants were invited to participate via email. Access to all information was protected with the individual participant’s username and password. After the web-based survey was sent to the nuclear medicine technologists, the laboratories were contacted for response. Once the required number of respondents was reached, the survey was discontinued. Only one survey was sent to each laboratory and only one respondent completed the survey.
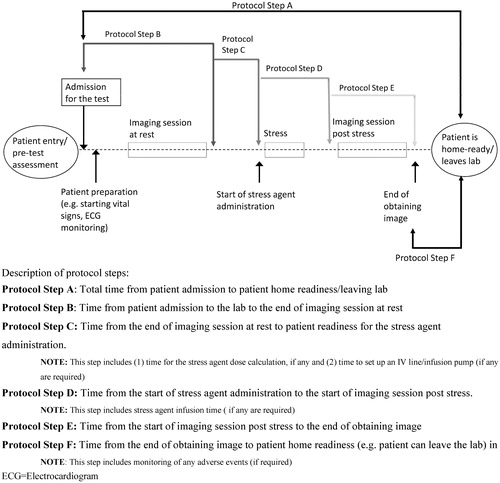
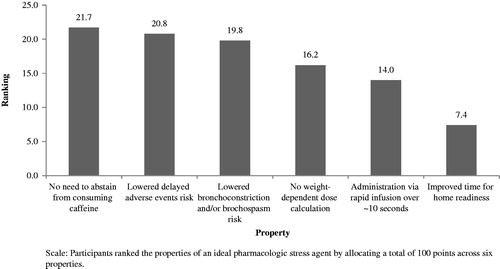
As part of the general laboratory profile, the survey collected data on the setting (hospital [academic or teaching] vs non-hospital), hours of operation (<40/week vs ≥40/week), number of SPECT cameras (1 vs ≥2), number of full-time equivalent (FTE) staff including nurses (0 vs 1 vs ≥2), number of FTE for nuclear technologists (1 vs 2 vs ≥3), number of FTE for physicians (0 vs 1 vs ≥2), and number of FTE for nurse practitioners/physician assistants (0 vs ≥1). Survey participants were asked to indicate the overall time and time for each laboratory staff member required to complete major MPI protocol steps, as depicted in . It was assumed that the MPI was conducted as a 1-day procedure, and a standard protocol was sent to all sites allowing for different response timeframes by site. Survey participants indicated the time necessary to treat and monitor the stress-agent-associated AEs that would delay the patient’s home readiness. The list of included adverse reactions does not include all AEs observed during use of the agents and is limited to those events for which the FDA listed as the most common adverse reaction to regadenoson in the labelCitation10,Citation11,Citation15. Participants were also queried on test cancellation and re-scheduling patterns to ascertain and compare patterns associated with the consumption of caffeine. Lastly, participants ranked the properties of an ideal pharmacologic stress agent by allocating a total of 100 points across six items (no weight-dependent dose calculation, administration via rapid infusion, no need to abstain from caffeine, lowered risk for bronchoconstriction, and/or bronchospasm, improved time for home readiness, lowered risk for delayed adverse events).
Data analysis
Descriptive statistical measures were generated for three agent-specific study cohorts corresponding to adenosine, dipyridamole, and regadenoson. Each cohort was defined as reporting at least one weekly use of the corresponding stress agent for MPI tests. The patients in the cohorts were mutually exclusive because each patient at each laboratory could not use more than one of the three study agents at one point in time. A series of bivariate analyses were generated to compare the overall and staff-specific time required to conduct an MPI test with each of the stress agents across different sites. The site-specific sub-group analyses were based on the setting, hours of operation, number of SPECT cameras, and number of FTE staff including nurses, nuclear technologists, physicians, and nurse practitioners/physician assistants. The group with the shortest total test time was selected as the reference group. The mean differences of test time between the other two agents and the reference group were then stratified by MPI protocol steps, number of cameras, operation hours per week, and number of staff involved. A comparison of time to manage stress-agent-related AEs was made between the other two agents and the reference group. The number of additional tests with regadenoson (increase in patient throughput) was calculated as a function of time to conduct one test with the use of regadenoson and either of the corresponding comparators (adenosine or dipyridamole) and the number of tests that laboratory can conduct on a weekly basis with the corresponding comparator. This calculation assumes a direct proportional relationship between the time per test and the number of weekly tests, e.g. shorter time per test corresponds to conducting more of such tests per week.
Regression analysis was conducted to explore the association between laboratory profile characteristics and the number of pharmacologic stress tests one laboratory can conduct on a weekly basis. Adjusted mean number of weekly tests for each of sub-group was calculated. 95% confidence intervals (CI) were estimated and statistical significance was p < 0.05. All analyses were conducted using SAS 9.2 software (SAS Institute, Cary, NC). Responses from the open-ended questions regarding the use of potentially saved time were reviewed. Similar responses were grouped into categories, with the indication of the frequency of each category recorded.
Results
Sample characteristics
A total of 141 distinct cardiovascular imaging laboratories located across the US completed the survey. The participants’ laboratory characteristics are presented in . The majority of the laboratory sites were located within a hospital or affiliated with a nearby hospital followed by non-hospital-affiliated diagnostic imaging centres. Of the 141 laboratories, 135 reported using SPECT cameras and conducted an average of 37 (28) MPI tests weekly, of which over half (56%) were performed using pharmacologic stress induction. Of the 135 respondents with a SPECT camera, 73 (54.1%) sites reported using adenosine, 48 (35.6%) use dipyridamole, and 62 (45.9%) use regadenoson in MPI testing. Across all laboratories, 69% utilized only one stress agent (28% adenosine, 20% regadenoson, and 21% dipyridamole), 27% reported using two agents, and 4% utilized all three stress agents.
Table 1. Laboratories’ profile characteristics.
Pharmacologic MPI Test Time Duration
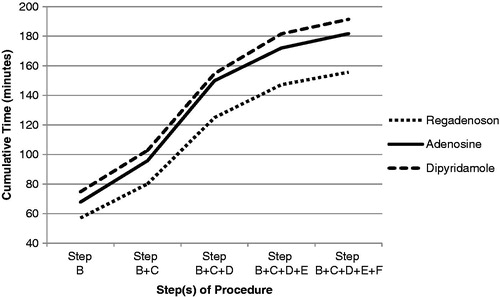
The total time to conduct an MPI test was shortest with regadenoson (156 [46] min) compared to adenosine and dipyridamole (182 [63] and 191 [61] min, respectively). The mean difference of total MPI test time for regadenoson compared to adenosine and regadenoson compared to dipyridamole, stratified by the number of cameras, operation hours per week, and number of staff is demonstrated in and . The mean duration of the pharmacologic tests (Step A in and and ) was longer with adenosine and dipyridamole when compared to regadenoson.
Table 2. Comparison of total time from patient admission to patient home readiness/leaving lab (Step A) between stress agents—regadenoson and adenosine.
Table 3. Comparison of total time from patient admission to patient home readiness/leaving lab (Step A) between stress agents—regadenoson and dipyridamole.
Table 4. Comparison in time (min) for each MPI protocol step between stress agents—regadenoson and adenosine.
Table 5. Comparison in time (min) for each MPI protocol step between stress agents—regadenoson and dipyridamole.
Sub-group analyses between regadenoson and adenosine show that, among laboratories with no more than 40 weekly hours of operation, the mean test time using regadenoson was shorter than those using adenosine. The mean test time for regadenoson was shorter than adenosine depending on the number of nurses, physicians, or nurse practitioners. The largest difference was seen among the nuclear technologist stratification; on average, the test duration was shorter when only one nuclear technologist was present when using regadenoson when compared to the nuclear technologist test duration for adenosine.
Differences observed between the regadenoson group and dipyridamole group were more obvious. Among sites with three or more cameras, the total test time with regadenoson was an hour shorter than dipyridamole. Stratifying by staff utilization, regadenoson saved the most time among sites with two nurses, one nuclear technologist, two physicians and no nurse practitioner, compared to dipyridamole. Total time decreased when two technologists, compared to one, were present ().
Pharmacologic MPI test time and staffing requirements
The trend of pharmacologic MPI test time and staffing requirements was similar for most of the individual protocol steps outlined in ; however, the steps at which more time was saved with regadenoson compared to the other two agents occurred during protocol Steps B (time from patient admission to the end of imaging at rest) and D (time from the start of stress agent administration to the start of imaging session post stress) ( and and ). Step B (admission for the test including explanatory process of the imaging test and possible occurrence and treatment of adverse events) and Step D (stress agent administration) were shorter during testing with regadenoson compared to adenosine and dipyridamole ( and ).
Figure 2. Average cumulative time (min) spent directly conducting one MPI test per step by tests using regadenoson, adenosine, and dipyridamole.
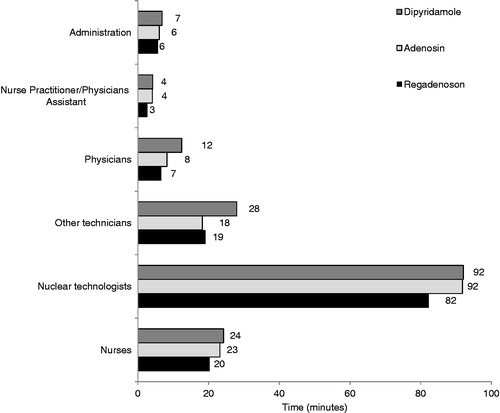
Nuclear technologists spent the most time directly conducting MPI tests compared to other laboratory personnel, and the findings were similar among all three stress agents. When examining the time spent by nuclear technologists by agent, however, regadenoson required the least amount of time per test compared to adenosine and dipyridamole (). Additionally, time that physicians were directly involved in conducting an MPI test (e.g. time spent with direct patient contact) was 12 min for dipyridamole, 8 min for adenosine, and 7 min for regadenoson. Time savings with regadenoson compared to other agents was also achieved for some of the other laboratory personnel ().
Time to manage adverse events (AEs)
The time spent in the management of AEs was shortest with regadenoson compared to both adenosine and dipyridamole and longest with dipyridamole compared to both adenosine and regadenoson for the majority of the AEs, with minor exceptions ( and ).
Table 6. Time (in min) per patient, per event to manage the reported stress agent-related adverse events (AEs) between regadenoson and adenosine patients.
Table 7. Time (in min) per patient, per event to manage stress agent-related adverse events (AEs) between regadenoson and dipyridamole patients.
Association of laboratory characteristics and weekly number of tests
Based on the multivariate analysis, the adjusted mean number of weekly MPI tests ranged from 16–31 across all laboratory characteristic sub-groups compared to an average of 21 (±19) tests/week estimated for the entire sample. The number of weekly MPI tests was significantly associated with the number of nurses and SPECT cameras (). Laboratories with ≥2 nurses or ≥2 SPECT cameras conducted significantly more tests that laborites with ≤1 nurse or ≤SPECT camera. Other laboratory characteristics were not significantly associated with the number of weekly tests.
Table 8. Estimated number of weekly MPI tests based on laboratory characteristics.
MPI test patterns
On average, most of the scheduled pharmacologic tests were completed as scheduled with a slightly higher completion rate with regadenoson compared to the other agents. Of all tests that were cancelled (3–5% across all agents), the test cancellation rate due to various events was generally similar across the three agents. Events that were assessed included cancellation due to not abstaining from caffeine consumption (regadenoson: 29%, dipyridamole: 34%, adenosine: 35%), having bronchoconstrictive or bronchospastic disease without active wheezing (regadenoson: 2%, dipyridamole: 4%, adenosine: 5%), smoking on the day of test (regadenoson: 2%, dipyridamole: 1%, adenosine: 1%), not abstaining from theophylline or dipyridamole medications (regadenoson: 1%, dipyridamole: 3%, adenosine: 1%), active wheezing (regadenoson: 8%, dipyridamole: 7%, adenosine: 6%), or systolic blood pressure <90 mmHG and/or heart rate <40 bpm (regadenoson: 4%, dipyridamole: 5%, adenosine: 3%).
Time savings and ideal stress agent
The time saving per test needed for respondents to switch to a different stress agent was 23.0 (15.1) min. The respondents also answered, in an open-ended response, what other tasks would be performed due to the time savings from using a different pharmacologic agent. Most nuclear medicine technologist respondents (80%) suggested that they would conduct other types of tests, process results, or complete paper work, and 25% mentioned conducting quality control procedures. About 33% of the nurse respondents reported that they would attend to other patient care activities, 25% would assist with other tests, and 12.5% would process paperwork or make patient phone calls. Among the other technologist respondents, 50% would conduct other types of tests, or process results.
Participants’ responses to the properties of an ideal pharmacologic stress agent are presented in . The most important property was no need for patients to abstain from consuming caffeine, followed by lowered risk for delayed AEs, and lowered risk for bronchoconstriction and/or bronchospasm. The lowest-ranked property was the improvement in time to home readiness.
Discussion
This study assessed operational tasks and staffing requirements for conducting MPI tests using adenosine, dipyridamole, and regadenoson. Previous clinical studies have suggested that regadenoson may be an efficient pharmacologic stress agent; however, this survey provided data from nuclear technicians to examine the actual time difference and staff requirements to conduct an MPI test, and found that regadenoson holds a time-saving and operational advantage over adenosine and dipyridamole.
Laboratories that are equipped with two or more SPECT cameras, employ two or more nurses (compared to zero or one) or two or more physicians (compared to zero or one), and operate no more than 40 h per week, benefit the most from the time saving per test perspective (i.e. laboratories with these operational characteristics were able to conduct an MPI test in less time). Specifically, laboratories with two or more nurses are equipped to complete twice as many tests per week compared to only one nurse and laboratories with two or more SPECT cameras can complete more tests per week after controlling for lab setting, hours of operation, and number of physicians on staff. Additionally, time that physicians were directly involved in conducting an MPI test was reduced by ∼2 h/week or 157 h/year with regadenoson compared to dipyridamole, resulting in higher cost savings compared to other laboratory personnel.
Previous studies examining laboratory staff and time allocation have reported that laboratory physicians spend the majority of their time in direct clinical work, administration, and documentationCitation29,Citation30. A time-and-motion study reported that if laboratory staff experience a decrease in time spent during a laboratory process, often administration, it is allocated towards direct patient care, allowing the laboratory staff to streamline the clinical testing process and expedite patient flowCitation31. Respondents from this survey also indicated this would allow for more time spent with patients and the opportunity for technologists to perform additional diagnostic tests.
One of the principle benefits of regadenoson is the dosing efficiency. Regadenoson administration is independent of patient weight, and is as efficacious in detecting ischemia regardless of weight, body mass index age, gender, and diabetes, unlike adenosineCitation21. The steps at which more time was saved with regadenoson compared to the other two agents occurred during protocol Step D, the time from the start of stress agent administration to the start of imaging session post-stress. Step D took ∼10 min less during testing with regadenoson compared to adenosine and 7.2 min less during testing with regadenoson compared to dipridamole. This time savings is most likely attributable to less time spent by the laboratory staff on dose adjustment. While the administration of regadenoson is attributed with direct time savings, the management of most AEs while using regadenoson also saves on time.
While this study cannot generate a hypothesis on the magnitude of hyperemic response of the pharmacologic agents, this study can confirm preliminary clinical data that suggested regadenoson offers an adequate hyperemic response with more quickly managed AEs compared to adenosine and dipyridamoleCitation20,Citation21. The effect of regadenoson on the pulmonary function of subjects with stable asthma or COPD is not clinically different from placebo with respect to the percentage of subjects experiencing a greater than 15% decrease in FEV1 up to the 24-h visitCitation32, and most pulmonary adverse reactions were resolved without therapyCitation24. In this study, the time to manage bronchospasm was 6.6 min less when using regadenoson as compared to adenosine, and 6.3 min less when using regadenoson than dipyridamole. Clinical studies also reported that regadenoson patients experienced a significantly lower mean summed score of chest painCitation20,Citation21. This study observed that the time to manage chest pain with regadenoson was 3.97 min less compared to adenosine and 9.65 min less compared to dipyridamole.
Study limitations
This study is subject to several limitations. Due to the nature of survey implementation, possible recall bias may limit the results. The times collected in the survey are not self-reported by staff members; they are reported by nuclear technologists on behalf of the staff. Therefore, the results are not based on any records of time. The average length of test time in our study ranged from 151–192 min from the admission of the test to the end of obtaining the image, so it is possible that patients experience AEs after the end-point of the survey testing procedure and, hence, may not be captured. Data from this study indicate that step B takes 10.73 min longer for adenosine than regadenoson patients and 17.63 min longer for dipyridamole than regadenoson patients. This is most likely attributable to differences in survey sites’ protocols and procedures including the explanatory process of the imaging test, operational ability, and patient characteristics (i.e. patients using adenosine or dipyridamole experience more adverse events than patients using regadenoson which may require additional explanation during Step B). Future consideration for a study is to evaluate the associations between lab characteristics and number of MPIs per week.
Conclusion
Despite these limitations, this study presents the operational advantages of regadenoson by showing the time efficiency and quality of operation during the MPI testing procedure. The survey suggests that overall time savings and time savings stratified by operational ability (number of staff, number of SPECT cameras, and hours of operation) translate to a more efficient utilization of laboratory resource use when using regadenoson compared to adenosine and dipyridamole. Due to fewer dosing adjustments, ease of administration, and less time to manage AEs, regadenoson is the most time-efficient pharmacologic stress agent option compared to adenosine and dipyridamole.
Transparency
Declaration of funding
This research was supported by Astellas Pharma US, Inc.
Declaration of financial/other relationships
James Spalding is an employee, and Smita Kothari is a former employee, of Astellas Pharma US, Inc. Michelle Friedman, You Wu, Elyse Gatt, and Luke Boulanger are employees of United BioSource Corporation, which has received research funds from Astellas Pharma US, Inc. to conduct this study. JME peer reviewers on this manuscript have no relevant financial relationships to disclose.
Acknowledgements
The authors would like to extend thanks to Kelly Lamothe and Svetlana Denevich from United BioSource Corporation for their valuable assistance in formulating this study as well as preparation of the manuscript.
References
- Greenland P, Alpert JS, Beller GA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2010;122:2748-64
- Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women–2011 update: a guideline from the american heart association. Circulation 2011;123:1243-62
- Kung HC, Hoyert DL, Xu J, et al. Deaths: final data for 2005. Natl Vital Stat Rep 2008;56:1-120
- Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation 2012;125:188-97
- Lloyd-Jones DM, Larson MG, Beiser A, et al. Lifetime risk of developing coronary heart disease. Lancet 1999;353:89-92
- Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation 2011;123:933-44
- Russell MW, Huse DM, Drowns S, et al. Direct medical costs of coronary artery disease in the United States. Am J Cardiol 1998;81:1110-5
- Vesely MR, Dilsizian V. Nuclear cardiac stress testing in the era of molecular medicine. J Nucl Med 2008;49:399-413
- Botvinick EH. Current methods of pharmacologic stress testing and the potential advantages of new agents. J Nucl Med Technol 2009;37:14-25
- Adenosine (adenosine) injection (Drug Label) Manufactured by Gland Pharma, Hyderabad, India. Revised 06/2007. DailyMed, U.S. National Library of Medicine, 2007. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=ab2edabd-e57d-4754-9822-93bd17af9e88. Accessed 2012
- Dipyridamole (dipridamole) solution. (Drug Label) Manufactured by Anazeo Health Corporation, Tampa, FL. Revised 05/2012. DailyMed, U.S. National Library of Medicine, 2012. http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=c76c3c6f-c3e0-4e35-ab01-5468585e0d9d. Accessed 2012
- Henzlova M, Cerqueira M, Taillefer R, et al. ASNC Imaging Guidelines For Nuclear Cardiology Procedures. J Nucl Cardiol 2009;16:331
- Cerqueira MD, Verani MS, Schwaiger M, et al. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol 1994;23:384-9
- Ranhosky A, Kempthorne-Rawson J. The safety of intravenous dipyridamole thallium myocardial perfusion imaging. Intravenous Dipyridamole Thallium Imaging Study Group. Circulation 1990;81:1205-9
- Lexiscan (regadenoson) injection (Drug Label). Manufactured by Astellas Pharma US, Inc. Revised 07/2012. DailyMed, U.S. National Library of Medicine, 2012. http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=75982. Accessed 2012
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, et al; Quality Assurance Committee of the American Society of Nuclear C. Stress protocols and tracers. J Nucl Cardiol 2006;13:e80-90
- Lapeyre AC 3rd, Goraya TY, Johnston DL, et al. The impact of caffeine on vasodilator stress perfusion studies. J Nucl Cardiol 2004;11:506-11
- Verani MS. Adenosine thallium 201 myocardial perfusion scintigraphy. Am Heart J 1991;122:269-78; discussion 302–6
- Bengalorkar GM, Bhuvana K, Sarala N, et al. Regadenoson. J Postgrad Med 2012;58:140-6
- Cerqueira MD, Nguyen P, Staehr P, et al; Investigators A-MT. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective A2A agonist regadenoson versus adenosine in myocardial perfusion imaging integrated ADVANCE-MPI trial results. JACC Cardiovasc Imag 2008;1:307-16
- Iskandrian AE, Bateman TM, Belardinelli L, et al. Adenosine versus regadenoson comparative evaluation in myocardial perfusion imaging: results of the ADVANCE phase 3 multicenter international trial. J Nucl Cardiol 2007;14:645-58
- Buhr C, Gossl M, Erbel R, et al. Regadenoson in the detection of coronary artery disease. Vasc Health Risk Manag 2008;4:337-40
- Leaker BR, O'Connor B, Hansel TT, et al. Safety of regadenoson, an adenosine A2A receptor agonist for myocardial perfusion imaging, in mild asthma and moderate asthma patients: a randomized, double-blind, placebo-controlled trial. J Nucl Cardiol 2008;15:329-36
- Thomas GS, Tammelin BR, Schiffman GL, et al. Safety of regadenoson, a selective adenosine A2A agonist, in patients with chronic obstructive pulmonary disease: a randomized, double-blind, placebo-controlled trial (RegCOPD trial). J Nucl Cardiol 2008;15:319-28
- Gordi T, Blackburn B, Lieu H. Regadenoson pharmacokinetics and tolerability in subjects with impaired renal function. J Clin Pharmacol 2007;47:825-33
- Hendel RC, Bateman TM, Cerqueira MD, et al. Initial clinical experience with regadenoson, a novel selective A2A agonist for pharmacologic stress single-photon emission computed tomography myocardial perfusion imaging. J Am Coll Cardiol 2005;46:2069-75
- Lexiscan Product Monograph. In. Deerfield, IL: Astellas Pharma US Inc,
- Johnson SG, Peters S. Advances in pharmacologic stress agents: focus on regadenoson. J Nucl Med Technol 2010;38:163-71
- Trotter MJ, Larsen ET, Tait N, et al. Time study of clinical and nonclinical workload in pathology and laboratory medicine. Am J Clin Pathol 2009;131:759-67
- Weigl M, Muller A, Zupanc A, et al. Participant observation of time allocation, direct patient contact and simultaneous activities in hospital physicians. BMC Health Serv Res 2009;9:110
- Banner L, Olney CM. Automated clinical documentation: does it allow nurses more time for patient care? Comput Inform Nurs 2009;27:75-81
- Prenner BM, Bukofzer S, Behm S, et al. A randomized, double-blind, placebo-controlled study assessing the safety and tolerability of regadenoson in subjects with asthma or chronic obstructive pulmonary disease. J Nucl Cardiol 2012;19:681-92