Introduction
Until recently, patients with severe aortic stenosis have had only one truly effective treatment option to address their life-threatening condition: surgical aortic valve replacement (AVR). With over 60 years of experience, cardiac surgical teams worldwide have made AVR highly successful and relatively safe for most patients. Unfortunately, aortic stenosis is by and large a disease of the elderly, and a non-trivial fraction of patients with severe aortic stenosis have either declined or not been offered AVR due to anatomical or clinical factors resulting in a markedly unfavorable balance of risk and benefitCitation1. Mortality in this group approximates 4% per month, and there is no medical therapy that can reduce this risk.
The development of systems permitting aortic valve replacement with a catheter device inserted through a peripheral artery or directly into the heart or aorta via a small thoracic incision (transcatheter aortic valve replacement, TAVR) without the use of cardiopulmonary bypass or opening the heart has introduced great new possibilities in the treatment of aortic stenosis. First, some patients unable to withstand open-heart surgery who would otherwise die from aortic stenosis can now be treated successfully. Second, in patients considered surgical candidates, TAVR offers the potential for faster and easier recovery and the avoidance of certain common surgical complications, albeit at the potential expense of a higher risk of other complications with the catheter-based approach.
Early registry experience with TAVR, mainly in Europe and Canada, suggested that 30-day mortality rates could be similar to or less than predicted surgical mortality in extremely high risk patients, despite the fact that the majority of the patients included were deemed inoperable by experienced surgeons, often for reasons not captured in the surgical risk scoresCitation2–4. Nonetheless, prior to approving any TAVR device in the US, the Food and Drug Administration (FDA) required a randomized trial of TAVR in inoperable patients in order to compare the risks and benefits of both the Edwards Sapien valve system and the clinical strategy itself with the outcomes of a control population whose valves were not replaced. It was clear before beginning the trial that the outcomes of the control group would be poor. It should also be noted that surgical aortic valve replacement was developed and instituted with little other than observational trials and with a risk that would preclude acceptance in today’s environment. Now, six decades later, it is consider standard live saving technology that is accepted worldwide. The PARTNER trial represents a singular scientific advance in the treatment of aortic valve disease because of its prospective randomized design and execution, and deserves careful consideration.
The results of ‘cohort B’ of the PARTNER trial, designed to answer those questions, were remarkableCitation5. In a patient population marked by advanced age and a high burden of co-morbid illness, TAVR resulted in a 20% absolute difference in 12-month survival. Further follow-up of this cohort has shown continued divergence in survival, with a 25% absolute difference at 2 yearsCitation6. It would be difficult, in the modern clinical literature, to find a larger treatment effect from any intervention for any disease state, and no such study exists for standard surgical aortic valve replacement.
TAVR has entered clinical practice in a milieu of worldwide economic fragility and escalating concerns about the overall costs of healthcare. In this era, it is incumbent on clinicians and the developers of new health technologies alike to scrutinize the value and the potential economic consequences of their interventions. It is, thus, no surprise that TAVR has been the subject of a burgeoning number of health technology assessments and health economic analyses. The current issue of the Journal of Medical Economics contains a new cost-effectiveness analysis for TAVR in inoperable patients from a Canadian perspectiveCitation7, as well as an unsolicited editorial from a group in Belgium that has also previously published on this topicCitation8.
The new paper from Hancock-Howard et al.Citation7 is no less than the sixth published report in which the cost-effectiveness of TAVR has been estimated for inoperable patients ()Citation9–13. The appropriate estimation of cost-effectiveness in this patient population presents some unique methodological challenges, as previously reviewedCitation14. Although the studies shown in were done with different country contexts and varied somewhat in their methods and key assumptions, the base case estimates—including from Neyt et al.Citation11—are all very similar. In all of these studies, TAVR was found to increase total healthcare costs and to increase life expectancy for the population by ∼1–2 years, resulting in cost-effectiveness ratios generally in the range of $20,000–$60,000 per life year (or QALY) gained. The results from Hancock-Howard et al.Citation7 fall directly in this range. It is worth mentioning that TAVR in this population not only extends survival, but also substantially improves quality-of-lifeCitation15.
Table 1. Cost Effectiveness Analyses of TAVR for Non-surgical Candidates.
Whether a health intervention is ‘cost-effective’ is a question that depends on a health system’s ability and willingness to pay for health benefits. Generally speaking, cost-effectiveness ratios in the range of those reported in are usually viewed as acceptable (or in some places, borderline) for most developed nations, and in our judgment are well within the range of many commonly accepted therapies in cardiovascular and non-cardiovascular medicine. To deny coverage and reimbursement for TAVR in truly inoperable patients with a reasonable chance of receiving benefit from it would be medical rationing of the worst kind.
Despite the consistency of the findings on this matter, Neyt and colleaguesCitation8,Citation16,Citation17 have found it necessary to repeatedly question the evidence base from which most of these cost-effectiveness studies have been derived, most importantly, the PARTNER trial. We believe a rebuttal to some of their major points is in order.
Neyt and Van Brabandt have stated that the clinical benefit observed in cohort B of the PARTNER trial may have been over-estimated due to imbalances in several baseline characteristics, which made the standard therapy control group sicker than the TAVR group. However, data published in the original clinical trial publicationCitation5 and presented to the FDACitation18 show no evidence that the very large benefit attributed to TAVR in the trial was significantly altered after adjustment for baseline patient characteristics. First, in the published manuscript, no sub-groups were identified with less than a 15% absolute reduction in 12-month all-cause mortality, and there were no statistically significant interaction terms found for any baseline variableCitation5. As presented to the FDA, an optimally fit multivariable Cox proportional hazards model, which adjusted for the 10 variables most strongly associated with 12-month survival, resulted in an adjusted hazard ratio for TAVR of 0.56 (95% CI = 0.410–0.756, p < 0.001)—almost indistinguishable from the unadjusted Kaplan-Meier hazard ratio of 0.55 reported in the academic publicationCitation5. The facts simply do not support the assertion that the treatment effect of TAVR was significantly over-estimated based on differences between the randomized treatment groups.
Neyt and Van Brabandt have also claimed that the PARTNER cohort B trial results over-stated the benefit of TAVR because different results were seen in a separte cohort of patients randomized between TAVR and standard therapy as part of a continued access program. This is based on a lack of understanding of what the randomized continued access program was and was not meant to be.
The PARTNER trial was conducted under an investigational device exemption (IDE) filed with the FDA. As with all major IDE trials, both the inoperable (cohort B) and high-risk operable (cohort A) portions of the program were initiated with detailed enrollment criteria and a fixed sample size and analysis plan, all of which were pre-specified and vetted by the FDA. It must be remembered that, prior to FDA approval of the SAPIEN device, patients with severe aortic stenosis who were deemed inoperable by their physicians had no access to TAVR in the US outside the trial, even though TAVR became available commercially in parts of Europe and in a Canadian compassionate use program. In early 2009, when the target enrollment for cohort B was completed, it was anticipated that commercial availability of TAVR in the US would have to wait another 2 years or more, since the trial’s minimum follow-up duration was 12 months.
In order to provide ongoing access to TAVR for patients facing a fatal illness as completion and a regulatory review of the PARTNER trial was taking place, the PARTNER sites were permitted to treat patients in a continued access program. However, since cohort B completed its enrollment prior to cohort A, for the first several months, ‘inoperable’ patients entered into the continued access program were randomized between TAVR and standard non-surgical therapy. This was neither an extension of the randomized portion of cohort B nor a separately planned randomized trial. Rather, it was simply the beginning of the continued access program, which was randomized for a short period of time only because patients were still being screened at the same sites for participation in cohort A, and there was a desire to avoid biasing the assessments of cohort A candidates by creating an enrollment mechanism (non-randomized continued access) that would ‘guarantee’ access to TAVR. As soon as cohort A completed its enrollment, the continued access program became fully non-randomized.
The randomized continued access portion of the PARTNER program thus had no scientifically-based enrollment target, and the data were never meant to be pooled with the ‘PMA’ (pre-market application) cohort. The randomized continued access cohort was small—about 25% the size of the PMA cohort—and, given its small size, no comparative testing of outcomes between groups was ever planned or conducted. While Neyt and Van Brabandt have correctly noted that 12-month mortality was higher in TAVR patients than control patients in this small cohort, they have not commented on the biggest difference in outcomes between this group and the PMA cohort, which was the fact that standard therapy group mortality at 12 months was more than 50% lower in the continued access cohort than the PMA cohort (21.6% vs 50.7%). In contast, 12-month mortality in the TAVR patients (34.3% vs 30.7%) was similar.
In our view, it is inappropriate to continue second-guessing the results of the PARTNER trial based on a small number of cases enrolled under different conditions than the PMA cohort, just as it would be inappropriate to focus in isolation on the results from roll-in patients. At each of the PARTNER sites which had not participated in an earlier pilot study of TAVR, the first two TAVR procedures were proctored by more experienced operators, and were not counted as part of the PMA cohort. As detailed in its FDA report, the outcomes for these 22 roll-in cases were actually better than for the trial as a whole: 30-day and 12-month mortality were, respectively, 0% and 14.3%Citation18. By Neyt and Van Brabandt’s logic, failure to incorporate the outcomes of these patients—who were enrolled and treated as part of the same trial program—under-estimates the benefit of TAVR in this patient population.
TAVR is a complex and demanding procedure with an already documented learning curveCitation19, and we agree that the dispersion of this therapy must be managed responsibly. This view is shared by all of the pertinent US professional societies as well as the US Medicare program, who have together formulated clear guidance on the types of facilities at which TAVR procedures should be performedCitation20,Citation21. Adherence to these guidelines is required for US reimbursement. Key principles of this policy include the evaluation of TAVR candidates by experienced multi-disciplinary heart teams, the maintenance of adequate procedural volumes, and an ongoing commitment to continuous quality improvement and scrutiny of patient outcomes through participation in a national registry (coverage with evidence development).
We believe that adoption of these principles, as well as rapid iterative improvements in the technology itself, will lead to improved, rather than worsened outcomes over time. Supporting this are preliminary data from the non-randomized portion of the PARTNER continued access program. For both the tranfemoral and transapical procedures in this program, with operable and inoperable patients combined, 12-month mortality and stroke rates were lower than those reported in the randomized portion of cohort A, which had better outcomes than cohort BCitation22,Citation23. Registries from around the world too numerous to review here have shown steady improvements in outcomes over time.
Although not the subject of the paper by Hancock-Howard et al.Citation7, Neyt et al.Citation11 have additionally commented on their virtual certainty that TAVR is not cost-effective relative to surgical AVR for high risk surgical candidates. This is an important issue, as ongoing and future clinical trials are focused on intermediate to high risk surgical candidates, and it is clear that, in parts of Europe, TAVR has already been adopted to varying extents in such patients.
Neyt et al.’sCitation24 comments on this patient group are based on their analysis of ‘real world’ cost data from Belgium, which do not agree with randomized, patient-level data collected from cohort A of the PARTNER trialCitation25. What is clear from cohort A of the PARTNER trial, which, like several other ongoing TAVR vs AVR trials, followed a non-inferiority design, is that, so far, there is no evidence of a survival difference between TAVR and AVR in high-risk patients. The benefit of TAVR expressed in health economic studies, therefore, stems from differences in patient-reported quality-of-life. Because these differences persist for less than 6 monthsCitation26, observed differences between TAVR and AVR have been and are likely to remain numerically small, on the order of 0.03–0.06 QALYs. As a consequence, TAVR will not be found to be cost-effective as an alternative to AVR unless it remains close to cost neutral.
In a patient level analysis of directly observed data collected as part of the PARTNER trial, Reynolds et al.Citation26 found that TAVR, on average, increased costs through 12 months by ∼$2000 per patient. As with the quality-of-life outcomesCitation26, an important difference was noted based on the procedural access site: patients eligible for treatment via the transfemoral route had on average a small cost savings relative to AVR (∼$1250 per patient at 12 months), while patients only eligible for TAVR via the trans-apical route had average costs which were significantly higher than their AVR controls (by ∼$9900 per patient).
In their technology assessment, Neyt and Van Brabandt reported 12-month clinical outcomes that were similar to those derived directly from the trial data, but, based on their review of Belgian claims data, conclude that TAVR was ∼€20,000 more expensive than AVR. Their cost estimates were derived from separate, non-randomized patient groups. Estimates of cost for TAVR were taken from the first 183 cases done in the country, presumably for a mixture of clinical indications (likely including both inoperable and high-risk operable patients), almost half of which were done via the trans-apical route. The average logistic Euroscore of this population was 30. They compared the costs in this patient group to the surgical AVR costs in Belgian patients treated from 2004–2007 who were ≥70 years in age (63% of the patient population) and had an illness severity of illness score of 3 or 4 out of 4 (80% of the patient population). In other words, they compared the costs of fairly average patients being treated with surgical AVR to the costs of learning curve TAVR cases being performed on patients who were in all likelihood much more severely ill.
It would be fair to concede that the economic results of a US-based study cannot be readily extrapolated to all countries due to differences in care patterns and cost structures as is noted in the PARTNER manuscript, and we therefore acknowledge the need for country-specific analyses and reporting of ‘real world’ data. It remains for the reader to decide if the early patients selected for TAVR in Belgium were similar to the cohort from whom they derived surgical AVR cost data.
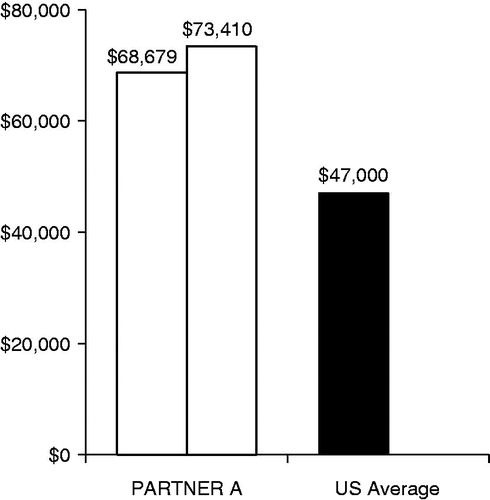
illustrates what might happen if a similar approach were taken in a US cost-effectiveness model of TAVR. The measured costs for the surgically treated patients in cohort A of the PARTNER trial were ∼$70,000 per patientCitation25, whereas, in 2010, the average reimbursement for surgical AVR in the US was ∼$47,000 per patientCitation27. Thus, a model utilizing average or even slighty above average surgical AVR reimbursement would have under-estimated the costs of the PARTNER surgical procedures by as much as $20,000. This under-scores the peril of using non-randomized data for cost inputs in a model where the results are largely driven by incremental costs.
Currently the Corevalve US pivotal IDE trial has been completed and, when analyzed, provides both effectiveness and financial data in great detail for a second TAVR valve system similar to the PARTNER data. These trials represent by far the most detailed examination of effectiveness and cost-effectiveness of treatment of severe symptomatic aortic stenosis ever conducted. Both of these catheter valve systems are 10 years old or less and will clearly undergo progressive improvement. Therefore, in many ways, TAVR remains a field still in the early stages of development. Although remarkable progress has been made in this field in a relatively short period of time, much work remains to fully define TAVR’s role in the treatment of aortic stenosis. Important current uncertainties related to health economics include the relative benefits and costs of TAVR and AVR in intermediate risk surgical candidates, and the outcomes and potential role of non-transfemoral access sites. As the field moves forward, these and other questions will need to be addressed with care and with rigor. Only then can doctors, patients, and policy-makers make well-informed decisions.
Transparency
Declaration of funding
This paper was not funded.
Declaration of financial/other relationships
MJR is a consultant for Medtronics and is a principal investigator for the CoreValve® US Pivotal Trial. MR has received grant support from Edwards Lifesciences, and is a conclustant for Medtronics.
References
- Bach DS, Cimino N, Deeb GM. Unoperated patients with severe aortic stenosis. J Am Coll Cardiol 2007;50:2018–9
- Moat NE, Ludman P, de Belder MA, et al. Long-term outcomes after transcatheter aortic valve implantation in high-risk patients with severe aortic stenosis: the U.K. TAVI (United Kingdom Transcatheter Aortic Valve Implantation) Registry. J Am Coll Cardiol 2011;58:2130–8
- Rodes-Cabau J, Webb JG, Cheung A, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 2010;55:1080–90
- Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation 2007;116:755–63
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597–607
- Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med 2012;366:1696–704
- Hancock-Howard R, Feindel C, Rodes-Cabau J, et al. Transfemoral aortic valve replacement versus medical management in high risk patients with symptomatic aortic valve stenosis: Canadian cost-effectiveness analysis based on the PARTNER trial cohort B findings. J Med Econ (in press)
- Neyt M, Van Brabandt H. TAVI: reveal the truth, the whole truth and nothing but the truth. J Med Econ (in press)
- Doble B, Blackhouse G, Goeree R, et al. Cost-effectiveness of the Edwards SAPIEN transcatheter heart valve compared with standard management and surgical aortic valve replacement in patients with severe symptomatic aortic stenosis: a Canadian perspective. J Thorac Cardiovasc Surg 2012; preprint doi:10.1016/j.jtcvs.2012.06.018. (Published online 16 July 2012.)
- Gada H, Kapadia SR, Tuzcu EM, et al. Markov model for selection of aortic valve replacement versus transcatheter aortic valve implantation (without replacement) in high-risk patients. Am J Cardiol 2012;109:1326–33
- Neyt M, Van Brabandt H, Devriese S, et al. A cost-utility analysis of transcatheter aortic valve implantation in Belgium: focusing on a well-defined and identifiable population. BMJ Open 2012;2. doi:10.1136/bmjopen-2012-001032.
- Reynolds MR, Magnuson EA, Wang K, et al. Cost-effectiveness of transcatheter aortic valve replacement compared with standard care among inoperable patients with severe aortic stenosis: results from the placement of aortic transcatheter valves (PARTNER) trial (Cohort B). Circulation 2012;125:1102–9
- Watt M, Mealing S, Eaton J, et al. Cost-effectiveness of transcatheter aortic valve replacement in patients ineligible for conventional aortic valve replacement. Heart 2012;98:370–6
- Hlatky MA, Simons CT. Cost-effectiveness of transcatheter aortic valve replacement. Circulation 2012;125:1076–7
- Reynolds MR, Magnuson EA, Lei Y, et al. Health related quality of life after transcatheter aortic valve replacement in inoperable patients with severe aortic stenosis. Circulation 2011;124:1964–72
- Neyt M, Van Brabandt H. Cost-effectiveness of transcatheter aortic valve replacement: overoptimistic study results and a call for publication of complete trial results. Heart 2012;98:1031–3
- Van Brabandt H, Neyt M, Hulstaert F. Transcatheter aortic valve implantation (TAVI): risky and costly. BMJ 2012;345:e4710
- U.S. Food and Drug Administration. The Edwards SAPIEN® THV Transcatheter Heart Valve System for patients with severe aortic stenosis who are not candidates for conventional open-heart aortic valve replacement surgery. Briefing document for the Circulatory Systems Device Panel Advisory Committee. http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM262935.pdf. Accessed October 25, 2012
- Alli OO, Booker JD, Lennon RJ, et al Transcatheter aortic valve implantation: assessing the learning curve. JACC. Cardiovasc Interv 2012;5:72–9
- Holmes DR, Jr., Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol 2012;59:1200–54
- Mack MJ, Holmes DR, Jr. Rational dispersion for the introduction of transcatheter valve therapy. JAMA 2011;306:2149–50
- Dewey TM. New data from PARTNER continued access (Non-randomized): transapical outcomes after TAVR. Transcatheter Therapeutics 2012;preprint (abstract presentation, October 22, 2012 (Miami, FL))
- Kodali S. New data from the PARTNER continued access (Non-randomized): Transfemoral outcomes after TAVR. Transcatheter Therapeutics 2012;preprint (abstract presentation October 22, 2012 (Miami, FL))
- Neyt M, Van Brabandt H, Van de Sande S, et al. Transcatheter Aortic Valve Implantation (TAVI): a health technology assessment update. KCE Reports. 2011. p 163. Available at http://www.kce.fgov.be/content/kce-reports. Accessed February 12, 2013
- Reynolds MR, Magnuson EA, Lei Y, et al. Cost effectiveness of transcatheter aortic valve replacement compared with surgical aortic valve replacement in high risk patients with severe aortic stenosis: results from the PARTNER Trial (Cohort A). J Am Coll Cardiol 2012;60:2683--92
- Reynolds MR, Magnuson EA, Wang K, et al. Health-related quality of life after transcatheter or surgical aortic valve replacement in high-risk patients with severe aortic stenosis: results from the PARTNER (Placement of AoRTic TraNscathetER Valve) Trial (Cohort A). J Am Coll Cardiol 2012;60:548–58
- Agency for Healthcare Research and Quality. http://hcupnet.ahrq.gov/. Accessed August 10, 2012