Abstract
Objectives:
This retrospective study aims to examine the association between prescribing information (PI)-concordant oral antidiabetic drug (OAD) treatment and clinical and economic outcomes in patients with type 2 diabetes mellitus and stages 3–5 chronic kidney disease (CKD).
Methods:
The study used a large, national administrative claims database with laboratory findings to identify patients with a diagnosis of diabetes and indication of stages 3–5 CKD (first observed indication as the index date) between 1/1/2005 and 30/06/2009. OADs prescribed during 6 months following the index date (baseline period) were evaluated and patients were considered non-PI-concordant if any did not meet the recommendations regarding patients with renal impairment. Glycemic control and measures of healthcare costs (standardized to 2010 US dollars using the Consumer Price Index) and resource utilization were assessed during the 12 months following the baseline period. Severe hypoglycemic events were assessed after the baseline period until lost to follow-up. Regression analyses were performed after adjusting for demographic and clinical characteristics.
Results:
Among the 3300 patients included in the study, 2454 (74.4%) were non-PI-concordant. The non-PI-concordant patients had higher risk of severe hypoglycemic events identified in all settings (HR = 1.35, 95% CI: 1.10–1.67) and events identified in inpatient hospital setting (HR = 2.51, 95% CI: 1.49–4.22), were more likely to have inpatient hospital admissions (OR = 1.27, 95% CI: 1.02–1.57), and were less likely to have glycemic control (OR = 0.56, 95% CI: 0.44–0.71). Annual diabetes-related cost was $1638 higher in the non-PI-concordant cohort (p = 0.0048).
Limitations:
The retrospective cohort design does not allow for conclusions to be drawn on the causal effect of PI-concordant use based on the associations observed.
Conclusion:
Our findings suggest PI-concordant treatment to be associated with better clinical and diabetes-associated economic outcomes. Future research is warranted to confirm the associations found in this study.
Introduction
Diabetes is a major public health concern affecting an estimated 346 million people worldwide and 25.8 million people in the United States (US)Citation1,Citation2. Nearly 1.9 million Americans aged ≥20 years were newly diagnosed with diabetes and an additional 79 million American adults were estimated to be prediabetic in 2010Citation2. Type 2 diabetes (T2DM) encompasses 90% of diabetes worldwideCitation1. Diabetes is identified as the seventh leading cause of death in the USCitation2 and diabetes-related deaths are projected to double between 2005 and 2030Citation1. Diabetes is also associated with a high economic impact. In 2007, the estimated total cost of diagnosed diabetes in the US was $174 billion; nearly $116 billion was the direct medical cost and $58 billion was the indirect costCitation3. The indirect cost included disability, work loss, and premature mortalityCitation2.
According to the Centers for Disease Control and Prevention estimates, more than 35% of patients with T2DM aged ≥20 years have chronic kidney disease (CKD) in the USCitation4. The important health consequences of CKD include cardiovascular disease, end-stage renal disease (ESRD), and premature deathsCitation4. In 2008, 44% of new cases of ESRD in the US were due to diabetesCitation3. CKD is also associated with significant increase in healthcare cost in patients with diabetesCitation5. In 2009, the total Medicare cost for CKD in patients with diabetes was $18 billion accounting for 26.1% of the total Medicare diabetes cost – an 11-fold rise since 1993Citation5. Higher healthcare resource utilization was reported in patients with coexisting T2DM and renal impairment in the form of higher ambulatory visits, emergency room visits, inpatient stays, glucose-monitoring visits, diabetic shoe fittings, and insulin injections than those with diabetes aloneCitation6.
The presence of CKD complicates the treatment of diabetes. In patients with diabetes and stages 3–5 CKD, hypoglycemia is a major concern due to the diminished kidney gluconeogenesis, impaired clearance of insulin, and some oral antidiabetic drugs (OADs)Citation7. According to the clinical guideline recommendations by the National Kidney Foundation (NKF), dosage of various OADs should be adjusted and several OADs should be avoided in patients with T2DM and moderate-to-severe CKDCitation7. A recent retrospective study has demonstrated that treatment of patients with T2DM and moderate-to-severe CKD (n = 6058) with OADs according to the NKF guidelines recommendations was associated with reduced risk of hypoglycemia, all-cause hospitalization, and better glycemic controlCitation8. In addition, the annual medical encounter costs (without prescription costs) were 10% higher for patients whose OAD treatment was not concordant with the NKF guidelines compared with those who were guideline concordantCitation8. Concordance to NKF recommendations was seen in only 55.5% of patients in the studyCitation8. However, it is noteworthy that concordance is not synonymous with compliance or adherence and does not relate only to the medicine intake behavior of the patient. Concordance also demonstrates the nature of interaction between clinicians and patients and can be synonymous with patient-centered careCitation9.
Recommended dose adjustments for patients with T2DM with renal insufficiency have also been provided in the prescribing information (PI) of several OADsCitation10–21. Thus, it would be informative to determine whether treatment according to PI recommendations in patients with coexisting type 2 diabetes and moderate-to-severe CKD yields better clinical and economic outcomes. Understanding the benefits of PI-concordance can help healthcare providers, payers, and policymakers decide on where to focus their efforts for improving management of T2DM among patients with comorbid stages 3–5 CKD, especially with newer agents that are not included in the NKF guidelines published in 2007. However, there are no published reports analyzing OAD use concordance with PI recommendations.
We conducted this retrospective study to examine the association between PI-concordant OAD treatment and clinical and economic outcomes in patients with T2DM and stages 3–5 CKD. We hypothesized that treating according to PIs would yield better clinical and economic outcomes.
Patients and methods
Data source
The study used a large, national administrative claims database with laboratory findings of approximately 100 large employers and health plans across the USCitation22 to analyze the available data from patients who had medical service claims with a diagnosis of diabetes mellitus (ICD-9-CM: 250.xx) between January 1, 2005 and December 31, 2010. The database covers healthcare administrative claims reimbursed by the health plans across all the settings such as inpatient and outpatient hospitals, emergency rooms, physicians’ offices, rehabilitation centers, and specialty centers for approximately 1.9 million unique commercially-insured individuals. The database also maintains separate records for pharmacy claims submitted by pharmacies for prescriptions reimbursed by the health plans and laboratory data (digital output for blood, urine, and other tissue samples) collected from several central reference laboratories. In the past 6 years, this database included more than 32.6 million laboratory test results. The specific tests were identified through the Logical Observation Identifiers Names and Codes (LOINC) nomenclature, which is a taxonomy of clinical laboratory test results that provides a very high level of specificity. The enrollment records contain demographic information, including age, gender, and geographic region, as well as information regarding health insurance payer type, employment status, and monthly enrollment status. Across all the data files, the identifiable patient information was encrypted and a unique patient identification number was given in order to comply with Health Insurance Portability and Accountability Act (HIPAA) regulations. The files were linked through this unique patient identification number.
Sample selection
Patients were selected based on having at least two medical claims with a diagnosis of T2DM on different dates and at least one record of stages 3–5 CKD (using laboratory value of estimated glomerular filtration rate <60 mL/min/1.73 m2 indicative of CKD or by the presence of an ICD-9-CM diagnosis code of 585.3–5 or associated procedure codes for dialysis) from January 1, 2005 to June 30, 2009. The first record of stages 3–5 CKD observed was set as the index date. Furthermore, patients were required to be aged 18–64 years on the index date, to have 18 months of continuous enrollment following the index date (the first 6 months of observation served as the baseline period followed by 12 months of follow-up in which outcomes were analyzed), and to have received at least one of the study OADs including glyburide, glipizide, glimepiride, acarbose, miglitol, metformin, repaglinide, nateglinide, rosiglitazone, pioglitazone, sitagliptin, or saxagliptin within the 6-month baseline period. Subjects also had to have laboratory test results necessary to determine OAD concordance with PI during the baseline period. Patients were excluded if they had secondary diabetes, gestational diabetes, or malignancy other than skin and prostate cancer, and if they were pregnant at any time during the study period.
PI-concordance
PI-concordance (Appendix) was assessed using the last prescription for each OAD during the 6-month baseline period. Since newly developed CKD could not be ascertained, a 6-month evaluation period was undertaken (baseline period) to allow time for physicians to make necessary changes in the OAD therapy. If the patients were on multiple OADs (combination therapy or alteration of regimen) during the 6-month baseline period, all OADs were evaluated. Patients were considered non-PI-concordant if any of the prescribed OADs did not meet PI recommendations regarding patients with renal impairment.
Study variables
Demographic and clinical characteristics
Demographic characteristics such as age, gender, insurance type, and region were identified at the index date from the patient’s enrollment data captured in the database. Using the primary or secondary ICD-9-CM diagnosis codes listed in medical claims during the 6-month baseline evaluation period following the index date, the Charlson Comorbidity Index (CCI) was estimatedCitation23,Citation24. Given that the entire study population had a diabetes diagnosis, the CCI score was modified by excluding diabetes. Intermediate clinical measures related to glycemic control during the baseline period and 12-month follow-up period were analyzed. Glycemic control was defined as an HbA1c < 7%. The first glycemic control measure (HbA1c) that occurred within the baseline period was considered as the baseline measure. The observation closest to the end date of the follow-up period was considered as the 12-month post-index measure. The percentage of patients with glucose levels under control at the baseline and follow-up periods were reported. Severe hypoglycemic events, identified from all settings and from inpatient setting, respectively, were also assessed during the post-index period until lost to follow-up using a published algorithmCitation25.
Healthcare cost and resource utilization
Healthcare costs per patient were assessed for all-cause and those related to diabetes. The healthcare resource utilization measures examined were rate of hospitalizations, concomitant use of insulin, and specialist visits to nephrologists, endocrinologists, ophthalmologists, or cardiologists. Proportion of use of these healthcare resources was reported at the 6-month baseline period. Total healthcare costs were summarized over the baseline and follow-up periods. The cost of specific categories of care, which included inpatient, outpatient, emergency room, and pharmacy, were also summarized. Diabetes-specific costs were identified based on medical services with associated diagnoses (250.xx) and pharmacy claims based on National Drug Codes (NDC) for blood glucose monitoring, OADs, noninsulin injectables, and insulin. All costs were adjusted to 2010 US dollars by using the annual medical care component of the Consumer Price Index to reflect inflation between 2005 and 2010.
Statistical analyses
The rate of concordance to the PI at the population level, as the proportion of patients in whom prescribed treatments were PI-concordant, was reported. In addition, we also reported the proportion of patients in whom the prescribed OAD was PI-concordant, for each OAD. The study measures were reported for overall population and compared between PI-concordant and non-PI-concordant cohorts. Means and standard deviations (SD) were reported for continuous variables, and frequency distributions with percentages were reported for categorical variables. Student’s t-tests were used to detect the differences for continuous variables, chi-square tests for categorical variables, and Wilcoxon rank-sum tests for cost variables. P-values < 0.05 were considered statistically significant based on two-tailed tests. Kaplan–Meier curves were used to descriptively assess the unadjusted time to severe hypoglycemic events, and a log-rank test detected the differences between Kaplan–Meier survival curves, with p < 0.05 being considered significant. Multivariate regression analyses were employed to control for differences in demographic and clinical characteristics when evaluating the association of OAD treatment concordance to the PI with clinical outcomes, healthcare costs, and utilization. Logistic regressions were employed to analyze the likelihood of hospital admissions and glycemic control. Odds ratios (ORs) were reported along with 95% confidence intervals (CIs). A generalized linear regression model with a log link function assuming a gamma distribution was used to examine the total and diabetes-related healthcare costCitation26. Coefficient and p-values were estimated for cost variables. Adjusted costs of non-PI-concordance, compared with PI-concordance, were estimated by computing the expected instantaneous change as a function of a change in variable, while keeping all the other covariates constantCitation27. The Cox proportional hazard regressions were employed to assess the risk severe hypoglycemic events associated with non-PI-concordance. The failure event of the Cox model was defined as the occurrence of the first severe hypoglycemic event. The hazard ratios (HR) and the 95% CI were reported for the variables.
Results
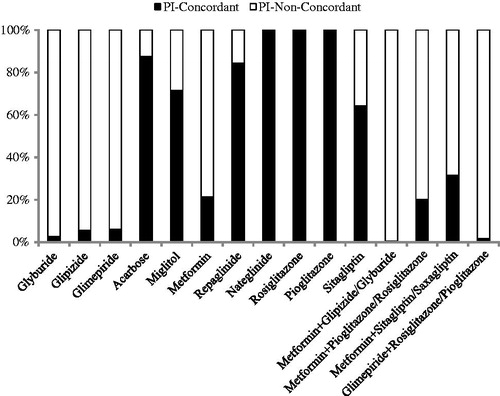
The study identified 3300 patients with T2DM and stages 3–5 CKD and that met the inclusion and exclusion criteria; of these, 846 (25.6%) patients had OAD treatments that were PI-concordant and 2454 (74.4%) patients that were non-PI-concordant. Concordance to PI recommendations by individual OAD is depicted in . During the 6 months baseline period, use of all thiazolidinediones were PI-concordant, whereas the majority of use of sulfonylureas (i.e., glyburide [97.3%], glipizide [94.4%], and glimepiride [94.0%]) were non-PI-concordant. The PI-concordance was also low with metformin (21.3%). Among the patients on sitagliptin, 64.2% of patients received therapy in accordance with PI recommendations. The majority of patients receiving combination therapy of two or more OADs were non-PI-concordant with at least one of the OADs.
Figure 1. Drug-level analysis of OAD usage according PI recommendations. OAD, oral anti-diabetic drug; PI, prescribing information.
Patient baseline characteristics
The mean age of the study population was 55.9 (SD: 6.2) years. The majority of the patients were males (62.1%), lived in the southern region (59.6%), covered under preferred provider organization managed-care planCitation28 (PPO; 56.9%), and had stage 3 CKD (83.2%) (). The proportion of males was significantly higher in the non-PI-concordant cohort (64.0%) compared with the PI-concordant cohort (56.7%; p < 0.001). A significantly higher proportion of patients in the non-PI-concordant group had stage 3 CKD compared with the PI-concordant group (84.7 vs. 78.8%; p < 0.001). During the baseline period, the average CCI score was higher in the PI-concordant cohort (1.8) compared with the non-PI-concordant cohort (1.4; p < 0.01). During the baseline period, among the patients with known baseline HbA1c value (n = 1939), mean HbA1c value was lower in PI-concordant patients (7.2%) compared with the non-PI-concordant patients (7.7%; p < 0.01). The proportion of patients with HbA1c <7% was also higher in the PI-concordant cohort (56.5%) compared with the non-PI-concordant cohort (42.4%, p < 0.01). ***During the 6-month baseline period, a higher proportion of patients in the concordant cohort were seen by nephrologists (35.5 vs. 25.6%; p < 0.01) or an endocrinologists (16.0 vs. 11.8%; p < 0.01) and used insulin (39.2 vs. 32.2%; p < 0.01) compared to the non-concordant cohort, while the rate of hospitalization was similar (14.8 vs. 13.9%; p = 0.54) During the 6-month baseline period, the mean all-cause healthcare costs were higher in the PI-concordant cohort compared to the non-PI-concordant cohort ($15,521 vs. $13,519; p < 0.01).
Table 1. Patient demographics, clinical characteristics, and healthcare costs and utilization at baseline evaluation period.
Clinical and economic outcomes during follow-up period
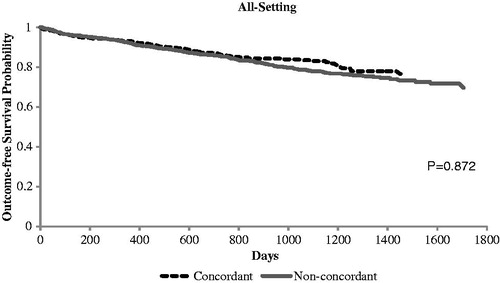
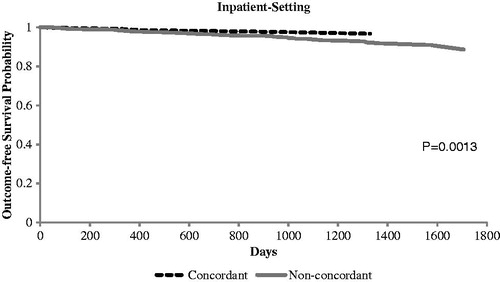
Of the patients with a known HbA1c value at the 12-month follow-up period (n = 1842), those in the PI-concordant cohort had a lower mean HbA1c (7.2 vs. 7.6%; p < 0.01) compared with those in the non-PI-concordant cohort (). The proportion of patients with HbA1c <7% remained significantly higher in the PI-concordant cohort (58.1%) compared with the non-PI-concordant cohort (43.8%; p < 0.01). The Kaplan–Meier curves ( and ) demonstrated no significant difference in time to severe hypoglycemic events identified in all settings between the study cohorts (p = 0.872). When we examined severe hypoglycemic events identified in inpatient setting, we found non-PI-concordant cohort had a higher risk (p = 0.0013).
Table 2. Crude healthcare cost and clinical outcomes among PI-concordant and non-PI-concordant stages 3–5 CKD patients during 12-month follow-up period.
During the 12-month follow-up period, the all-cause healthcare costs remained significantly higher in the PI-concordant cohort versus the non-PI-concordant cohort ($30,227 vs. $26,317; p < 0.01) (). No significant difference was observed between the cohorts for diabetes-related healthcare costs ($10,843 vs. $10,433; p = 0.10). Outpatient costs continued to contribute the most for all-cause and inpatient costs continued to contribute the most for diabetes-related healthcare costs in both the cohorts. Rate of inpatient hospitalization was also similar (17.9 vs. 19.7%; p = 0.23).
Multivariate regression analyses
Results from Cox proportional regression demonstrated a higher risk of severe hypoglycemic events identified in all settings in the non-PI-concordant cohort (HR = 1.35, 95% CI: 1.10–1.67) after adjusting for age, gender, geographical region, CCI score, CKD stage, visits to specialists, insulin use, and any inpatient hospitalization during the baseline period (). The risk of hypoglycemic events was similar in patients aged between 18 and 54 years compared with patients aged between 55 and 64 years (HR = 0.99, 95% CI: 0.82–1.19). Female patients had a lower risk of hypoglycemic events (HR = 0.83, 95% CI: 0.69–0.99). According to the geographical region, the risk of hypoglycemia was higher in patients living in West (HR = 1.57, 95% CI: 1.24–2.00) compared with patients living in South. The CCI score ≥3 (HR = 2.14, 95% CI: 1.62–2.82), any inpatient hospitalization during the baseline period (HR = 1.30, 95% CI: 1.03–1.63), and use of insulin (HR = 1.99; 95% CI: 1.66–2.38) were the other characteristics associated with higher risk of hypoglycemic events. When assessing severe hypoglycemic events identified from inpatient setting, we found non-PI-concordance was associated with 151% higher risk (HR = 2.51, 95% CI: 1.49–4.22). Other risk factors included older age, living in West region, higher CCI, and prior inpatient hospitalization.
Table 3. Regression results for risk of severe hypoglycemic event.
The logistic regression analysis demonstrated that the likelihood of all-cause inpatient hospitalizations was higher in the non-PI-concordant patients (OR = 1.27, 95% CI: 1.02–1.57) compared with the PI-concordant patients (). Factors associated with greater likelihood of inpatient hospitalizations included Northeast region (OR = 1.38, 95% CI: 1.03–1.86), CCI score ≥3 (OR = 1.55, 95% CI: 1.18–2.04), stages 4 and 5 CKD (OR = 1.77, 95% CI: 1.41–2.21), insulin use (OR = 1.74, 95% CI: 1.44–2.10), and any inpatient hospitalizations (OR = 2.66, 95% CI: 2.12–3.34) during the 6-month baseline period. Among the patients with HbA1c data available during the follow-up period (n = 1842), those with OAD treatment non-concordant to PI guidelines were less likely to have glycemic control (OR = 0.56, 95% CI: 0.44 – 0.71). Patients with stages 4 and 5 CKD were more likely to have glycemic control (OR = 1.43, 95% CI: 1.06–1.92). Living in Midwest (OR = 1.74, 95% CI: 1.16–2.59) and West (OR = 1.42, 95% CI: 1.06–1.92), and visiting a nephrologists during the baseline period (OR = 1.35, 95% CI: 1.02–1.79) increased the likelihood of glycemic control. Insulin use during the baseline period, however, was associated with a lower likelihood of glycemic control (OR = 0.29, 95% CI: 0.23–0.36).
Table 4. Regression results for glycemic control and annual inpatient admissions.
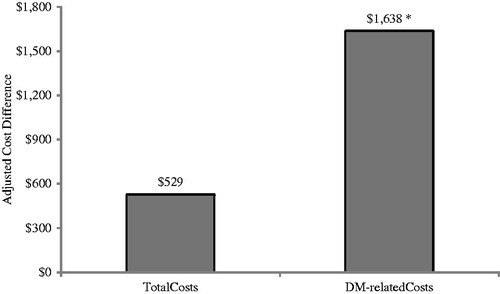
After adjusting for covariates similar to previous models, we found that patients who were non-PI-concordant had similar annual all-cause healthcare costs during the 12-month follow-up period to concordant patients (adjusted difference = $529, p = 0.66). When we evaluated diabetes-related costs during the 12-month follow-up period, the non-PI-concordant cohort’s costs were higher by $1638 (p = 0.0048) when compared to the PI-concordant cohort ().
Figure 4. Adjusted 1-year cost differences associated with non-concordance to prescribing information. Model adjusted for age, gender, geographical region, CCI score, CKD stage, visits to specialists, insulin use, and any inpatient hospitalization during the baseline period. *Statistically significant at p < 0.05 compared to PI-concordant cohort.
Discussion
CKD is a common comorbidity in patients with T2DM; however, published literature on how these patients are treated in clinical practice is very limited. Insulin and many of the currently used OADs are excreted through renal system. The NKF has provided recommendations on the adjustment of prescribed dosage of several OADs in patients with T2DM and moderate-to-severe CKD. The NKF guidelines also list the OADs that should be avoided completely in moderate-to-severe CKDCitation7. A recent retrospective analysis evaluated the clinical and economic consequences of concordance with the NKF treatment guidelines for OAD use in patients with T2DM and stages 3–5 CKD using electronic medical records from an integrated health systemCitation8. Almost 45% of patients in the study were not treated concordantly with the NKF guidelines. Findings from the study suggested a higher risk of hypoglycemic events, hospitalization, uncontrolled glycemic level, and higher medical costs associated with non-concordance of NKF recommendationsCitation8.
We conducted this retrospective analysis of the large administrative Thomson Medstat MarketScan Commercial and Laboratory Databases to assess the association of PI-concordant OAD treatment with clinical and economic outcomes among patients with T2DM and CKD. To our knowledge, this is the first study that assesses the outcomes associated with the use of OADs in concordance with their PI. In addition, our study used data from a more geographic diverse population which could better represent the variation across prescribing patterns. We found that only 25% of the study population was using OADs in concordance with PI recommendations which suggests unawareness on the part of healthcare providers regarding the PI recommendations. Consistent with the previous results on NKF concordance, we found that patients with PI-concordance demonstrated better clinical outcomes. Glycemic control, defined as the proportion of patients with HbA1c <7.0%, was higher in the concordant patients both during the baseline evaluation and follow-up periods. The multivariate analysis further demonstrated greater likelihood of having glycemic control in the PI-concordant cohort in the follow-up period. The likelihood of inpatient hospitalizations was also lower in the PI-concordant cohort. Fewer inpatient hospitalizations in the PI-concordant cohort may be attributed to better glycemic control. A previous study has shown that poor glycemic control is associated with a greater likelihood of inpatient hospitalization for several short-term complications (i.e., infections, hyperglycemia, hypoglycemia, and electrolyte disturbances)Citation29. Hypoglycemia, possibly attributed to impaired renal clearance of OADs and diminished kidney gluconeogenesis, represents a major concern in the management of patients with T2DM and stages 3–5 CKD. We observed a lower risk of hypoglycemic events in the PI-concordant patients. This observation further supports the association between guideline-concordant treatment and a lower risk of hypoglycemic events reported in the earlier studyCitation8. However, we acknowledge the limitation of the study which did not consider the incidence or frequency of hypoglycemic events prior to the index date. The prior hypoglycemic events are strong predictor of the subsequent events.
We also evaluated the economic outcomes associated with PI-concordance. After using multivariate regression analysis to adjust for demographic characteristics, CCI score, CKD stage, visits to specialists, inpatient hospitalization, and insulin use during baseline period, the total annual healthcare costs during the follow-up period was similar between the cohorts. The adjusted annual total diabetes-related costs were significantly higher in the non-PI-concordant cohort, an observation similar to the previous report where non-NKF concordance was associated with significantly higher diabetes-related healthcare costsCitation8. The economic benefit observed associated with PI-concordant treatment may have important policy implications. Health plans should consider such costs offset when allocating resources to improve quality of care for diabetes patients with CKD.
The better clinical and diabetes-related economic outcomes associated with PI-concordance highlight the importance of regular screening of CKD in patients with diabetes and careful planning of the treatment regimen. The current standard of diabetes care recommends annual screening of CKD among patients with diabetesCitation30. This is particularly important in the current scenario where the presence of CKD in patients with T2DM may be undiagnosed by physicians. In a recent retrospective analysis of a large national health plan database in the United States, the prevalence of renal impairment determined by claims analysis (physician diagnosed) was approximately one-third the prevalence determined according to laboratory values (11.9 vs. 34.3%)Citation6. Undiagnosed patients are more likely to progress to more severe stages of CKD. Earlier detection of CKD allows the physician to adjust the dosage when necessary and avoid contraindicated OADs according to NKF guidelines or PI recommendations, especially in patients with stages 3–5 CKD. Healthcare professionals need to be more aware of the PI recommendations in addition to NKF guideline recommendations, especially for the recently approved drugs which are not yet included in the NKF guidelines.
Limitations
The findings of this study must be evaluated within the limitation of the data and study design. The retrospective cohort design does not allow for conclusions to be drawn on the causal effect of PI-concordant use based on the associations observed. Records of OAD prescriptions were used to determine concordance with PI. Although this method of gathering the information has been shown to be reliable, it has several limitations including (1) whether patients actually took their OADs as prescribed could not be determined; and (2) variability of insulin regimens on a day-to-day basis could not be accounted for. Similarly, we could not determine how long the patients had been on OAD treatment prior to the index date due to the inclusion of prevalent cases in the design, nor could it be determined how long the patient(s) had been diagnosed with diabetes. Further, claims-based analyses rely upon diagnostic codes that do not necessarily capture the patients’ medical information accurately owing to possible coding errors, coding for the purpose of ruling out rather than diagnosis of actual disease, and under-coding. The findings from this study were based on a sample of commercially insured enrollees from multiple payers and aged less than 65 years only. Hence, the results may not be generalizable to larger populations that include elderly patients, other payers, or the uninsured. Given that the prevalence of type 2 diabetes and CKD increases with age, future studies are needed to confirm these findings in older population, specifically, aged >65 years.
Conclusions
This retrospective study demonstrated that the treatment of patients with T2DM and comorbid stages 3–5 CKD in accordance with PI recommendations is associated with better glycemic control, a reduced risk of hypoglycemic events, a lower risk of inpatient hospitalizations, and lower diabetes-related costs. However, future studies are warranted to generalize our results and to assess long-term effects beyond 1 year.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim Pharmaceuticals, Inc.
Declaration of financial/other relationships
M. A. is an employee of Boehringer Ingelheim Pharmaceuticals, Inc. S.-Y.C, Y.C.L, and V.A are employees of United BioSource Corporation and were contracted to conduct this study by Boehringer Ingelheim Pharmaceuticals, Inc. G.O. is an employee of University of Utah college of Pharmacy.
Supplementary Material
Download PDF (45.1 KB)Acknowledgments
The authors also acknowledge Rahul Birari (MS Pharm, PhD) and Dr Amit Bhat (MPharm, PhD), Indegene Lifesystems Pvt Ltd, for providing the necessary writing assistance and editorial support towards the development of the manuscript.
References
- Diabetes. WHO. Available at: http://www.who.int/mediacentre/factsheets/fs312/en/ [Last accessed April 19, 2012]
- National Diabetes Fact Sheet: National estimates and general information on diabetes and prediabetes in the United States, 2011. Centers for Disease Control and Prevention. 2012. Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf [Last accessed 11 April 2012]
- Diabetes Statistics. American Diabetes Association. 2012. Available at http://www.diabetes.org/diabetes-basics/diabetes-statistics/ Last accessed 11 April 2012]
- National Chronic Kidney Disease Fact Sheet 2010. Centers for Disease Control and Prevention. 2012. Available at http://www.cdc.gov/diabetes/pubs/factsheets/kidney.htm. [Last accessed 11 April 2012]
- USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease in the United States Chapter 6. United States Renal Data System 2012. Available at http://www.usrds.org/atlas10.aspx. [Last accessed 11 April 2012]
- Burke J, Kovacs B, Borton L, et al. Health care utilization and costs in type 2 diabetes mellitus and their association with renal impairment. Postgrad Med 2012;124:77-91
- National Kidney Foundation. KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 2007;49:S12-180
- Chen SY, Siu K, Kovacs B, et al. Clinical and economic outcomes associated with National Kidney Foundation guideline-concordant oral antidiabetic drug treatment among type 2 diabetes patients with chronic kidney disease. Curr Med Res Opin 2012;28:1-9
- Bell JS, Airaksinen MS, Lyles A, et al. Concordance is not synonymous with compliance or adherence. Br J Clin Pharmacol 2007;64:710-11
- Glynase PresTab (Micronized Glyburide Tablets) [PI] New York: Pharmacia & Upjohn Company, Division of Pfizer, Inc., 2010
- Glucotrol (Glipizide) [PI] New York: Roerig Division of Pfizer Inc., 2010
- Amaryl (Glimeperide) [PI] New Jersey: Sanofi-Aventis U.S. LLC., 2012
- Precose (Acarbose) [PI] Wayne, New Jersey: Bayer Healthcare Pharmaceutical Inc., 2011
- Glyset (Miglitol) [PI] New York: Pharmacia & Upjohn Company, Division of Pfizer, Inc., 2011
- Glucophage (Metformin) [PI] Princeton, New Jersey: Bristol-Meyers Squibb Company, 2009
- Prandin (Repaglinide) [PI] Princeton, New Jersey: Novo Nordisk Inc., 2011
- Starlix (Nateglinide) [PI] East Hanover, New Jersey: Novartis Pharmaceutical Corp., 2012
- Avandia (Rosiglitazone) [PI] Research Triangle Park, North Carolina: GlaxoSmithKline, 2011
- Actos (Pioglitazone) [PI] Deerfield, Illinois: Takeda Pharmaceuticals America, Inc., 2012
- Januvia (Sitagliptin) [PI] Whitehouse Station, New Jersey: Merck and Co, Inc. 2012
- Onglyza (Saxagliptin) Princeton, New Jersey: Bristol-Myers Squibb Company, 2011
- Self-Insured Group Health Plans. Self-Insurance Institute of America, Inc. Available at http://www.siia.org/i4a/pages/index.cfm?pageid=4546. [Last accessed 11 January 2013]
- D'Hoore W, Bouchaert A, Tilquin C. Practical consideration on the use of the Charlson comorbidity index with administrative databases. J Clin Epidemiol 1996;49:1429-33
- Schneeweiss S, Maclure M. Use of comorbidity scores for control of confounding in studies using administrative databases. Int J Epidemiol 2000;29:891-8
- Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4-10
- Mihaylova B, Briggs A, O'Hagan A, et al. Review of statistical methods for analysing healthcare resources and costs. Health Econ 2011;20:897-916
- Greene WH, ed. Econometric Analysis. Fifth edn. Upper Saddle River, New Jersey: Pearson Education, Inc., 2003
- Managed Care. Medline Plus website. Available at: http://www.nlm.nih.gov/medlineplus/managedcare.html. Last accessed 21 January 2013
- Menzin J, Langley-Hawthorne C, Friedman M, et al. Potential short-term economic benefits of improved glycemic control: a managed care perspective. Diabetes Care 2001;24:51-5
- Standards of medical care in diabetes – 2011. Diabetes Care 2011;34(Suppl 1):S11-61