Abstract
Objective:
Epidemic Kaposi’s sarcoma (KS) is one of the most common acquired immune deficiency syndrome (AIDS) defining malignancies, a disease with stigmatized clinical features that characterizes the diagnosis of AIDS. This study aims to perform a cost-effectiveness analysis between liposomal doxorubicin and paclitaxel in treating AIDS-KS.
Methods:
A 21 week decision tree analysis was created using a hospital perspective to compare treatment patterns with liposomal doxorubicin and paclitaxel. All costs were calculated in 2011 US dollars and obtained from an academic treatment center. Acquisition costs were obtained from public estimates using wholesale acquisition cost (WAC). Effectiveness was estimated based on a Phase 3 study of liposomal doxorubicin and paclitaxel (Von-Roenn et al.). Adverse events (AEs) associated with treatment and not the disease were included in the analysis. One-way sensitivity analysis was performed to test the robustness of the results.
Results:
Cost minimization analysis showed that treatment with liposomal doxorubicin was $18,125 whereas paclitaxel costs $12,347. After accounting for response rate, the results showed that liposomal doxorubicin costs $39,403 versus $21,661 for paclitaxel. This study has some limitations. Clinical data were derived from different clinical trials. In addition, many assumptions were made.
Conclusion:
Paclitaxel is dominant due to its lower acquisition cost and high response rate. Acquisition cost of liposomal doxorubicin and paclitaxel are significantly different. After accounting for all the factors that contribute to cost and response rate, paclitaxel is more cost effective than liposomal doxorubicin.
Introduction
Epidemic Kaposi’s sarcoma (AIDS-KS) is a disease associated with human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS). Patients with AIDS-KS present with painful vascular tumors that are disfiguring and debilitatingCitation1. These stigmatizing clinical features marked by the lesions on the skin often characterize the diagnosis among the AIDS populationCitation2.
Before the highly active antiretroviral therapy (HAART) era, patients with AIDS were 20,000 times more likely to have KS than the general populationCitation3. After the approval of HAART, the incidence of this type of cancer in the US declined significantly (2 per 10,000 age-adjusted incidence rate in 2006 compared to 17.2 per 10,000 age-adjusted incidence rate in 1990, the peak of the AIDS epidemic), though approximately 15% of patients with AIDS still develop the disease in the USCitation4,Citation5. In the 1990s, due to the increase in the incidence of AIDS-KS, the Food and Drug Administration (FDA) approved chemotherapy drugs to treat the different stages of AIDS-KS. For patients with advanced disease, liposomal anthracyclines (pegylated liposomal doxorubicin or liposomal daunorubicin – currently under patent) are used as first-line treatmentCitation6. In the case of patients with disease progression or anthracycline resistance paclitaxel, currently available as generic, has been approved as second-line therapyCitation7.
Chemotherapy treatments are usually expensive because of acquisition cost, administration costs, premedication, cost of managing adverse events associated with the treatment and treatment failure. Four studies evaluated whether a treatment with liposomal anthracyclines for AIDS-KS is cost effective. All of them compared liposomal doxorubicin to liposomal daunorubicin in different countriesCitation8–11. Three of them found that liposomal doxorubicin was more cost effective than liposomal daunorubicinCitation8–10.
To date, only one Phase 3 clinical trial comparing liposomal doxorubicin with paclitaxel has been completedCitation12. The study included 36 patients in the paclitaxel arm and 37 in the liposomal doxorubicin arm. In this study, researchers found no statistical difference in efficacy for paclitaxel (57% [95% CI: 39%, 74%]) compared with liposomal doxorubicin (46% [95% CI: 29%, 63%]), yet the study was terminated early due to poor accrual rates. The 2 year overall survival rate was 79% (95% CI: 62%, 96%) for paclitaxel and 78% (95% CI: 56%, 84%) for liposomal doxorubicinCitation12,Citation13.
Wholesale acquisition cost (WAC) data from 2011 shows that the available liposomal doxorubicin (Doxil; Doxil is a registered trade name of Janssen Pharmaceuticals, Titusville, NJ, USA) cost is more than 20 times higher than the cost of paclitaxel, currently available as genericCitation14. However, to date, no study has performed a cost effectiveness analysis comparing liposomal doxorubicin and paclitaxel even though a large difference exists between the two drugs in terms of acquisition costs. Therefore, it is unclear, after accounting for this factor and others, as to how these variables influence the total cost and effectiveness in the treatment of AIDS-KS. Hence, this study aims to perform a cost-effectiveness analysis of liposomal doxorubicin versus paclitaxel for the treatment of advanced AIDS-KS.
Methods
Study treatments
The model included two drugs used to treat patients with AIDS-KS. The dosage of Doxil was of 20 mg/m2 every 3 weeks for 7 cycles and paclitaxel 100 mg/m2 every 2 weeks for 8 cycles, derived from the only head-to-head Phase 3 trial comparing the drugsCitation12,Citation13. The literature showed that liposomal doxorubicin was more cost effective than liposomal daunorubicinCitation8–10; hence, liposomal daunorubicin was removed from this analysis.
Analysis
In the absence of a head-to-head Phase 3 trial data of sufficient sample size, the effectiveness of liposomal doxorubicin and paclitaxel were based on the response rate results of Von Roenn et al.Citation10 and tested based on the reported confidence intervals (CIs) to verify the impact of the variation of effectiveness in the model. As a first analysis for this study a cost minimization was done, accounting only for the variation in total treatment costs, and as a second analysis effectiveness was added to the model to perform a complete cost-effectiveness analysis.
For this study, a cancer institute perspective was employed, where only direct medical costs were considered including medication costs, administration costs, office visits and hospitalization. The results indicate the total variation among direct costs of treatment of advanced AIDS-KS for liposomal doxorubicin and paclitaxel, without accounting for indirect costs and intangible costs. The reference case was a white male 38 years of age, based on epidemiology data on AIDS-KS, average body surface area (BSA) of 1.75 m2. The model accounts for an entire treatment of each drug for a total of 21 weeks.
Costs
shows all costs included in this analysis. The total costs were defined as acquisition costs, preparation costs, the management costs of adverse events (AEs), and the cost of treatment failure. For the model, the acquisition costs were derived from the wholesale acquisition cost (WAC), according to an online pricing tool (Analy$ource) as of October, 2012Citation1Citation4. Administration costs of the drugs included in the study as well as costs of AE management (i.e., hypersensitivity and grade 3/4 neutropenia with hospitalization) were estimated based on data provided from an academic medical center and the work of Brixner et al. on the mean cost of the events to the academic medical centerCitation15,Citation16. Administration costs and preparation included pharmacy costs, healthcare professionals’ time, and all infusion associated materials.
Table 1. Cost data.
Paclitaxel has a longer administration time, and requires a polyvinyl chloride (PVC) free infusion line, filter, and PVC free infusion bag, therefore contributing to a higher cost associated with the administration/preparation of the drug when compared with doxorubicin. The cost of treatment failure was calculated based on discontinuation of treatment followed by the introduction of another therapy. All the costs were in 2011 US dollars, and adjusted according to the consumer price index (CPI) medical expendituresCitation17.
Cost calculation
The cost calculation was performed based on the following equation:
where C is the cost of one cycle, N is the number of cycles, and Cevent and pevent are respectively the cost and probability of AEs for the event of neutropenia, hypersensitivity and discontinuation. The equation included the full cost of treatment for patients who did not discontinue the drug at any point before the end of the treatment period. For patients who discontinued and switched drugs, it was assumed that these patients would only receive one treatment cycle before discontinuation/switching to another agent. The cost of discontinuation is calculated as the cost of on one cycle of the assigned drug plus the cost of a full-length therapy with the other agent. All the costs and probabilities of the AEs (grade 3/4 neutropenia and/or hypersensitivity) were also accounted for in this model.
Administration/preparation cost includes all costs associated with administration and preparation of drugs including healthcare professional wages and medical equipment.
Assumptions
All grade 3 or 4 neutropenia required hospitalization, and the costs were estimated from an academic medical centerCitation15;
Vomiting/nausea/diarrhea would not have required major treatment; hence, the costs of these AEs were not included;
Erythrodysesthesia (hand and foot syndrome), most common in patients receiving the treatment with liposomal doxorubicin, had no impact in cost since treatment is cold water and vitamin B6Citation18;
Opportunistic infection was considered to be AIDS-related not chemotherapy-related and with similar frequency (54% for paclitaxel and 50% for liposomal doxorubicin), thus, removed from this cost analysisCitation18,Citation19; in addition, with the HAART treatment, patients are at a lower risk of developing opportunistic infections;
Patients would not discontinue the treatment due to adverse events;
In case of treatment failure patients would switch drugs, and the number of cycles received from the first drug will be assumed to be smaller than the total number of cycles (number of cycles = 1);
Treatment failure (discontinuation) could happen for only one of the competitors;
All patients survived the total chemotherapy treatment period.
Clinical outcomes
The effectiveness was measured as response rate (percentage of complete and partial response defined as the absence of any detectable disease, persisting for at least 1 month; and a 50% or greater decrease in the number/size of lesions lasting for at least a month or flattening of nodular lesions in at least 75% of nodules and without any sign of new lesion or progression respectively), based on the small and only head-to-head Phase 3 trial comparing liposomal doxorubicin and paclitaxelCitation12. The probabilities of AEs were derived from the package insert (PI) based on clinical trials, prepared by the manufacturer and approved by the FDA. All trials selected for this analysis had similar patient characteristics and demographicsCitation18,Citation20.
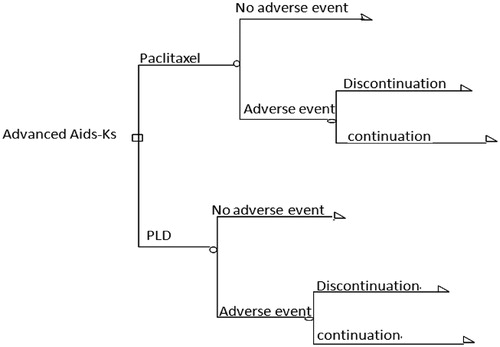
Decision tree analysis
A decision tree analysis was used to create the model with the probabilities and costs associated with each pathway (). Based on the assumptions and probabilities of an AE, costs were calculated for treatment with each drug for a period of 7 cycles (21 weeks) for liposomal doxorubicin and 8 cycles (16 weeks) for paclitaxel, with a longer period in case of discontinuation and switching, after which costs were compared among the two agents. Finally, the response rate was included in the analysis. The incremental cost-effectiveness ratio (ICER) was also calculated to determine the incremental cost to have one more response.
Sensitivity analysis
A sensitivity analysis was performed to test the robustness of the results obtained from the decision tree analysis for the cost minimization and the cost-effectiveness analysis, since estimations and assumptions were made. We considered a range of 50% lower and upper bound to perform the sensitivity analysis of this study for all the cost variables and probabilities assuming that this range is reasonable and includes a vast majority of possible values for the variables, similarly to the work of Aujesky et al. (2004)Citation21. For the cost of cycles, a range of 20% was used around the base case cost of one cycle based on the pricing variation available in the US. Given that the effectiveness of the drugs is based on a clinical trial of few patients, sensitivity analysis for response rate was performed based on the CI presented in Von Roenn et al.Citation12.
Our sensitivity analysis was performed as a one-way sensitivity analysis, where one variable is changed at a time while all the other variables remain in the base case scenario values to evaluate the impact of each variable in the model. The model and all cost calculations were performed using Microsoft Excel Office.
Results
Patients characteristics
For this model, safety data was obtained from each treatment’s package insert (PI)Citation18,Citation20. All patients had advanced disease according to the AIDS Clinical Trial Group (ACTG) criteria, patients were predominately male, Caucasian, and in their late 30 s. The use of HAART for the underlying condition was permitted. Patients receiving paclitaxel had a worse overall prognosis (poor risk, ACTG criteria), and received prior chemotherapy treatment for KS. Overall, patients on doxorubicin had fewer cases of grade 3/4 neutropenia and alopecia compared to paclitaxel. The infusion time for paclitaxel was longer and more frequently administered (3 hours every 2 weeks compared to 1 hour for doxorubicin every 3 weeks).
Cost minimization and cost effectiveness
The cost minimization analysis (CMA) based on our assumptions, trial probabilities, and hospital cost data is shown in . The data showed it costs $12,347 to treat a patient with paclitaxel compared to $18,125 to treat a patient who received liposomal doxorubicin, a total difference of $5778. Although paclitaxel has a higher number of cycles, more episodes of grade III/IV neutropenia, higher rate of hypersensitivity reactions and a more expensive administration cost associated with longer administration hours, the lower acquisition cost makes paclitaxel the less expensive option, assuming the effectiveness of both drugs are the same.
Table 2. Cost minimization and cost effectiveness analysis.
The cost-effectiveness analysis, using the response rate data from Von Roenn et al.Citation12, found that paclitaxel costs $21,661 per response while liposomal doxorubicin costs $39,403 for the same outcome. Hence, the comparison of the cost-effectiveness ratios indicated that paclitaxel was cost effective when compared to liposomal doxorubicin. The incremental cost effectiveness ratio (ICER) of using paclitaxel versus liposomal doxorubicin is dominated (−$52,535). When ICER calculations generate a negative result, it indicates that one treatment is both less costly and more effective (dominant), and the other is more costly and less effective (dominated).
Sensitivity analysis
A one-way sensitivity analysis was performed using ranges artificially created (50% around the point estimate). shows the sensitivity analysis of the CMA, indicating the impact of each variable in the final cost compared to the base case. When the cost of cycles of paclitaxel was increased to 20% the total treatment cost increased by $89, which means that the total cost of a treatment with paclitaxel would be $12,436. If the costs negotiated are approximately the total cost of one cycle (−20%), then the total cost of treatment with paclitaxel would be $10,866, a savings of $1481. When the same analysis was done for liposomal doxorubicin, the difference in cost using the 20% increase in cost compared to the base case scenario was $1650 and the total cost of the total treatment with liposomal doxorubicin would be $19,740. Using the base case cost of one cycle (−20%), the cost of treatment with liposomal doxorubicin would be $14,900 (−$3225). shows that the cost of cycles had the largest and most expressive impact on the final cost.
Table 3. Sensitivity analysis – CMA.
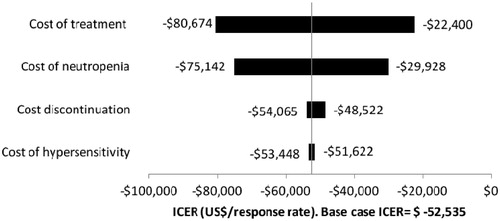
The tornado diagram is shown in . Including all cost variables and probabilities, the total cost of treatment, including cost of cycles and number of cycles administered, had the biggest impact on the model (range −$80,674 to −$22,400), and widest range of possible ICERs, followed by neutropenia. Discontinuation and hypersensitivity do not have a big impact our model across the range of possible values.
To test the robustness of our point estimates for response rate, we used the range reported as the confidence interval by Von Roenn et al. ()Citation12. All the costs were kept constant at the base case. shows the impact of the difference in response rate between the two drugs on the CER. In the case where the two drugs have the lowest response rate, paclitaxel is more cost effective than liposomal doxorubicin. At base case or in the case where both drugs have the highest response rate, paclitaxel is still more cost effective than liposomal doxorubicin.
Table 4. Cost effectiveness ratio – sensitivity analysis.
In the case where paclitaxel had the lowest response rate and liposomal doxorubicin was represented at the base case, paclitaxel was more cost effective than doxorubicin. For paclitaxel to have the same CER as liposomal doxorubicin in the base case, the response of paclitaxel would be 0.36, which is outside of the reported CI. In the case where liposomal doxorubicin had the highest response rate and paclitaxel remained at base case, paclitaxel was more cost effective than liposomal doxorubicin. For liposomal doxorubicin to have the same CER as paclitaxel in the base case, the response of liposomal doxorubicin would be 0.84, which was also outside of the reported CI of liposomal doxorubicin. The only situation where liposomal doxorubicin was more cost effective than paclitaxel was when paclitaxel had a response rate of 0.43 or lower and liposomal doxorubicin has the highest response rate reported.
Discussion
The results from this study showed that paclitaxel was less costly and more cost effective than liposomal doxorubicin. The large difference in acquisition cost (paclitaxel less expensive than liposomal doxorubicin) accounts for only one piece of the total treatment cost. The complete treatment costs for each drug should include the probabilities of neutropenia grade 3/4, hypersensitivity, and discontinuation for toxicities and costs associated with treatment as well. Another important variable that this study considered was the response rate. After accounting for the differences in response rates, paclitaxel was less expensive and more cost effective than the competitor.
The sensitivity analysis showed that treatment costs have the biggest impact in the model. Wholesale acquisition costs are commonly used in economic models; therefore, it was assumed to be a good estimation of drug acquisition cost. The authors understand that different providers may negotiate prices varying from the reported WAC, hence the sensitivity analysis results allowed for a better interpretation of the results accordingly.
Neutropenia was the second variable that most commonly impacted the model. One reason for this influence could be the high incidence of grade 3/4 neutropenia among patients undergoing chemotherapy treatment, especially among immunosuppressed patients. Although the costs of neutropenia in this study were based on an academic medical center15 the data were comparable to other studies that evaluated the cost of neutropenia associated with chemotherapeutic treatment. Strokes et al. (2009)Citation22 found that in 2005 the cost of neutropenia was $12,148, while $7598 was from hospitalization-related neutropenia. Adjusted to 2011 costsCitation17 $9410 was associated with hospitalization and $15,044 was the total cost of neutropenia. Kuderer et al. (2006)Citation23 estimated the median cost of neutropenia hospitalization to be $8376 in 1999 ($13,378 in 2011). The Healthcare Costs and Utilization Project (HCUP) estimated that in 2008 the total cost of neutropenia associated with chemotherapy was $11,079 ($12,180 in 2011)Citation24. Based on other sources of neutropenia costs, the cost estimation in this study of $10,297Citation1Citation5 was believed to be reasonable and comparable with others in the literature.
For hypersensitivity, this model considered a point estimate of $1029 as the cost of hypersensitivityCitation15 which is lower and more conservative than the cost value found by HCUP of $3632 in 2008, $3983 after being adjusted to 2011 US dollarsCitation17,Citation24. For this model, the response rates were based on a small Phase 3 studyCitation12 that concluded that, although not statistically different, paclitaxel has a higher response rate than liposomal doxorubicin. Our base-case analyses showed that when paclitaxel was more effective (ORR = 57%) than liposomal doxorubicin (ORR = 46%), paclitaxel was more cost effective than liposomal doxorubicin. To account for the scenario where paclitaxel and liposomal doxorubicin have the same effectiveness, a CMA was completed. The analyses indicated that liposomal doxorubicin was more expensive and clearly dominated. In a scenario where liposomal doxorubicin was more effective than paclitaxel at base case, still liposomal doxorubicin is dominated. Our sensitivity analysis showed that the cost effectiveness of the two drugs was comparable and highly sensitive to the variation in response rate.
Our results cannot be compared with other studies for AIDS-KS since it is the first study comparing liposomal doxorubicin with paclitaxelCitation8–11. Of the other studies that compared liposomal doxorubicin to liposomal daunorubicin, threeCitation8–10 studies concluded that liposomal doxorubicin was more cost effective, while Egan and HenryCitation11 published the only data that concluded otherwise. Goebel et al. in 1999Citation2Citation4 showed that liposomal doxorubicin was also more cost effective than liposomal daunorubicin, bleomycin and vincristine, and doxorubicin, bleomycin and vincristine.
Despite the economic profile, physicians might find more clinical incentive in prescribing liposomal doxorubicin for patients with AIDS-KS for many reasons. First, paclitaxel requires a premedication that include steroids, due to the high risk of hypersensitivity associated with the use of paclitaxel. Second, after the approval and widespread utilization of HAARTs for patients with HIV/AIDS, a concern was raised about possible drug interactions due to the same metabolic pathwayCitation25,Citation26. Recent studies have shown that the use of paclitaxel appears to be safe when used concomitantly with HAARTs; still, the possibility of drug interactions plays a role in the treatment choiceCitation25,Citation27,Citation28. Third, the AE profile of paclitaxel, including a higher incidence of neutropenia and alopecia in combination with a longer infusion time, makes paclitaxel less attractive for patients, even though the sustained response of paclitaxel may be one of the longest, according to Cheung et al.Citation2. Thus, we hypothesized that more doxorubicin data are available in the literature and therefore physicians are more comfortable prescribing liposomal doxorubicin for patients with AIDS-KS in contrast to paclitaxel.
Physicians might find an economical incentive in prescribing liposomal doxorubicin for patients with AIDS-KS as well due to the reimbursement policy of ‘buy and bill’. In this policy physicians buy the medication at a lower cost than what they charge insurers. This current reimbursement approach for chemotherapy drugs provides no incentive to search for less expensive treatment optionsCitation29.
Our work had limitations. First, clinical data were derived from different clinical trials due to the lack of a large head-to-head trial comparing doxorubicin with paclitaxel and response rates were based on a terminated Phase 3 study. We acknowledge the large impact on the response rate in our conclusions and the impact in our results. Yet, since AIDS-KS is rare and both drugs are already FDA approved, a clinical trial is warranted unlikely to be designed. Second, all trials available were curative not palliative, although the treatment for AIDS-KS is palliative; hence we recognize that the reported rates, including tolerance to AEs, might have been different in a palliative trial. In addition, data from clinical trials are extremely controlled while in the real-world scenario compliance rates are smaller and that could have impacted our results. Third, many assumptions needed to be made; however, the sensitivity analysis has shown that our results are robust and consistent with a conservative approach. Fourth, our study was completed from a hospital perspective; thus, our results are accurate for this scenario only; other studies in different scenarios are warranted to allow these results to be extrapolated outside the hospital scenario.
This work explored the use of two different drugs for the treatment of AIDS-KS in a simplistic model, by making assumptions and simplifications. Future work includes the incorporation of utility to the model, to assess the impact of the treatments in the quality of life of patients, to account for differences in adverse event profiles and administration time.
Conclusion
Although patients who take paclitaxel experience more AEs than patients who receive liposomal doxorubicin, which increases total costs, the present results showed that the treatment of AIDS-KS with paclitaxel is less expensive and more cost effective than liposomal doxorubicin. This is the first study that compares resource utilization of liposomal doxorubicin and paclitaxel for the treatment of AIDS-KS. Similar studies in different scenarios are warranted to show external validity or to refute this study’s results. This study does not intend to help physicians make decisions or to advocate the use of any specific drug regimen for the treatment of AIDS-KS. Instead, it can be used to show the importance of making treatment decisions that account for an economic analysis in the health care setting.
Transparency
Declaration of funding
The authors received no funding for the interpretation or elaboration of this manuscript. All authors participated in the definition of study objectives, development of model, interpretation of results, and wrote and critically reviewed the manuscript. All of the authors have read and approved the content of the entire manuscript, and believe it represents honest work and adheres to ICMJE requirements.
Declaration of financial/other relationships
K.R. has disclosed that she has no significant relationships with or financial interests in any commercial companies related to this study or article. C.A. was awarded grants from AHRQ R18 and CDC Small Community Transformation Grants, and has served as a consultant for Astellas and Bayer. J.B., M.G. and S.S. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article or financial interests in any commercial companies related to this study or article.
References
- Northfelt DW, Dezube BJ, Thommes JA et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 1998;16:2445-51
- Cheung MC, Pantanowitz L, Dezube BJ. AIDS-related malignancies: emerging challenges in the era of highly active antiretroviral therapy. The Oncologist 2005;10:412-26
- Engels EA, Pfeiffer RM, Goedert JJ et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006;20:1645-54
- Altekruse SF, Kosary CL, Krapcho M et al. SEER cancer statistics review, 1975–2007. 2010. Available at: http://seer.cancer.gov/csr/1975_2007/ [Last accessed November 2009]
- Bryan BA, Dyson OF, McCubrey JA, Akula SM. Biology of Kaposi’s sarcoma-associated herpes virus. Front Biosci 2005;10:2882-91
- Liposomal KS drugs approved. GMHC Treat Issues 1996;4:8-9
- Paclitaxel for treating KS. Proj INF Perspect 1997;23(15)
- Bennett CL, Golub RM, Stinson TJ et al. Cost-effectiveness analysis comparing liposomal anthracyclines in the treatment of AIDS-related Kaposi’s sarcoma. J Acquir Immune Defic Syndr Hum Retrovirol 1998;18:460-5
- Hjortsberg C, Persson U, Lidbrink E, et al. Cost-effectiveness analysis of pegylated-liposomal doxorubicin and liposomal daunorubicin treatments in patients with Kaposi’s sarcoma. Acta Oncologica 1999;38:1063-7
- Vanni T, Fonseca BAL, Polanczyk CA. Cost-effectiveness analysis comparing chemotherapy regimens in the treatment of AIDS-related Kaposi’s sarcoma in Brazil. HIV Clin Trials 2006;7:194-202
- Egan T, Henry H. Cost-effectiveness of liposomal daunorubicin versus liposomal doxorubicin in Kaposi sarcoma. Value Health 1998;1:39
- Von Roenn J, Lee S, Cianfrocca M et al. Phase III study of paclitaxel (Pac) versus pegylated liposomal doxorubicin (PLD) for the treatment of advanced human immunodeficiency virus (HIV)-associated Kaposi’s sarcoma (KS): an eastern cooperative oncology group (ECOG) and AIDS malignancy consortium. J Clin Oncol–ASCO Annual Meeting Proceedings Part I 2007;25(20503)
- Cianfrocca M, Lee S, Von Roenn J et al. Randomized trial of paclitaxel versus pegylated liposomal doxorubicin for advanced human immunodeficiency virus-associated Kaposi sarcoma: evidence of symptom palliation from chemotherapy. Cancer 2010;116:3969-77
- Analysource. Available at: https://www2.analysource.com/qry/as_menu.taf?_nc=5766067819826c07b3c0e0e324b03495 [Last accessed October 2012]
- Academic Medical Center. Confidential data. 2010
- Brixner DI, Oderda GM, Nickman NA, et al. Documentation of chemotherapy infusion preparation costs in academic- and community-based oncology practices. J Natl Compr Canc Netw 2006;4:197-208
- United States Department of Labor. CPI inflation medical care. Available at: http://www.bls.gov/cpi/ [Last accessed October 2012]
- Solimando DA. Paclitaxel package insert. Canc Investig 1997;15:503
- Steward S, Jablonowski H, Goebel FD et al. Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International pegylated liposomal doxorubicin study group. J Clin Oncol 1998;16:683-91
- Centocor Ortho Biotech Products LP. Doxil package insert. Revised 09/2012
- Aujesky D, Smith KJ, Cornuz J, et al. Cost-effectiveness of low-molecular-weight heparin for secondary prophylaxis of cancer-related venous thromboembolism. Thromb and Haemostasis 2005;93:592-9
- Stokes ME, Muehlenbein CE, Marciniak MD et al. Neutropenia-related costs in patients treated with first-line chemotherapy for advanced non-small cell lung cancer. J Manag Care Pharm 2009;15:669-82
- Kuderer NM, Dale DC, Crawford J et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer 2006;106:2258-66
- Goebel FD, Jablonowski H. Therapy of special HIV-associated diseases: HCV-HIV-co-infection and AIDS-related Kaposi's sarcoma -- official satellite to the 7th European Conference on Clinical Aspects and Treatment of HIV-infection, October 23, 1999 in Lisbon, Portugal. Eur J Med Res. Dec 16 1999;4:507-13
- Tulpule A, Groopman J, Saville MW et al. Multicenter trial of low-dose paclitaxel in patients with advanced AIDS-related Kaposi sarcoma. Cancer 2002;95:147-54
- Schwartz JD, Howard W, Scadden DT. Potential interaction of antiretroviral therapy with paclitaxel in patients with AIDS-related Kaposi’s sarcoma. AIDS 1999;13:283-4
- Gill PS, Tulpule A, Espina BM et al. Paclitaxel is safe and effective in the treatment of advanced AIDS-related Kaposi’s sarcoma. J Clin Oncol 1999;17:1876-83
- Welles L, Saville MW, Liteau J et al. Phase II trial with dose titration of paclitaxel for the therapy of human immunodeficiency virus-associated Kaposi’s sarcoma. J Clin Oncol 1998;16:1112-21
- Newcomer L. Changing physician incentives for cancer care to reward better patient outcomes instead of use of more costly drugs. Health Aff (Millwood) 2012;31:780-5