Abstract
Objectives:
To estimate average annual cost per multiple sclerosis (MS) patient in the US using published estimates from the literature.
Methods:
A search was performed of English-language literature published between 2007 and June 2012 in PubMed and Embase using the term ‘multiple sclerosis’ and the subject heading ‘healthcare costs’. Included articles were primary studies with MS cost figures that could be converted to per patient per year values. Costs were inflated to 2011 dollars using the medical component of the Consumer Price Index.
Results:
Fifteen studies met the inclusion criteria. Eight presented only direct cost calculations; the remaining seven presented estimates of total cost, broken down into direct and indirect costs. Total all-cause healthcare costs for MS as reported by studies that included direct and indirect costs ranged from $8528–$54,244 per patient per year. On average, direct costs comprised 77% (range 64–91%) of total costs. Prescription medications accounted for the majority of direct costs. On average, indirect costs comprised 23% (range 9–36%) of total costs. Compared with direct all-cause medical costs for other chronic conditions reported in the literature, MS ranked second behind congestive heart failure.
Limitations:
Data sources in these studies were dated, ranging from 1999–2008, and therefore do not include some of the newer, more costly therapies. In addition, this review does not include either assessment of the decrements in quality-of-life associated with MS or costs associated with increasing levels of disability or early retirement. Furthermore, variations in study designs, populations, methodologies, and cost inputs preclude more precise cost estimates.
Conclusions:
MS is a costly chronic disease. Further research is needed to understand: costs by MS type, costs associated with increasing disability and early retirement, and the potential impact of new treatments expected to launch in coming years.
Introduction
Multiple sclerosis (MS) is a neurologic autoimmune disease in which the immune system attacks and degrades myelin sheaths on axons in the brain, spinal cord, and optic nerves, eventually causing irreparable axonal damage as well as neuronal death. It is the leading cause of non-traumatic neurologic disability in young adults in the US and EuropeCitation1. While the precise immunologic mechanisms underlying disease onset and progression are still being elucidated, it is clear that CD4-positive T-cells play a prominent roleCitation2. Accumulating evidence has also highlighted B-cells as being critical to MS pathogenesis, and studies suggest that B-cell-depleting therapies may provide a unique therapeutic avenueCitation3.
MS pathology and the resulting slowing or blockade of neuronal signaling can manifest in multiple ways, including difficulty with vision, muscle co-ordination, strength, sensation, and other bodily functionsCitation4, as well as fatigue, cognitive and psychological impairment, sleep disorders, and pain. There are four main forms of MS. The most common type is relapsing-remitting MS (RRMS), in which patients experience acute neurologic symptoms producing temporary disability (relapses) followed by periods of remission, or recovery, where acute symptoms subside and disability may or may not persist. This form can be preceded by clinically isolated syndrome (CIS), in which there has been only a single MS-type event, and no second event has yet been detected to confirm the diagnosis. Most patients with RRMS will ultimately develop secondary progressive MS (SPMS) in which relapsing-remitting disease transitions to a steady accumulation of disability such that patients either do not experience or do not notice relapses due to this high burden of background disability. Primary progressive MS (PPMS) manifests as a slow and steady increase in disability throughout the affected person’s life in the absence of acute relapses. Finally, progressive-relapsing MS is a rare form in which the disease is progressive from the start, but the patient also experiences periodic relapses.
Disease modifying therapies (DMTs) have been found to reduce relapses and slow disability progression due to relapses with incomplete recoveryCitation5–9. They are also known to reduce relapse-associated costsCitation10,Citation11. In addition, DMTs have been found to slow the conversion from CIS to RRMS and SPMSCitation12–15. MS is associated with high direct and indirect costs. Direct costs consist of medical expenditures, such as hospitalizations, inpatient and outpatient care, and pharmaceuticals. Indirect costs consist of those associated with short- and long-term disability, disease-related absences from work, workers’ compensation, and early retirement. MS is a disease that starts early, with most patients diagnosed when aged 20–40 yearsCitation4. Because of this early onset, MS can occur during the patient’s most productive working years, presenting huge potential societal costs associated with productivity loss.
The purpose of this literature review was to estimate the average annual cost per MS patient in the US, comprising both direct and indirect costs by broad disease type (RRMS, PPMS), based on published economic studies.
Methods
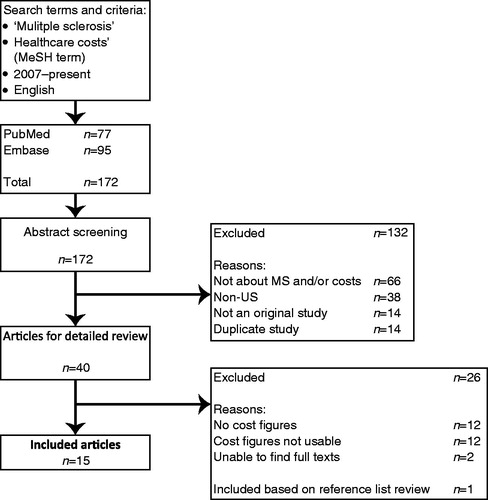
This literature review was conducted by searching both PubMed and Embase using the search term ‘multiple sclerosis’ in conjunction with the medical subject heading ‘healthcare costs’. The search was limited to English-language studies published between January 1, 2007 and June 21, 2012 (in order to capture 5 full years of literature). Duplicate articles, review articles, and studies for which the full text was not available were excluded. Studies were also excluded if they were not based in the US, were not cost focused, or did not include data that could be converted to annual per patient costs. Reference lists from the remaining studies were hand searched to identify any additional articles meeting the above-mentioned criteria. The initial list of studies was reviewed by two investigators (G.A, K.F.V.); the abstract review was conducted by one investigator (G.A.) with ambiguities discussed and resolved by two (G.A, K.F.V.). Cost data were abstracted primarily by one investigator (G.A.) with a sample duplicated by another (K.F.V.). contains a detailed explanation of all inclusions and exclusions.
Figure 1. Flowchart for articles included in the systematic literature review: January 1, 2007 to June 21, 2012. MS, multiple sclerosis.
Many of the studies presented costs by patient sub-groups, including: medication adherence statusCitation10; treatment statusCitation16; and type of treatmentCitation17–22. Others simply presented averages from a more general MS population (e.g., all MS patients in PharMetrics Integrated Patient-centric Database)Citation22–25. In the studies with multiple sub-groups, costs for each sub-group were averaged together to estimate costs for the whole sample. If a study had a reported sample size for each patient sub-group, a weighted average was calculated based on the number of patients in each group. If no sample size was reported, the values for each sub-group were averaged with equal weight (see Supplementary Appendix A). Descriptive data were captured for each study in order to evaluate their comparability. Specifically, study type and data source were reported as well as ‘cost year’ (the year in which cost figures were reported), and the number of years for which data were collected.
Cost figures were extracted from the identified publications and, where possible, separated based on disease type (RRMS and PPMS) and cost type (direct and indirect). Other types of MS, such as SPMS and PRMS, were not included because of an absence of cost literature at that level of disease detail. Articles that detailed specific components of the cost were separated in an effort to reveal the main cost drivers of MS. Cost data extracted from the literature were evaluated in three different ways: Firstly, mean total, direct, and indirect costs were calculated (see Supplementary Appendix B); secondly, direct and indirect costs were calculated as a percentage of the total cost; and thirdly, the percentage of total cost attributable to each specific cost component was calculated. Costs were converted from ‘cost year’ to 2011 dollars using the medical component of the Consumer Price Index.
Results
Literature search
The search criteria resulted in 172 articles being identified: 77 articles from the PubMed database and 95 from Embase (). The abstracts for each of these 172 citations were then reviewed and compared against the previously mentioned inclusion and exclusion criteria, after which 40 articles remained. Twenty-six articles were eliminated because they had no cost figures or the cost figures were not useable, and the full texts of two of the articles could not be sourced. One additional article was included based on a review of the reference lists. The 15 remaining articles were included in this literature review.
Total, direct, and indirect costs
In the first analysis, total, direct, and indirect costs were extracted, where possible. Twelve of the 15 total studies were included in this initial analysisCitation10–26 (). These articles were included because of their standardized reporting of aggregated (direct and indirect) costs for all MS cases (not broken down by disease type). Five of the 12 articles presented only direct cost calculationsCitation16,Citation20–22,Citation25, while the remaining seven presented estimates of total cost broken down into direct and indirect costs.
Table 1. Total, direct, and indirect costs in the US for multiple sclerosis from published literature.
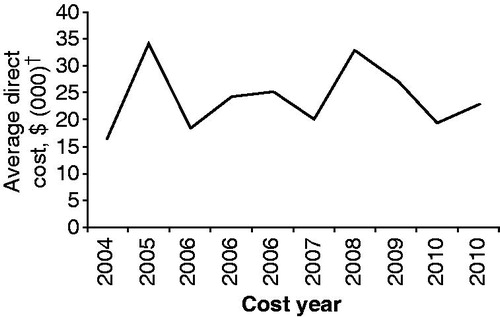
Total mean costs for patients with MS ranged from $8528–$54,244 per patient per year in 2011 dollars; direct costs ranged from $6144–$34,511 in 2011 dollars (). There was no clear relationship between the average direct cost by year among the 10 studies that included DMTs in the direct cost estimates (). The two lowest direct-cost estimatesCitation10,Citation26 were derived from studies that did not consider MS medication costs. Indirect costs ranged from as low as $1896 in a study that only included costs associated with sick leave, disability, and workers’ compensation, to as high as $19,733 in a study that included costs of missed work time, under-employment, and episodes of unemployment due to disability.
Figure 2. Average direct cost by year (2004–2010) among 10 studies that included DMTs in the direct cost estimatesCitation16–25. The studies used are the 10 studies from that included DMTs in the direct cost estimates. †Costs presented in 2011 US dollars. DMT, disease-modifying therapy.
Direct and indirect costs as a percentage of total costs
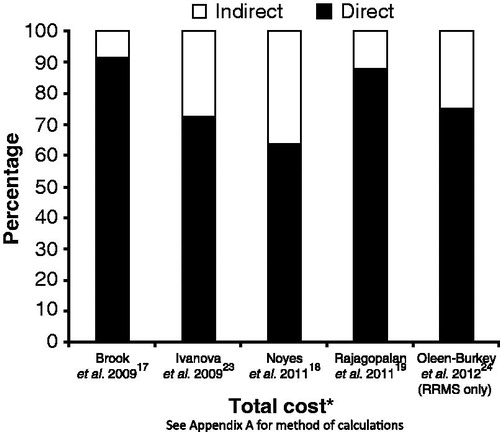
Calculation of the percentage of costs attributable to direct and indirect costs was based only on studies that included such a breakdown, as well as the cost of DMTs as a component of direct costCitation17–19,Citation23,Citation24. Based on the five studies that met these criteria, direct costs comprised 64–91% of total annual costs (average 77%) and indirect costs comprised 9–36% of total annual costs (average 23%) ().
Figure 3. Direct and indirect costs as a percentage of total costs for multiple sclerosis for articles identified during the systematic literature reviewCitation17–19,Citation23,Citation24. *Data year for Brook = 2007; Ivanova = 2006; Noyes = 2010; Rajagolpalan = 2010; Oleen-Burkey = 2009. RRMS, relapsing-remitting multiple sclerosis.
Detailed cost components
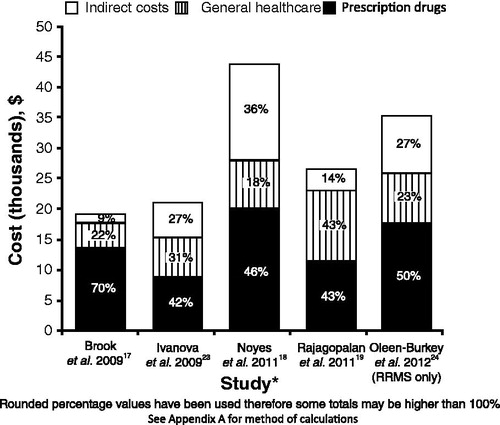
Inclusion criteria for this analysis were the same as the previous analysis (i.e., to be included, studies had to report direct costs, including DMTs, as well as indirect costs), thus the same five studies were analyzed. Prescription drugs and indirect costs were the biggest single cost drivers of MS, representing an average of 50% and 23% of total costs, respectively. General healthcare costs represented an average of 27% of the total costs, but represent a multitude of cost categories ().
Figure 4. Breakdown of multiple sclerosis costs for articles identified during the systematic literature reviewCitation17–19,Citation23,Citation24. * Data year for Brook = 2007; Ivanova = 2006; Noyes = 2010; Rajagolpalan = 2010; Oleen-Burkey = 2009. RRMS, relapsing-remitting multiple sclerosis.
Studies presenting alternative cost categories
Studies that met the overall systematic review search criteria, but did not include direct/indirect cost breakdowns and/or presented costs by patient sub-groups, were evaluated separately. Only one (Gilden et al.Citation27) presented cost by disease type (in 2006 dollars), reporting that the direct costs associated with PPMS were ∼4-times higher than RRMS ($23,630 compared with $5887, respectively)Citation27. The per-patient annual direct costs associated with RRMS during relapse reportedly averaged $17,016, RRMS during remission averaged $7296, and stable RRMS averaged $3972 ()Citation27. Lad et al.Citation28 used 14 years of data from the Nationwide Inpatient Sample to estimate the average cost of an MS hospitalization alone at $20,07628 in 2006 dollars. Lastly, Tan et al.Citation29 reported MS-specific direct health costs based on adherence to DMTs. Adherent patients averaged $3421 in direct medical expenditure over a 12-month period compared with $5179 for non-adherent patientsCitation29 in 2009 dollars.
Table 2. Studies presenting alternative cost categories.
Direct cost comparison with other chronic conditions
The direct costs for other major chronic conditions were compared with the direct costs for MS estimated from this literature review. Direct costs associated with other major chronic conditions were taken from a study done by the Center for Healthcare Research and Transformation (CHRT)Citation30. The direct cost of MS was calculated at $21,238 by taking an average of the costs reported in the original cost years across studies that included DMTs (). The average direct cost value across studies serves only as a rough estimate to allow general comparisons between MS and other chronic conditions. Using the CHRT study as a benchmark, MS was the second most costly chronic condition behind congestive heart failure (). MS was ∼7.5-times as costly as having no chronic conditions ().
Figure 5. Average annual direct multiple sclerosis costs compared with costs for other chronic conditions. Adapted from Health Care Cost Drivers. Chronic disease, comorbidity, and health risk factors in the US and MichiganCitation30. * Data represent spending for privately insured adults (generally 18–64 years old) covered by one private insurer in one state only (Blue Cross Blue Shield of Michigan [BCBSM]). While BCBSM does insure a majority of Michigan adults, data do not represent every non-Medicare adult in Michigan. Reprinted by permission from Center for Healthcare Research & Transformation (CHRT), University of MichiganCitation30.
![Figure 5. Average annual direct multiple sclerosis costs compared with costs for other chronic conditions. Adapted from Health Care Cost Drivers. Chronic disease, comorbidity, and health risk factors in the US and MichiganCitation30. * Data represent spending for privately insured adults (generally 18–64 years old) covered by one private insurer in one state only (Blue Cross Blue Shield of Michigan [BCBSM]). While BCBSM does insure a majority of Michigan adults, data do not represent every non-Medicare adult in Michigan. Reprinted by permission from Center for Healthcare Research & Transformation (CHRT), University of MichiganCitation30.](/cms/asset/c32b97b0-747c-4719-a7bc-0d2b7bb918d7/ijme_a_778268_f0005_b.jpg)
Discussion
The purpose of this review was to estimate the average annual cost burden per MS patient in the US based on data from the most recent published literature. The original focus of this review was to quantify the largest potential cost drivers – relapse and early retirement – as well as the cost of disability as measured by Expanded Disability Status Scale (EDSS) score. However, data of this type were largely unavailable in the US. Overall, few MS cost reviews have been published in recent years. Two international systematic review articles of the costs of MS were published in 2010Citation31,Citation32. Both of these studies examined literature for early through mid-2009 and emphasized comparisons in MS costs by country. Sharac et al.Citation32 reviewed 32 studies from Europe, Australia, and the Netherlands, as well as seven from the US. With all direct and indirect costs included, the average cost per patient per year across all countries ranged from ∼$7000–$78,000 in 2008 US dollars. Another systematic review focusing on the intangible costs associated with MS found 13 studies, but only one was from the USCitation33. This review reports that intangible costs represent about one-quarter to one-half of the costs of MS internationally. A 2011 German study included the cost burden of RRMS, SPMS, and PPMS, and also included the cost of the disease by EDSS scoreCitation34. Early retirement was included in the indirect cost estimate in these analyses. The total estimate in this comprehensive cost analysis was €41,316 per patient per year (approximately equivalent to $53,000 in 2009 dollars), with early retirement making up ∼32% of the total cost. They also found SPMS and PPMS to be more costly than RRMS. An earlier multi-country reviewCitation35 evaluated 10 studies (two from the US) to estimate MS costs by EDSS score and found that both direct and indirect costs increased with increasing disabilityCitation35.
Our systematic literature review evaluated recent estimates of the direct and indirect costs associated with MS in the US. The majority of the studies included in this review estimated annual direct costs at between ∼$16,000–$34,000 and indirect costs under $20,000 per patient per year in 2011 dollars. Ivanova et al.Citation10 and Birnbaum et al.Citation26 reported much lower direct costs than other studies included in this review owing to exclusion of the cost of DMTs from their analyses. Noyes et al.Citation18 reported both direct and indirect costs that were much higher than the other included studies. The indirect costs in this study were higher because, in addition to missed work days and disability, they also included under-employment due to MS (part-time work instead of full-time work) and episodes of unemployment due to MS. Overall, according to the studies in this review the largest cost drivers of the disease are prescription drugs and indirect costs. There was no apparent trend for costs and publication year, cost year, or the years from which data were obtained across the included studies.
The variations in costs – both direct and indirect – across the included studies are largely a result of differences in study designs and cost categories. Consequently, it is difficult to provide an accurate estimate of the comprehensive costs associated with MS.
Studies that did not meet the inclusion criteria
There are some studies that did not meet our search parameters but which should be mentioned when assessing the current MS cost structure. A study by Earnshaw et al.Citation36 estimated costs by EDSS score. They derived their costs from a study undertaken in 2003, and inflated those costs to 2007 US$. The costs were analyzed using a patient-reported survey on MSwatch.com, and included both direct costs and indirect costs, which included devices and investments to adapt living conditions. Costs in this study were reported on a monthly basis and were multiplied by 12 to give a per patient per year value. The annual cost was reported at $5353 per patient with an EDSS score of 0.0–2.5; at $11,110 in patients with an EDSS score of 3.0–5.5; at $27,807 in patients with an EDSS score of 6.0–7.5; and at $49,823 in patients with an EDSS score of 8.0–9.5. Thus, the estimate for the highest level of disability was almost 10-times more costly than for the lowest level of disability. The increase in costs for the two highest EDSS categories likely reflects loss of ambulation and a patient’s need for a wheelchair at an EDSS score of 7. It is also important to note that the EDSS score is not currently used in routine clinical practice. Furthermore, it has limitations in defining disability including a bias toward locomotor function, and an insensitivity to change in the patient’s disability. The study by Earnshaw et al.Citation36 also included the cost of relapses. Relapse with an EDSS score of 0.0–2.5 was estimated to cost $5725 per patient per year and relapse with an EDSS score of 3.0–5.5 cost $14,855 per patient per year. Oleen-Burkey et al.Citation37 estimated the annual cost of relapse at $4449, but no specifications were made for EDSS score.
Another important study, published in 2006Citation38, gathered data from self-reported patient surveys using the North American Committee on Multiple Sclerosis Patient Registry. Average cost of all MS types was reported, and early retirement was included in the analysis. Indirect costs made up 56% of total costs, with early retirement accounting for 34% of total costs. In comparison, the cost of drugs made up only 28% of the total. In this study, costs (direct and indirect) were also stratified by EDSS score ranges. In 2004 US$, EDSS score <4.0 was reported with a cost just over $30,000 per patient per year; EDSS 4.0–6.0 was ∼$50,000; and EDSS >6.0 was almost $70,000; costs were ∼$50,000 across all MS patientsCitation38. These figures are much higher than the studies included in this review as a result of the inclusion of early retirement, which can have a major effect on total costs. This study was not included in the current analysis as it was published outside the search parameters (2006). With insights from a recent retrospective cohort study based on prospectively collected data (1985–2008) from British Columbia, Canada, which showed that current DMTs such as interferon beta have very little effect on the progression of disabilityCitation39, it is important to capture early retirement as an economic end-point as new therapies seek to delay the onset of disability.
Limitations
Our ability to quantify the total per patient cost of MS was limited by a lack of available data in the literature. Specifically, the costs associated with increasing levels of disability as well as early retirement have not been reported. In addition, variations in study designs, populations, methodologies, and cost inputs make it difficult to confidently estimate the actual direct and indirect costs of MS. As an example, Ivanova et al.Citation23 split general healthcare into only inpatient and outpatient care, while Noyes et al.Citation18 split general healthcare into inpatient care, outpatient care, physical/occupational therapy, and home healthcare. The use of differing methodologies and inputs was a common thread across the studies for both direct and indirect costs.
The data sources in these studies tended to be older, ranging from 1999–2008, and therefore do not include some of the newer and more costly drug therapiesCitation40, which could greatly increase direct and total costs. In addition, this review does not include assessment of the decrements to quality-of-life associated with MS. Considering the magnitude to which disability and relapse can affect the quality of a person’s life could add a considerable burden to this already costly disease.
Implications for a future study
The current literature review demonstrates a need for an updated, comprehensive, economic burden study for MS. Such a study should include the direct and indirect costs of MS by disease type (RRMS, PPMS, and SPMS), disability score, and by cost components (including outpatient care, inpatient care, long-term care, prescription drugs, and missed days of work). In addition, more research is needed to estimate the costs of an individual relapse, the cost of early retirement, which may be especially important in PPMS, or periods of withdrawal from the work force.
Based on our review, this type of comprehensive study has not been done in the US since the introduction of the newer MS treatments into the market. An ideal cost study would lay the groundwork to help healthcare practitioners understand the potential impact of new treatments that have recently been approved and/or are expected to launch in this disease area over the coming years. Indeed, these new therapies have the potential to challenge existing treatment paradigms, and subsequently treatment cost considerations, in several fundamental waysCitation41. Firstly, in addition to fingolimod, other new agents are entering the marketplace (such as teriflunomide, an alternative once-daily oral immunomodulatory therapy approved by the US Food and Drug Administration in September 2012)Citation42, and there are likely to be additional new oral alternatives (e.g., BG-12) that have different, and possibly more convenient, dosing and administration schedules. It is unknown how the convergence of efficacy, side-effect profiles, and costs of these agents will be viewed compared with well-established, yet perhaps more cumbersome injectables. Secondly, multiple emergent monoclonal antibody therapies (e.g., alemtuzumab, daclizumab, ofatumumab, and ocrelizumab) will likely drive new risk–benefit calculations for all therapeutic algorithms. Finally, combination therapy with approved agents appears to be a clinical possibility in a market that, until now, has not been presented with this scenario.
Conclusion
MS is a costly chronic disease, with prescription drug costs and indirect costs being the largest cost drivers according to reports in the literature. Compared with a study that estimated and ranked the direct costs of a number of chronic diseases, our review suggests that MS is the second most costly chronic condition after congestive heart failure. Ultimately, if new therapies could slow the progression of disability and decrease the number of relapses for patients with MS, they would be vitally important in reducing the cost burden of the disease.
Transparency
Declaration of funding
All activities relating to this study were conducted internally within Genentech, Inc. GA and KV were responsible for the literature review. All authors contributed to the interpretation of the data and writing of the manuscript.
Declaration of financial/other relationships
KV and SGR are employees of Genentech, Inc. GA was an employee of Genentech, Inc. at the time of authorship.
Supplementary Material
Download PDF (54 KB)Acknowledgments
Support for third-party writing assistance for this manuscript was provided by F. Hoffmann-La Roche Ltd.
References
- Dutta R, Trapp B. Pathogenesis of axonal and neuronal damage in multiple sclerosis. Neurology 2007;68(22 Suppl 3):S22-31
- Kasper LH, Shoemaker J. Multiple sclerosis immunology: the healthy immune system vs the MS immune system. Neurology 2010;74(Suppl 1):S2-8
- Disanto G. The evidence for a role of B cells in multiple sclerosis. Neurology 2012;78:823-32
- Multiple sclerosis. PubMed Health. US National Library of Medicine, 2011. www.ncbi.nlm.nih.gov/pubmedhealth/PMH0001747/. Accessed July 31, 2012
- The IFNB Multiple Sclerosis Study Group and the University of British Columbia MS/MRI Analysis Group. Interferon beta-1b in the treatment of multiple sclerosis: final outcome of the randomized controlled trial. Neurology 1995;45:1277-85
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis: The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- PRISMS. PRISMS-4: long-term efficacy of interferon beta-1a in relapsing MS. Neurology 2001;56:1628-36. Erratum in Neurology 2001;57:1146
- Johnson KP, Brooks BR, Cohen JA, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability: Copolymer 1 Multiple Sclerosis Study Group. Neurology 1998;50:701-8
- Polman C, O’Connor RW, Havrdova E, et al. A randomized placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899-910
- Ivanova JI, Phillips AL, Bergman RE, et al. Impact of medication adherence to disease-modifying drugs on severe relapse, and direct and indirect costs among employees with multiple sclerosis. J Med Econ 2012;15:601-9
- Goldberg LD, Edwards NC, Fincher C, et al. Comparing the cost-effectiveness of disease-modifying drugs for the first-line treatment of relapsing-remitting multiple sclerosis. J Manag Care Pharm 2009;15:543-55
- Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. CHAMPS Study Group. N Engl J Med 2000;343:898-904
- Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006;67:1242-9
- Comi G, Martinelli V, Rodegher M, et al; PreCISe Study Group. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet 2009;374:1503-11
- Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis. Lancet 2001;357:1576-82
- Curkendall SM, Wang C, Johnson BH, et al. Potential health care cost savings associated with early treatment of multiple sclerosis using disease modifying therapy. Clin Ther 2011;14:61-9
- Brook RA, Rajagopalan K, Kleinman NL, et al. Absenteeism and health-benefit costs among employees with MS. Curr Med Res Opin 2009;25:1469-76
- Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based evaluation. Neurology 2011;77:355-63
- Rajagopalan K, Brook RA, Beren IA, et al. Comparing costs and absences for multiple sclerosis among US employees: pre- and post-treatment initiation. Curr Med Res Opin 2011;27:179-88
- Castelli-Haley J, Oleen-Burkey MA, Lage MJ, et al. Glatiramer acetate and interferon beta-1a for intramuscular administration: a study of outcomes among multiple sclerosis intent-to-treat and persistent-use cohorts. J Med Econ 2010;13:464-71
- Castelli-Haley J, Oleen-Burkey MA, Lage MJ, et al. Glatiramer acetate and interferon beta-1b: a study of outcomes among patients with multiple sclerosis. Adv Ther 2009;26:552-62
- Prescott JD, Factor S, Pill M, et al. Descriptive analysis of the direct medical costs of multiple sclerosis in 2004 using administrative claims in a large nationwide database. J Manag Care Pharm 2007;13:44-52
- Ivanova JI, Birnbaum HG, Samuels S, et al. The cost of disability and medically related absenteeism among employees with multiple sclerosis in the US. Pharmacoeconomics 2009;27:681-91
- Oleen-Burkey M, Castelli-Haley J, Lage MJ, et al. Burden of a multiple sclerosis relapse. Patient 2012;5:57-69
- Asche CV, Singer ME, Jhaveri M, et al. All-cause health care utilization and costs associated with newly diagnosed multiple sclerosis in the United States. J Manag Care Pharm 2010;16:703-12
- Birnbaum HG, Ivanova JI, Samuels S, et al. Economic impact of multiple sclerosis disease-modifying drugs in an employed population: direct and indirect costs. Curr Med Res Opin 2009;25:869-77
- Gilden DM, Kubisiak J, Zbrozek AS. The economic burden of Medicare-eligible patients by multiple sclerosis type. Value Health 2011;14:61-9
- Lad SP, Chapman CH, Vaninetti M, et al. Socioeconomic trends in hospitalization for multiple sclerosis. Neuroepidemiology 2010;35:93-9
- Tan H, Cai Q, Agarwal S, et al. Impact of adherence to disease modifying therapies on clinical and economic outcomes among patients with multiple sclerosis. Adv Ther 2011;28:51-61
- Center for Healthcare Research and Transformation. Health care cost drivers: chronic disease, comorbidity, and health risk factors in the U.S. and Michigan. Issue Brief July 2010. Center for Healthcare Research & Transformation. Ann Arbor, MI. http://www.chrt.org/publications/price-of-care/issue-brief-2010-08-health-care-cost-drivers/. Accessed November 14, 2012
- Naci H, Fleurence R, Birt J, et al. Economic burden of multiple sclerosis; A systematic review of the literature. Pharmacoeconomics 2010;28:363-79
- Sharac J, McCrone P, Sabes-Figuera R. Pharmacoeconomic considerations in the treatment of multiple sclerosis. Drugs 2010;70:1677-91
- Wundes A, Brown T, Bienen EJ, et al. Contribution of intangible costs to the economic burden of multiple sclerosis. J Med Econ 2010;13:626-32
- Reese JP, John A, Wienemann G, et al. Economic burden in a German cohort of patients with multiple sclerosis. Eur Neurol 2011;66:311-21
- Patwardhan MB, Matchar DB, Samsa GP, et al. Cost of multiple sclerosis by level of disability: a review of literature. Multi Scler 2005;11:232-9
- Earnshaw SR, Graham J, Castelli-Haley J, et al. Cost-effectiveness of glatiramer acetate and natalizumab in relapsing-remitting multiple sclerosis. Appl Health Econ Health Policy 2009;7:91-108
- Oleen-Burkey M, Kobelt G, Borgstrom F. Costs and quality of life of patients with relapsing-remitting multiple sclerosis currently on immunomodulatory therapy in the United States. International Committee on Databases in Multiple Sclerosis, 2003; Abstract Hackensack, NJ
- Kobelt G, Bery J, Atherly D, et al. Costs and quality of life in multiple sclerosis: a cross-sectional study in the United States. Neurology 2006;66:1696-702
- Shirani A, Zhao Y, Karim ME, et al. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA 2012;308:247-56
- Von Schaper E, Kresge N. Novartis’s $48,000 pill spurs U.S. price increases for older MS treatments. 2011. www.bloomberg.com/news/2011-03-21/novartis-s-48-000-pill-spurs-u-s-price-increases-for-ms-drugs.html. Accessed July 23, 2012
- Fox EJ, Rhoades RW. New treatments and treatment goals for patients with relapsing-remitting multiple sclerosis. Curr Opin Neurol 2012;25(1 Suppl):S11-19
- US Food and Drug Administration. FDA approves new multiple sclerosis treatment Aubagio. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm319277.htm. Accessed November 2, 2012