Abstract
Objective:
This time-and-motion study aimed to quantify healthcare personnel time associated with routine anemia-management tasks for maintenance therapy with C.E.R.A. (continuous erythropoietin receptor activator) that treats anemia with once-monthly injections versus other erythropoiesis-stimulating agents (‘Other ESAs’), including shorter-acting ESAs (epoetin alfa, epeotin beta) and darbepoetin alfa.
Methods and design:
This was a non-interventional, observational study where patients were treated for anemia according to individual center practices. Time taken to complete frequent anemia-management tasks for both groups (C.E.R.A. vs. ‘Other ESAs’) was recorded and potential annual time savings per patient and per center following assumed 100% uptake of C.E.R.A. once monthly were estimated.
Results:
For ‘Other ESAs’, the average total time spent per patient per year on frequent anemia management-related tasks ranged from 48 minutes in Spain to 265 minutes in Poland. For C.E.R.A. once monthly, the average total time spent per patient per year ranged from 12 minutes in Spain to 39 minutes in Poland, a reduction in actual time spent of 76–89% versus ‘Other ESAs’. 100% adoption of C.E.R.A. once monthly may result in average annual time savings of 26–553 hours, a reduction of 67–95% depending on center size and frequency distribution of ‘Other ESAs’.
Limitations:
Due to variability in treatment practices between centers (differences in task, description and frequency distribution of ‘Other ESAs’) and the small numbers of centers participating in each country, it is difficult to generalize annual per patient time results to reflect each country. Per center results should be interpreted with caution as they were derived based on specific center sizes that may not reflect typical center sizes in the country.
Conclusions:
Adoption of C.E.R.A. once monthly could offer substantial time savings on frequent anemia management-related tasks versus ‘Other ESAs’; allowing re-allocation of scarce resources to other aspects of patient care.
Introduction
Chronic kidney disease (CKD) is a highly prevalent, complex and progressive conditionCitation1. The cost of treating patients with end-stage renal disease (ESRD) (CKD stage 5 requiring dialysis) is considerable; in England, the cost estimate in 2000 was £600 millionCitation2. As the prevalence of ESRD grows, driven partly by increased life expectancy, treatment costs are expected to rise exponentiallyCitation3. However, healthcare costs are coming under greater scrutiny, and increasing pressure is being placed on dialysis units, clinics and hospitals to shoulder the growing economic burden, without compromising the quality of patient careCitation4,Citation5.
Renal anemia is a common complication of CKD that manifests early on and increases in severity as the disease progressesCitation6. Anemia is associated with reduced quality of life and contributes to increased morbidity and mortality in patients with ESRDCitation7–10. Anemia management is a pivotal aspect of care for patients with ESRD, and erythropoiesis-stimulating agents (ESAs) have long been the mainstay of anemia therapy in patients on dialysis, correcting and maintaining stable hemoglobin (Hb) levels, slowing the rate of decline of renal function, improving patients’ quality of life and lowering the risk of hospitalization and deathCitation11,Citation12. However, a Canadian study highlighted the substantial time taken to prepare and administer these drugsCitation13. Owing to their short half-lives, ESAs such as epoetin alfa and epoetin beta require frequent administration (from three times weekly to once weekly) while darbepoetin alfa can be administered once weekly to once monthly to maintain stable Hb levels within the desired target rangeCitation12. Thus, use of ESAs which require frequent administration may have a significant impact on the workload of renal healthcare teamsCitation5.
MIRCERA (methoxy polyethylene glycol epoetin beta) is a continuous erythropoietin receptor activator (C.E.R.A.), which has a pharmacological profile that allows for extended dosing intervals. Evidence from a number of phase III trials in CKD patients on dialysis demonstrates that C.E.R.A. once monthly is well-tolerated and maintains stable Hb levels within the recommended target range with fewer dose adjustmentsCitation14–18.
Results from a number of previous studiesCitation3,Citation5,Citation19 suggested that adoption of a once-monthly ESA could provide considerable time savings for dialysis centers. In this study, we aimed to quantify the healthcare personnel time associated with routine anemia-management tasks for maintenance therapy with ESAs such as epoetin alfa, epoetin beta and darbepoetin alfa which require frequent administration and C.E.R.A. once monthly in 20 hemodialysis centers across five European countries. The real-life data generated were used to model the potential time savings associated with 100% adoption of C.E.R.A. once monthly in these centers.
Methods
Study design
This was a prospective, observational, non-interventional study conducted in 20 hemodialysis centers across five European countries (France, Germany, Italy, Poland and Spain). All hemodialysis centers participating in the study used C.E.R.A. once monthly to treat some of their ESRD patients who had renal anemia and were able to allocate local staff such as research nurses and students for data collection. In addition, all centers were amenable to external observations and monitoring of frequent anemia-management tasks. All patients were treated according to standard practices at each center, including the concomitant use of intravenous iron, and consequently no exclusion criteria were applied.
The patients with ESRD at each center were treated for anemia with epoetin alfa, epoetin beta, darbepoetin alfa or C.E.R.A once monthly. The purpose of this study was to assess the potential impact of switching patients from their current ESA therapy to C.E.R.A. once monthly in a real-world setting. Within this paper, the group of non-C.E.R.A. drugs are referred to as ‘Other ESAs’, including shorter-acting ESAs (epoetin alfa and epoetin beta) which are administered three times, twice or once weekly, and darbepoetin alfa which is generally administered once or twice weekly or every 2 or 3 weeks, although in some instances this ESA can be administered once monthly. Time-and-motion methodology was used to observe and measure frequent anemia-management tasks and to document the time taken by healthcare staff to perform these tasks. The main end point of the study was time per patient per task, and the composite time for all anemia-related tasks performed during the hemodialysis session. Patient consent was not required, as the intention of the study was to observe healthcare staff rather than patients. All data were anonymized by the study centers in line with the study protocol.
Data collection
To define specific tasks to be observed in this time-and-motion study, interviews were carried out with relevant healthcare staff in each center to identify the frequent and observable tasks involved in anemia management. These were defined as tasks associated with ESA treatment with clear start and end points for which time could be clearly measured and which were not intertwined with hemodialysis-related tasks. For this study, frequent and observable anemia management-related tasks were defined as preparation, distribution and injection of ESAs. Preparation and distribution were performed either per group of patients or for each individual patient, while injection was performed for each individual patient.
In the dialysis centers, time necessary to perform each identified task was collected a defined number of times. For group tasks, a target sample of 20 observations was set for ‘Other ESAs’ and 15–20 for C.E.R.A. once monthly. For individual-patient tasks, the sample size was set at 40 observations for ‘Other ESAs’ and 30–40 for C.E.R.A. once monthly. Target samples for C.E.R.A. once-monthly groups were lower as there were fewer opportunities to observe injections due to the once-monthly administration schedule. All observations were carried out by trained observers using a stopwatch and recorded onto paper case-record forms. No patient demographic data or clinical measures were collected. All data were collected between February 2009 and October 2010.
Two brief questionnaires were also completed by one healthcare staff member at each dialysis center to obtain qualitative information on potential changes in center anemia-management practices and additional potential time savings generated by the adoption of C.E.R.A. once monthly. The first questionnaire was completed by the head nurse or the nephrologist to obtain information on each center’s experiences with C.E.R.A. once monthly. The second questionnaire aimed to capture feedback from the head nurse on potential changes in practices that could occur following the introduction of C.E.R.A. once monthly and to obtain an understanding of where freed time could be allocated.
Statistical analysis
This was a descriptive study of real-world observations for tasks associated with administration of ‘Other ESAs’ and C.E.R.A. once-monthly anemia treatments. Sample sizes as described above for ‘Other ESAs’ and C.E.R.A. once-monthly tasks were not determined by using power calculations, as a priori statistical differences in healthcare practitioner time were not expected between drug treatments.
Descriptive statistics were calculated for each task sample for ‘Other ESAs’ and C.E.R.A. once-monthly groups separately at each center. Summary statistics on time observations included: number of observations, mean, standard deviation, 95% confidence interval around the mean, median, and minimum and maximum. For tasks that were typically conducted for a group of patients (e.g., preparation), time data were adjusted to ‘time per patient per ‘Other ESA’’ or ‘time per patient per C.E.R.A. once monthly’ by dividing task time by the number of patients in the group. As the numbers of patients for group tasks were typically larger for ‘Other ESAs’ than for C.E.R.A. once monthly, a generalized linear model was used to test whether group size was a predictor of time per patient. If group size was found to be a predictor, mean task time after adjustment for group size was used for ‘Other ESAs’ and C.E.R.A. once-monthly samples. Time data were assumed to follow a gamma distribution and were analyzed using Statistical Analysis Software (SAS Institute Inc., Cary, North Carolina, USA).
Average time for each task (preparation, distribution and injection) was summed to obtain an estimate of average total time per hemodialysis session in each center for ‘Other ESAs’ and C.E.R.A. once-monthly groups, respectively. Additionally, mean time for each task was averaged across all centers within a country to yield an estimated country mean time per task, thus giving each center equal weight. Country mean task times were also summed to yield an estimate of average total time per session in the country. Time data were not pooled across countries due to differences in care processes.
For each center, an average administration frequency (defined as ‘number of sessions per patient per year’) of ‘Other ESAs’ and C.E.R.A. once-monthly administration per patient was calculated, based on information obtained from staff interviews prior to the start of data collection; this was used to calculate estimated annual time for anemia management per patient for ‘Other ESAs’ and C.E.R.A. once monthly, respectively.
Considering that the annual time per patient for ‘Other ESAs’ is determined by the average number of ESA sessions, two hypothetical scenarios for drug frequency distribution were carried out to assess the range of potential time savings achieved following a switch to once-monthly C.E.R.A. as follows: all patients in each center receiving shorter-acting ESAs administered three times weekly (e.g., epoetin alfa) and all patients receiving ESAs administered every 2 weeks (e.g., darbepoetin alfa).
Modeling the impact of C.E.R.A. once monthly per center
For each of the 20 dialysis centers in this study, the total number of patients per center (as reported at the time of interview) was used to calculate estimated annual time per center on anemia management with ‘Other ESAs’ and C.E.R.A. once monthly, according to the C.E.R.A. once-monthly uptake level at the time of the interview. The potential annual time savings that could be achieved following 100% adoption of C.E.R.A. once monthly compared with 100% use of ‘Other ESAs’ were modeled, and results were simulated for levels of C.E.R.A. once-monthly uptake ranging from 0% to 100%.
Results
Characteristics of hemodialysis centers
A total of five French (three units based in public hospitals and two based in private clinics), four German (one unit based in a hospital and three independent clinics), five Italian (private clinics), three Polish (hospital-based) and three Spanish dialysis centers (one hospital-based center and two non-hospital ambulatory centers) participated in this time-and-motion study. All participating centers had up to four types of ESA available for the treatment of renal anemia (epoetin alfa, epoetin beta, darbepoetin alfa and C.E.R.A. once monthly).
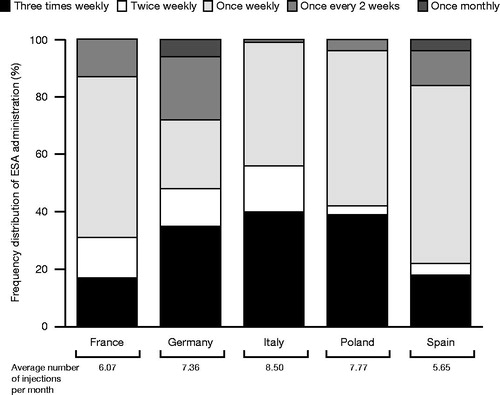
The number of patients with ESRD receiving ESA treatment ranged from 39 to 105 in French, 103 to 240 in German, 56 to 130 in Italian, 60 to 136 in Polish and 39 to 100 in Spanish centers. The average number of ‘Other ESA’ injections administered per patient per month ranged from 5.65 across Spanish centers to 8.50 across Italian centers, as determined by the drug frequency distribution (). A correlation can be observed between the average number of monthly injections and the higher proportion of increased drug frequency in Poland and Italy. The average level of C.E.R.A. once-monthly uptake across all dialysis centers at the time of initial interviews ranged from 20% to 34%, and the average number of ‘Other ESA’ administrations that could be avoided per patient per year following a switch to C.E.R.A. once monthly ranged from 56 to 90 ().
Table 1. Characteristics of ESA administration per country*.
Observed time for ‘Other ESAs’ versus C.E.R.A. once monthly per patient
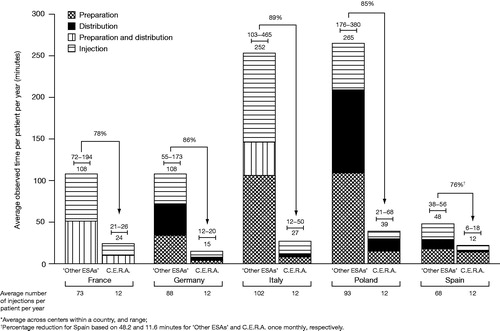
Average total time per session with ‘Other ESAs’ was 0.78 minutes in Spain, 1.29 minutes in Germany, 1.53 minutes in France, 2.52 minutes in Italy and 3.25 minutes in Poland. For C.E.R.A. once monthly, the average total time was 0.97 minutes, 1.25 minutes, 1.93 minutes, 2.29 minutes and 3.03 minutes in Spain, Germany, France, Italy and Poland, respectively. The average number of injections per patient per year for ‘Other ESAs’ ranged between 68 in Spain and 102 in Italy compared with 12 injections of C.E.R.A. once monthly (). These multiplied by the average time per session yielded an estimated annual time per patient, which ranged from 48 to 265 minutes for ‘Other ESAs’ and 12 to 39 minutes for C.E.R.A. once monthly (). Therefore, estimated annual time savings per patient treated with C.E.R.A. once monthly instead of ‘Other ESAs’ were 78% (range between centers of 67–86%) in France, 86% (79–91%) in Germany, 89% (83–95%) in Italy, 85% (82–88%) in Poland and 76% (69–84%) in Spain ().
Impact of switching to C.E.R.A. once monthly from ‘Other ESAs’ administered three times weekly or every 2 weeks
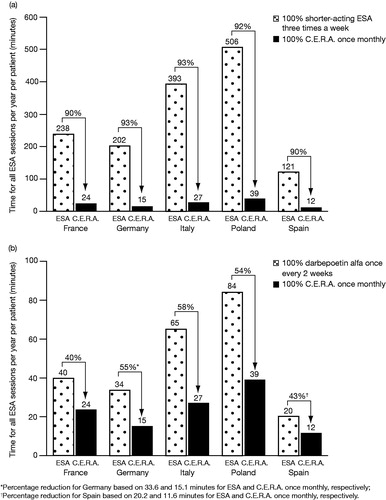
For a patient receiving shorter-acting ESAs administered three times weekly (i.e., 156 injections per year), estimated annual time savings with a switch to C.E.R.A. once monthly would increase to between 90% in France and Spain and 93% in Germany and Italy (). A comparison between ESAs administered every 2 weeks (i.e., 26 injections per year) and C.E.R.A. once monthly indicated that there was still a substantial reduction in estimated annual time savings, ranging between 40% in France and 58% in Italy ().
Modeling the impact of C.E.R.A. once monthly at the center level
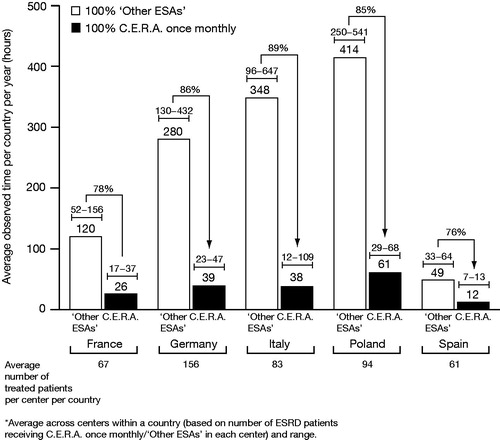
The analysis predicted that 100% adoption of C.E.R.A. once monthly across the centers participating in this study would result in average annual time savings of 94 hours (range between centers of 35–119 hours) in five French, 241 hours (108–394 hours) in four German, 310 hours (84–553 hours) in five Italian, 353 (221–477 hours) in three Polish and 37 hours (26–54 hours) in three Spanish centers, depending on the frequency distribution of ‘Other ESA’ administration and the overall number of patients treated at each center ().
Qualitative results
Interview responses were heterogeneous across centers. Many centers indicated that the time spent on non-observed tasks such as inventory and ordering of ESA treatment was expected to decrease only marginally when switching to 100% C.E.R.A. once monthly. However, the time spent on these activities could be substantially reduced across Polish centers due to the lower volume of drugs administered when switching to 100% C.E.R.A. once monthly. The reduced volume of drugs required following conversion to C.E.R.A. once monthly would also result in a substantial decrease in refrigeration space occupied in centers across all five countries. Healthcare staff in Polish, German and Italian dialysis centers considered that C.E.R.A. once-monthly maintenance therapy may also have some impact on reducing the frequency of assessing Hb levels and associated dose adjustments.
Healthcare staff interviewed felt that time savings achieved as a result of switching to C.E.R.A. once monthly would be beneficial to both staff and patients across dialysis centers, as time being freed up could be spent on better documenting patient parameters and instructing or re-instructing patients on dialysis access care and diet.
Discussion
The findings from this study demonstrate that anemia management is associated with substantial time spent on frequent observable tasks, including preparation, distribution and injection. The use of ‘Other ESAs’ requires frequent dosing to maintain stable Hb levels in patients with CKD, thus increasing the workload of healthcare staff. Our analysis suggests that 100% adoption of C.E.R.A. once-monthly maintenance therapy could offer substantial time savings for healthcare staff on frequent anemia-management tasks, with average per country savings ranging from 76% to 89%.
However, some limitations of this time-and-motion study should be noted. Firstly, variability in time for pre-specified tasks was observed between centers within a country (e.g., time for ESA preparation ranged from 0.08 minutes to 0.46 minutes across three Spanish centers, and similarly from 0.13 minutes to 0.42 minutes for C.E.R.A. once monthly). This exemplifies different treatment practices across centers within a country, making pooling of data difficult and therefore justifying the approach of giving each center equal weighting when averaging task time. Also, in some countries no clear differentiation could be made between preparation and distribution tasks, especially in centers where the ESA was collected directly from the refrigerator and distributed to the individual patient at their bedside. Between-center variability in task time could also be due to potential inter-rater variability in manual stopwatch measurement. This was mitigated by defining clear and unambiguous start and stop points for pre-specified tasks in each center in order to reduce scope for subjectivity in task interpretation, and through appropriate training. It should also be noted that no formal statistical hypothesis was tested around a difference in staff time between ‘Other ESAs’ and C.E.R.A. once monthly, as processes were not expected to differ by drug.
Secondly, as the absolute results in time per patient per year are dependent on center characteristics, it is less meaningful to compare results across centers. Indeed, annual time for administration tasks is driven by the frequency of administration of the various ‘Other ESAs’ ranging from three times weekly to once every 2 or 3 weeks. Differences in ESA drug frequency administration between centers resulted in average number of administrations per patient per year ranging between 68 sessions in Spain and 102 sessions in Italy, with a substantially smaller proportion of administrations three times weekly in Spain (18%) compared with Italy (40%). Within each country, variability in drug frequency distribution across centers was equally high (e.g., between 0.7 and 2.4 sessions per patient per week in French centers). As a result, the estimated time savings identified in this study are not a reflection of a given hypothetical administration frequency, but rather what is observed (and can be expected) in a real-world setting with the usage of different ESAs. Of course, a center with a higher proportion of patients receiving injections three times weekly would have increased scope for time savings when converting to 100% C.E.R.A. once monthly compared with a center where most patients receive ESA agents once every 2 weeks; this was explored through scenario analyses (). While the time savings between ESAs administered every 2 weeks and C.E.R.A. once monthly (40–58%) were not as substantial as those between ESAs administered three times weekly and C.E.R.A. once monthly (90–93%), they can still be considered substantial enough to be of benefit to healthcare staff, particularly those in busy hemodialysis centers.
Thirdly, time per center per year is driven by the total number of patients treated with an ESA. The range of total observed time for average country center size () gives an indication of the annual resource requirements in terms of staffing, and highlights the opportunity for reallocating staffing resources. However, the percentage reduction in annual time when switching from ‘Other ESAs’ to 100% C.E.R.A. once monthly is a more useful metric, as it demonstrates the relative impact on time (as opposed to a potentially wide-ranging absolute value).
Finally, the number of hemodialysis centers that participated in each country was relatively small, thus generating results that reflect the different regional locations and treatment settings (public hospital versus private practice settings) of the dialysis centers, and may not be representative of the countries in this study as a whole. Therefore, it is essential to highlight that the purpose of this study was to provide an indication of potential time savings and not to generate results that could be generalized across the countries. In addition, as the main focus of this study was to establish the time savings associated with C.E.R.A. once monthly, cost was not investigated. Indeed, transferring staff time into monetary value through fully loaded salary cost reflects ‘opportunity cost’ as opposed to real budget savings, and may therefore be less meaningful. Cost-effectiveness is not considered an appropriate methodology as no differences in efficacy are expected between ‘Other ESAs’ and C.E.R.A. once monthly. Cost minimization would be more appropriate but was outside the realm of this study. It was considered that such a calculation may not produce meaningful results as it would be driven by distribution of products used, the frequency distribution for each drug at each center and also differences in drug prices.
Our data support the findings from a previous time-and-motion study carried out in dialysis centers in Germany and the UK, which suggested that an ESA administered once monthly would offer annual time savings of 117 and 169 minutes per patient, respectively (corresponding to a 79% and 84% reduction in healthcare staff time compared with when patients were receiving a mix of other ESA products)Citation5. Our data are also in accordance with other studies suggesting that using a once-monthly ESA to correct anemia in dialysis patients may provide quantifiable time, labor and associated cost savings when compared with current treatment practicesCitation3,Citation19,Citation20.
The time savings generated by converting to C.E.R.A. once monthly reflect the opportunity for dialysis centers to invest freed-up healthcare staff time on other tasks that could improve patient care, allowing clinicians and nurses to spend more time on clinical assessment and disease managementCitation5. The additional time available to help patients achieve guideline targets for key clinical parameters such as maintenance of Hb levels within the recommended target range may exert beneficial effects on morbidity and mortality in patients with CKDCitation5. These potential benefits coincide with the views expressed by healthcare staff in this study who considered that time being freed up could be spent on documenting patient parameters and instructing or re-instructing patients on dialysis access care and diet.
Conclusions
Hemodialysis centers spend a significant proportion of time on tasks related to anemia management, especially those tasks required with every ESA administration. Data from this time-and-motion study show that use of C.E.R.A. once monthly could lead to important time savings, allowing scarce healthcare resources to be reallocated to focus on other important aspects of patient care at a critical point in the dialysis procedure.
Transparency
Declaration of funding
This study was sponsored by F. Hoffmann-La Roche Ltd, Basel, Switzerland. All authors had full access to the data and participated in reviewing and interpreting the data and the paper.
Declaration of financial/other relationships
E.D.C. and M.R-C. are full-time employees of United BioSource Corporation; F.D., K.K., W.K., F.M. and G.V. declare that they have no conflict of interest.
Acknowledgments
The authors would like to thank Tanya Chaudry at Complete HealthVizion, UK, for her assistance with writing and revising the draft manuscript, based on detailed discussion and feedback from all authors. Writing assistance was funded by F. Hoffmann-La Roche Ltd. Primary responsibility for opinions, conclusions and interpretation of data lies with the authors. All authors read and approved the final version of this manuscript.
Notes
*MIRCERA is a registered trademark of F. Hoffmann-La Roche Ltd, Basel, Switzerland
References
- Nugent RA, Fathima SF, Feigl AB, et al. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron Clin Pract 2011;118:c269-77
- Roderick P, Nicholson T, Armitage A, et al. An evaluation of the costs, effectiveness and quality of renal replacement therapy provision in renal satellite units in England and Wales. Health Technol Assess 2005;9:1-178
- Schiller B, Doss S, De Cock E, et al. Cost of managing anemia with erythropoiesis-stimulating agents during hemodialysis: a time and motion study. Hemodial Int 2008;12:441-9
- Kerr PG. Renal anaemia: recent developments, innovative approaches and future directions for improved management. Nephrology 2006;11:542-8
- Saueressig U, Kwan JTC, De Cock E, et al. Healthcare resource utilization for anemia management: current practice with erythropoiesis-stimulating agents and the impact of converting to once-monthly C.E.R.A. Blood Purif 2008;26:537-46
- Hsu CY, McCulloch CE, Curhan GC. Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 2002;13:504-10
- Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis 2005;45:658-66
- Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004;19:121-32
- Silverberg D, Wexler D, Blum M, et al. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant 2003;18(Suppl 8):vii7-12
- Foley RN, Parfrey PS, Harnett JD, et al. The impact of anemia on cardiomyopathy, morbidity, and mortality in end-stage renal disease. Am J Kidney Dis 1996;28:53-61
- Gouva C, Nikolopoulos P, Ioannidis JPA, et al. Treating anemia early in renal failure patients slows the decline of renal function: a randomized controlled trial. Kidney Int 2004;66:753-60
- Locatelli F, Aljama P, Bárány P, et al. Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant 2004;19(Suppl 2):ii1-47
- Churchill DN, Macarios D, Attard C, et al. Costs associated with erythropoiesis-stimulating agent administration to hemodialysis patients. Nephron Clin Pract 2007;106:c193-8
- Levin NW, Fishbane S, Valdés Cañedo F, et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA). Lancet 2007;370:1415-21
- Locatelli F, Mann JFE, Aldigier J-C, et al. C.E.R.A. safety profile: a pooled analysis in patients with chronic kidney disease. Clin Nephrol 2010;73:94-103
- Sulowicz W, Locatelli F, Ryckelynck JP, et al. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol 2007;2:637-46
- Carrera F, Lok CE, de Francisco A, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrol Dial Transplant 2010;25:4009-17
- Mann JF, de Francisco A, Nassar G, et al. Fewer dose changes with once-monthly C.E.R.A. in patients with chronic kidney disease. Clin Nephrol 2011;76:9-15
- Klatko W, Felisiak J. Time and motion study of anaemia management with erythropoiesis stimulating agents in haemodialysis units in Poland. JHPOR 2012;1:116-21
- Burnier M, Douchamps JA, Tanghe A, et al. Less frequent dosing of erythropoiesis stimulating agents in patients undergoing dialysis: a European multicentre cost study. J Med Econ 2009;12:77-86