Abstract
Background:
The prevalence of severe hypertriglyceridemia (TG > 1000 mg/dl) is estimated at 150–400 per 100,000 individuals in North America. Severe hypertriglyceridemia in the fasting state is associated with increased acute pancreatitis risk and is a sign of chylomicronemia which reflects the accumulation in the bloodstream of chylomicrons, the large lipoprotein particles produced in the gut after a meal.
Objective:
To assess medical resource use and costs associated with chylomicronemia.
Methods:
Patients with chylomicronemia of different causes (≥2 diagnoses with ICD-9 code 272.3) were identified from a large US claims database (years 2000 to 2009) and matched 1:1 to controls free of chylomicronemia based on age, gender, demographics, comorbidities, and use of lipid lowering drugs. During a 1-year study period, medical resource use and costs associated with chylomicronemia or acute pancreatitis were compared between matched cases and controls.
Results:
Among 6472 matched pairs, annual per-patient medical costs, calculated independently of the occurrence of acute pancreatitis, were significantly greater by $808 for chylomicronemia cases vs controls ($8029 vs $7220, p < 0.01), half of which was attributable to chylomicronemia-related services (p < 0.01). Chylomicronemia cases with a history of acute pancreatitis (n = 46) had greater rates of inpatient visits (p < 0.05) and greater average costs for subsequent acute pancreatitis or abdominal pain (p < 0.01) as well as greater total medical costs ($33,587 vs $4402, p < 0.01) vs matched controls. The average episode of acute pancreatitis (n = 104 episodes) generated medical costs of $31,820, almost entirely due to inpatient stays.
Limitations:
Triglyceride levels were not available to characterize disease severity.
Conclusions:
Patients with chylomicronemia, and especially those with a history of acute pancreatitis, incurred significantly greater total medical costs compared with individuals without chylomicronemia but with an otherwise comparable health profile.
Introduction
Chylomicrons are large lipoproteins produced in the gut wall following a meal and deliver dietary triglycerides (TG) from the gut to other parts of the body. Chylomicrons are usually rapidly cleared from the bloodstream as a consequence of TG hydrolysis by the enzyme lipoprotein lipase (LPL), a process that supplies body tissues with fatty acids for metabolic fuel. When LPL is defective or otherwise saturated, chylomicrons accumulate in the plasma. Chylomicronemia or hyperchylomicronemia (i.e., the presence of chylomicrons in the fasted state) starts to develop as chylomicrons begin to persist in fasting serum, with triglycerides >400 mg/dl. With TG levels of 1000 mg/dl most patients have chylomicronemiaCitation1. Chylomicronemia is associated with a number of complications collectively referred to as the chylomicronemia syndrome. The most serious of these complications is acute pancreatitis, which can be life threatening and recurring, sometimes leading to chronic pancreatic insufficiency. Other findings associated with chylomicronemia include plasma lactescence, lipemia retinalis, eruptive xanthomas, abdominal pain, hepatosplenomegaly, neuro-cognitive deficits, dyspnea, or cardiometabolic complicationsCitation1–3.
The prevalence of chylomicronemia (TG > 1000 mg/dl) is estimated at 150–400 per 100,000 individuals in North AmericaCitation4,Citation5. The vast majority of these patients have plasma accumulation of both chylomicrons and very low-density lipoproteins (Fredrickson type V hyperlipoproteinemia and other phenotypes)Citation6. The majority of affected individuals typically have a genetic predisposition toward high triglycerides exacerbated by one or more acquired risk factors including disease states (e.g., type 2 diabetes mellitus, hypothyroidism)Citation7, medications (e.g., estrogens, glucocorticoids), elements of the metabolic syndrome (e.g., obesity, insulin resistance), life habits (e.g., excess dietary fat intake), and other conditions (e.g., alcohol intake, pregnancy)Citation8–11. These patients have a risk of acute pancreatitis in proportion to their chylomicronemia and plasma triglyceride levels, and generally respond to a reduction in dietary fat or TG-lowering drugs such as fibrates, fish oil, and niacinCitation12. TG can also be controlled by optimizing treatment of diabetes or other exacerbating factors and avoiding alcohol or offending medications. However, despite optimal or maximally tolerated therapy, some patients continue to have elevated TG levels and episodes of acute pancreatitisCitation13.
A tiny fraction of patients with chylomicronemia have the familial chylomicronemia syndrome (FCS; Fredrickson type I hyperlipoproteinemia), a rare autosomal recessive disease associated with gene defects severely affecting lipoprotein lipase activity. With an estimated worldwide prevalence of 0.1–0.2 per 100,000Citation14, FCS accounts for only a small proportion of patients with chylomicronemia, but affected individuals have the highest risk of pancreatitis among patients with chylomoicronemia. FCS patients typically present in childhood with massively elevated triglyceride levels (>2000 mg/dL) and recurrent bouts of abdominal pain. Into adulthood, both plasma TG levels and risk of acute pancreatitis and other complications remain very elevated. Because standard TG-lowering medications are generally ineffective in FCS, the standard of care therapy is a very restrictive low fat dietCitation15,Citation16.
With the growing epidemic of type 2 diabetes and obesity, the number of patients with other causes of chylomicronemia than FCS is expected to increase in coming yearsCitation17–19. Hence, the medical care of chylomicronemia and its complications will require a larger share of resources from healthcare systems. We have conducted the present study for the purpose of quantifying the cost of chylomicronemia and chylomicronemia associated pancreatitis. Using recent data from healthcare payers throughout the US, we show here that there is a substantial incremental medical cost associated with a diagnosis of chylomicronemia, especially in affected patients who have acute pancreatitis.
Methods
Sample selection
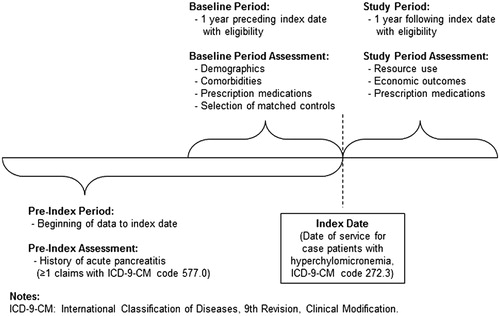
The Thomson MarketScan US Commercial Claims and Encounters and Medicare Supplemental DatabaseCitation20 contains health insurance eligibility and medical service claims from ∼100 different insurance providers and third party administrators in the US. The data represent the medical claims of insured employees and their dependents from over 40 national employers and Medicare-eligible retirees with employer-provided Medicare Supplemental plans for ∼40 million covered lives. Among enrollees with at least 1 year of continuous eligibility between 2000–2009, chylomicronemia cases were identified as having two or more medical claims at different dates of service with International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM)Citation21 code 272.3, hyperchylomicronemia, which includes FCS and non-FCS. Specifically, ICD-9-CM code 272.3 comprises Bürger-Grütz syndrome, Fredrickson type I and or V hyperlipoproteinemia, hyperlipidemia group D, and mixed hyperglyceridemiaCitation22. Therefore, it is not possible to distinguish between FCS and non-FCS, and cases may include a combination of FCS and non-FCS chylomicronemia patients. An index date for each case was defined as the first chylomicronemia claim that was preceded and followed by at least 1 year of continuous health plan eligibility (). Since an objective of this study was to assess the economic burden of patients with chylomicronemia and a history of acute pancreatitis, follow-up time was divided into a pre-index period (for assessing diagnosis history) and a post-index period (for assessing costs). History of acute pancreatitis was assessed during all periods of health plan eligibility prior to the index date.
Chylomicronemia cases were matched to two sets of control patients to meet different analytical objectives: (1) to assess pre-index comorbidities associated with chylomicronemia and (2) to assess the incremental economic burden of chylomicronemia. To assess pre-index comorbidities associated with chylomicronemia, each chylomicronemia case was exactly matched 1:1 to a control patient free of claims with ICD-9-CM 272.3 on the basis of age (±1 year), sex, geographic region, index year, and health plan type (e.g., preferred provider organization, health maintenance organization). To assess the incremental economic burden of chylomicronemia, cases were matched 1:1 to controls on the aforementioned characteristics with additional exact matching on all of the following: history of diagnoses for diabetes, hypertension, and obesity (see Appendix 1 for ICD-9-CM codes), and any prescription fills for the following classes of lipid-lowering medications: fibric acid derivatives, HMG CoA reductase inhibitors, intestinal cholesterol absorption inhibitors, nicotinic acid derivatives, and lipid-lowering combinations. Classes of lipid-lowering medications were identified using generic product identifier (GPI) codes (see Appendix 2). In each matched sample, controls were assigned the same index date as their matched case, and were required to have at least 1 year of eligibility preceding and following that index date. In addition to matching on history of diabetes in the core analyses, a sensitivity analysis was conducted to assess the burden of chylomicronemia in the absence of diabetes.
Outcome measures
Health resource use and costs during the 1-year post-index period were assessed from claims for inpatient, emergency room, outpatient, and other services (e.g., lab visits, home healthcare visits), and from prescription fills. Medical service claims were classified as chylomicronemia-related, acute pancreatitis-related, abdominal pain-related, or cardiovascular-related based on associated ICD-9-CM codes (see Appendix 1). Prescription fills were assessed for fibric acid derivatives, HMG CoA reductase inhibitors, intestinal cholesterol absorption inhibitors, nicotinic acid derivatives, bile acid sequestrants, and lipid-lowering combinations. Total medical costs are reported in 2009 US dollars and included paid amounts for inpatient, outpatient, emergency room, and other services. Total costs included all medical and prescription drug costs.
Statistics
Demographics, comorbidities, medication use, and medical service use in the pre-index period were compared between chylomicronemia cases and matched controls using McNemar’s test for categorical characteristics and the Wilcoxon signed rank test for continuous characteristicsCitation23,Citation24. Assessed comorbidities included factors known to be secondary causes of chylomicronemia (e.g., alcohol abuse, hypothyroidism, infections)Citation4,Citation7,Citation8 and outcomes associated with chylomicronemia (acute pancreatitis, anxiety, hepatomegaly, splenomegaly)Citation25. During the 1-year outcome period, health resource use and costs were compared for matched cases vs controls using the same tests. Analyses were conducted for all chylomicronemia cases and matched controls and for the sub-group of chylomicronemia cases with a history of acute pancreatitis (). Medical service use, costs, and diagnoses were also summarized among all episodes of acute pancreatitis (pre- and post-index) for sampled chylomicronemia cases, with an episode defined as the time from an inpatient diagnosis for acute pancreatitis to the end of the first 30-day period free of claims for acute pancreatitis at any place of service. Statistical significance was assessed at the 5% level. All statistical analyses were carried out using SAS® 9.2Citation26.
Results
Sample selection and baseline characteristics
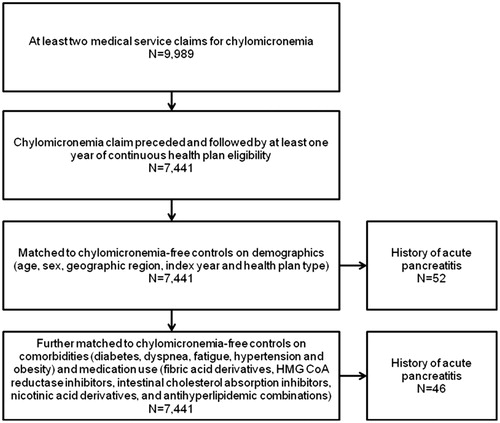
Among over 40 million total claimants, 9989 had at least two claims for chylomicronemia and, among these cases, 7441 had a claim for chylomicronemia that was preceded and followed by at least 1 year of continuous healthcare coverage. A total of 7254 chylomicronemia cases were matched to chylomicronemia-free controls on demographic factors. Fifty-two of these matched cases had a history of acute pancreatitis. Further matching on comorbidities and medication use in addition to demographics resulted in 6472 matched pairs, among which 46 chylomicronemia cases had a history of acute pancreatitis ().
Among the cases and controls matched on demographics, the mean age was 54.6 years and 52% were male. During the pre-index periods, chylomicronemia cases had significantly higher rates of diabetes, hypertension, hypothyroidism, obesity, and other dyslipidemia compared to control patients. Cases also had greater use of lipid lowering drugs. Diagnoses of acute pancreatitis were rare in the period prior to index date, but significantly more prevalent among cases than controls (0.7% vs 0.3%; p < 0.01). Inpatient diagnoses for acute pancreatitis in the pre-index period were infrequent, affecting 0.2% of cases and 0% of controls. Among the 52 chylomicronemia cases with a history of acute pancreatitis and their matched controls, the mean age was 50.2 years and 48.1% were male. Cases had significantly higher rates of hypertension (50% vs 28.8%, p < 0.05) and fibric acid use (19.2% vs 3.8%, p < 0.05) compared to controls. Comorbidities (e.g., HIV, lupus, organ transplant) that could contribute to secondary chylomicronemia were rare among all cases and among those with a history of acute pancreatitis, most often affecting less than 1% of cases and showing no statistically significant differences between cases and controls. Hypothyroidism, metabolic syndrome, and renal disease were significantly more prevalent among cases than controls in the full cohort, each by less than 3%. Hypothyroidism remained significantly more prevalent among cases in the sub-group with a history of acute pancreatitis (five patients vs one patient, p < 0.05).
After further matching on comorbidities and medication use, the mean age among 6472 case-control pairs was 54.8 years and 51.7% were male. Chylomicronemia cases remained significantly more likely to have histories of acute pancreatitis and hypothyroidism (). In the sub-group of 46 chylomicronemia cases with a history of acute pancreatitis, the mean age was 51.2 years and the percentage of males was 45.7%. Due to the matching on medication use, there were no significant differences in the use of lipid lowering drugs between the groups.
Table 1. Baseline characteristics for chylomicronemia cases and controls matched on demographics, comorbidities, and prescriptions.
Outcomes
During the 1-year study period, there were no statistically significant differences between chylomicronemia cases and controls matched on age, gender, demographics, comorbidities, and prescriptions in the full sample (n = 6472) in terms of rates of all-cause inpatient visits (8.2 vs 7.8%, p = 0.41), acute-pancreatitis related events (0.2% vs 0.1%, p = 0.18) or abdominal pain (1.3% vs 1.2%; p = 0.42, ). However, rates of chylomicronemia-related resource use were significantly greater among cases vs controls for inpatient events (0.3% vs 0%; p < 0.01), emergency room visits (0.2% vs 0%, p < 0.01) and outpatient visits (88.1% vs 0%; p < 0.01). Almost 70% of chylomicronemia cases had an outpatient visit with a primary diagnosis claim for chylomicronemia during the 1-year post-index period. Chylomicronemia cases also had significantly more visits to cardiologists (18.1 vs 14.5%; p < 0.01) or endocrinologists (3.2% vs 2.0%, p < 0.01). Despite baseline matching of lipid lowering prescription fills during the pre-index period, greater proportions of chylomicronemia cases filled prescriptions for these medications (fibrates, HMG CoA reducatase inhibitors, cholesterol absorption inhibitors, nicotinic acid derivatives, bile acid sequestrants and combination therapies) during the post-index period (43.9 vs 33.0%; p < 0.01) ().
Table 2. Health resource use outcomes for chylomicronemia cases and matched controls.
Total costs during the 1-year post-index period were significantly greater for matched chylomicronemia cases vs controls ($8029 vs $7220, p < 0.01), a relative increase of 11% and an absolute increase of $808, half of which was attributable to chylomicronemia-related services (p < 0.01) (). Costs were higher in cases vs controls for chylomicronemia-related inpatient services (p < 0.01) and cardiovascular-related inpatient services ($1090 vs $786, p < 0.05), and for outpatient visits related to abdominal pain ($114 vs $90, p < 0.01), visits to cardiologists or endocrinologists (p < 0.01). Although lipid-lowering medication costs were significantly higher for cases vs controls ($386 vs $616, p < 0.01), total prescription costs were comparable ($1672 vs $1691, p = 0.21). In the sensitivity analysis excluding patients with diabetes, total costs were lower for both cases and controls ($7337 vs $6331, p < 0.01), but the incremental burden of chylomicronemia remained similar to that of the full population.
Table 3. Cost outcomes for chylomicronemia cases and matched controls.
Among chylomicronemia cases with a history of acute pancreatitis, rates of inpatient visits during the post-index period were significantly greater compared to matched controls (23.9% vs 6.5%, p < 0.05), largely attributable to increased inpatient visits related to acute pancreatitis (15.2% vs 0%, p < 0.05) and abdominal pain (19.6% vs 0%, p < 0.01) (). ER visits were also higher among cases vs controls, but the differences were not statistically significant.
Total costs during the 1-year post-index period were significantly greater for matched chylomicronemia cases with a history of acute pancreatitis vs controls ($33,587 vs $4403, p < 0.01) (). The difference was attributable to greater costs among cases vs controls for all-cause inpatient services ($9325 vs $411, p < 0.01). Costs among cases were numerically greater for inpatient services related to chylomicronemia ($3235 vs $0), acute pancreatitis ($6647 vs $0), abdominal pain ($7725 vs $0), and cardiovascular events ($958 vs $176), but only the differences in acute pancreatitis and abdominal pain-related costs were statistically significant (p < 0.05). Outpatient costs were significantly greater for cases vs controls in total ($7612 vs $2113, p < 0.01), partly attributable to visits for chylomicronemia ($349 vs $0, p < 0.01), acute pancreatitis ($124 vs $0, p < 0.01) and abdominal pain ($835 vs $68, p < 0.01). Average costs for endocrinologist visits among cases were high ($2300), but were not statistically different from the absence of these costs among controls (p = 0.06). Cases were not associated with a statistically significant difference in total prescription costs ($2515 vs $1521, p = 0.14) or costs for lipid lowering drugs compared to controls ($206 vs $230, p = 0.87). In the sensitivity analysis excluding patients with diabetes, total costs were lower for both cases and controls ($32,346 vs $3472, p < 0.01), but the incremental burden of chylomicronemia with a history of acute pancreatitis remained similar to that of the full population.
Acute pancreatitis episodes
Among the chylomicronemia patients, we identified 104 episodes of acute pancreatitis. Ninety-seven episodes (93.3%) involved an inpatient stay, with an average of 10 days spent in an inpatient setting (median = 6 days; range = 2–117 days). Ten per cent of episodes had re-admissions within 30 days of discharge. Thirteen per cent of episodes involved an emergency room visit. There was an average of three outpatient visits per episode. Average total medical costs were $31,820 per episode of acute pancreatitis, with inpatient stays accounting for $30,408.
Discussion
In this retrospective analysis of US data combining claims for both FCS and non-FCS causes of chylomicronemia, the average patient with a diagnosis for chylomicronemia, with or without history of acute pancreatitis, incurred ∼$808 in additional costs per year relative to matched controls. These additional costs associated with chylomicronemia were in excess of those attributable to age, diabetes, or other comorbidities, and occurred in the presence of current treatment patterns with lipid lowering prescriptions. Among patients with chylomicronemia and a history of acute pancreatitis, excess costs were substantially higher, approaching $30,000 per patient per year, with over one third attributable to chylomicronemia- or acute pancreatitis-related medical services. For comparison, the direct cost of hypertension in the US was ∼$1100 per patient in 2006Citation27, and the direct cost of diabetes was ∼$8000 per patient in 2002Citation28.
It has been reported that the risk of acute pancreatitis in patients with chylomicronemia is increased by >50-fold compared to normolipidemic controlsCitation10,Citation29, the risk being the highest (increased by 350-fold) in the sub-group of FCS patients with complete LPL deficiency (LPLD). This study combined both FCS and non-FCS causes of chylomicronemia and the annual incidence of inpatient care for acute pancreatitis was 0.2% in the presence of chylomicronemia. For patients with chylomicronemia and a history of acute pancreatitis, the annual incidence of inpatient admission with acute pancreatitis reached 15.2%, representing a 150-fold increase compared to the full control sample, and more than a 300-fold increase compared to published incidences of acute pancreatitis in the US populationCitation25,Citation30. Although elevated, this incidence is lower than the 35 events per 100 person-years of acute pancreatitis previously reported among patients with FCS only, and is compatible with published data on the increased risk of acute pancreatitis in FCS and non-FCS patientsCitation10,Citation29. Among patients with sustained hypertriglyceridemia (TG ≥ 500 mg/dL), annual risk of acute pancreatitis has been reported as 1.1%, compared to 0.5% among patients with controlled triglyceride levels <500 mg/dLCitation31.
In the present study of the broader population of patients with chylomicronemia diagnoses, the annual incidence of an inpatient admission with acute pancreatitis among control patients free of chylomicronemia was 0.1%. This higher risk is likely due to matching the controls to chylomicronemia cases on history of diabetes, hypertension, and use of lipid lowering medications. As a consequence, controls in the present study are likely to be more severely affected by chronic metabolic diseases or at higher metabolic risk than the general population. Thus, the results presented herein reflect the incremental cost of chylomicronemia vs controls without chylomicronemia, but with an otherwise comparable health profile, and may under-estimate the economic burden of chylomicronemia compared to health services consumption in the general population.
Medical costs per episode of acute pancreatitis exceeded $30,000 in the present study, with an average inpatient stay of 10 days following admission. This economic impact per episode was greater than observed in a prior study of inpatient care for all-cause acute pancreatitis, which found an average cost of ∼$10,000 per admission and stays averaging 6 daysCitation32. However, the economic impact of an episode of acute pancreatitis observed in the present study (>$30,000) represents the category of chylomicronemia-associated acute pancreatitis only (not all-cause acute pancreatitis). In comparison, the average in-hospital costs of an episode of acute pancreatitis in Canada among FCS patients affected by LPLD were $50,000, according to a study published in 1997Citation33. The longer stays and greater costs in the present study are similar to our inclusion of initial admission and re-admissions, which occurred for 10% of patients, into a single episode of acute pancreatitis. The high costs of acute pancreatitis contributed over 20% of the excess costs for patients with chylomicronemia and history of acute pancreatitis vs matched controls. Acute pancreatitis resulting in hospitalization represents the most severe end of a spectrum of symptomsCitation25. Chylomicronemia patients with a history of acute pancreatitis also generated higher costs for services related to abdominal pain, in the outpatient and emergency department settings compared to controls. A recent study in a more severe patient population has estimated the cost of persistent hypertriglyceridemia as $1400 in the first year, compared to patients with controlled hypertriglyceridemiaCitation31.
Chylomicronemia patients incurred ∼$800 more in medical costs compared with controls. Chylomicronemia-related medical services accounted for one half of these excess costs and costs related to cardiovascular disease accounted for most of the remainder. Cardiovascular-related inpatient and outpatient services were also drivers of higher cost among chylomicronemia patients with a history of acute pancreatitis. It was not possible to determine whether cardiovascular risk was associated with chylomicronemia itself or explained by other risk factors associated with chylomicronemia or other classes of TG-rich lipoproteins in the present retrospective study of claims data. Other studies have documented that FCS patients with complete LPLD may present atherosclerosis and cardiovascular complicationsCitation3, although increased risk of cardiovascular events is not considered as a feature of FCS. However, it is documented that cardiovascular risk in the presence of chylomicronemia and lactescent plasma is significantly increased in non-FCS patients compared to FCS, moderate hyper TG, or normolipidemic controlsCitation10. Although reduction of cardiovascular risk could result in reduced medical service utilization and costs, further study is needed to assess the impact of chylomicronemia per se on cardiovascular risk, and the extent to which that impact can be reduced by controlling chylomicron-TG or total TG levels.
Since chylomicronemia can be exacerbated by diabetes, the present study design matched cases and controls with a history of diabetes diagnosis. However it was not possible to match precisely on diabetes severity due to the limited information available in claims data. To ensure that differences in diabetes severity were not contributing to the apparent burden of chylomicronemia, sensitivity analyses were conducted in the sub-group of cases and controls free of diabetes diagnosis. These sensitivity analyses identified similar incremental costs of chylomicronemia, and of chylomicronemia with a history of acute pancreatitis, as seen in the full population, suggesting that differences in diabetes severity were not a confounding factor when comparing costs between chylomicronemia cases and their matched controls. However, it should be noted that the present analysis, by matching on diabetes diagnosis, does not capture the impact of chylomicronemia on increased risk of diabetes and associated costs.
Chylomicronemia of any cause also confers an increased risk of acute pancreatitis. Among those patients who already experienced an episode of acute pancreatitis, interventions that could reduce the risk of future recurrences could have substantial economic and clinical benefits. Among chylomicronemic patients without history of acute pancreatitis, future efforts at risk-stratification may help identify the patients that would benefit most from interventions to reduce the risk.
The present study has limitations. Firstly, inclusion was based on ICD-9 codes not on laboratory test results, genotyping nor fine clinical lipid phenotyping. Secondly, the results do not distinguish between the underlying cause of chylomicronemia: FCS (Frederickson Type I, LPLD) or non-FCS (Frederickson type V or others)Citation22, since these conditions share the same ICD-9 code (which also contains Bürger-Grütz syndrome, another name for Frederickson’s Type 1, hyperlipidemia Group D, and mixed hyperglyceridemia). As a consequence, the present study could not stratify nor distinguish the costs between patients with Frederickson’s Types I and V, and also likely included patients with chylomicronemia and high TG levels who are of neither Fredrickson Type I nor VCitation22. Thirdly, there is also a risk of misclassification or miscoding of diagnoses in claims. In order to reduce the effect of missing data or data errors, inclusion in our study sample required at least two claims for chylomicronemia to avoid selection of patients who may be miscoded or experiencing only a brief and transient episode of chylomicronemia. Finally, the data used in this study consists of claims for medical services and prescription fills provided throughout the US and reimbursed by private insurers (including Medicare supplemental insurance). The economic findings may not be generalizable to public payers or non-US populations, and does not evaluate the specific economic impact of chylomicronemia due to FCSCitation33.
Conclusion
Using a large claims database, this study found that chylomicronemia was associated with incremental annual costs of ∼$800 per patient compared with non-chylomicronemic individuals having an otherwise comparable medical profile. A history of acute pancreatitis in patients with chylomicronemia was associated with additional costs of ∼$29,000 per patient per year, with over 20% of this added cost attributable to recurrent episodes of acute pancreatitis.
Transparency
Declaration of funding
Novartis Pharmaceuticals Corporation provided financial support for this study.
Declaration of financial/other relationships
Daniel Gaudet held the Canada Research Chair in preventive genetics and community genomics (www.chairs.gc.ca), which is supported by a CIHR team grant (# CTP-82941). Karine Tremblay is a Université de Montréal post-doctoral and CCRP fellow and receives grant support from the Canadian Heart and Stroke Foundation. Diane Brisson has no relevant financial relationships to disclose. During the study, James Signorovitch, Elyse Swallow, and Liangyi Fan are salaried employees of Analysis Group, Inc., which received funding from Novartis for this study. Charles Meyers and Jean-Bernard Gruenberger have disclosed that they are employees of Novartis and own stock in the company. JME Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
Supplementary Material
Download PDF (39.2 KB)References
- Chait A, Brunzell JD. Chylomicronemia syndrome. Adv Intern Med 1992;37:249-73
- Brunzell JD. Clinical practice. Hypertriglyceridemia. N Engl J Med 2007;357:1009-17
- Benlian P, De Gennes JL, Foubert L, et al. Premature atherosclerosis in patients with familial chylomicronemia caused by mutations in the lipoprotein lipase gene. N Engl J Med 1996;335:848-54
- Johansen CT, Kathiresan S, Hegele RA. Genetic determinants of plasma triglycerides. J Lipid Res 2011;52:189-206
- Ford ES, Li C, Zhao G, et al. Hypertriglyceridemia and its pharmacologic treatment among US adults. Arch Intern Med 2009;169:572-8
- Bathnagar D. Hypertriglyceridemia. In: Betteridge DJ, Illingworth DR, Shepherd J, eds. Lipoproteins in health and disease. London: Arnold Publishers and Oxford University Press, 1999. p 737-53
- O'Brien T, Dinneen SF, O'Brien PC, et al. Hyperlipidemia in patients with primary and secondary hypothyroidism. Mayo Clin Proc 1993;68:860-6
- Brunzell JD, Schrott HG. The interaction of familial and secondary causes of hypertriglyceridemia: role in pancreatitis. Trans Assoc Am Physicians 1973;86:245-54
- Stone NJ. Secondary causes of hyperlipidemia. Med Clin North Am 1994;78:117-41
- Brunzell JD, Deeb SS. Familial lipoprotein lipase deficiency, apo CII deficiency and hepatic lipase deficiency. In: Scriver CR, Beaudet AL, Sly WS, Valle D, eds. The metabolic and molecular bases of inherited disease. 8th edn. New York, NY: McGraw-Hill, 2001. p 2789-16
- Tremblay K, Methot J, Brisson D, et al. Etiology and risk of lactescent plasma and severe hypertriglyceridemia. J Clin Lipidol 2011;5:37-44
- Fung MA, Frohlich JJ. Common problems in the management of hypertriglyceridemia. CMAJ 2002;167:1261-6
- Brunzell JD, Bierman EL. Chylomicronemia syndrome. Interaction of genetic and acquired hypertriglyceridemia. Med Clin North Am 1982;66:455-68
- Christian JB, Bourgeois N, Snipes R, et al. Prevalence of severe (500 to 2000 mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol 2011;107:891-7
- Brisson D, Methot J, Tremblay K, et al. Comparison of the efficacy of fibrates on hypertriglyceridemic phenotypes with different genetic and clinical characteristics. Pharmacogenet Genomics 2010;20:742-7
- Santamarina-Fojo S. The familial chylomicronemia syndrome. Endocrinol Metab Clin North Am 1998;27:551-67, viii
- Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9
- Okosun IS, Chandra KM, Boev A, et al. Abdominal adiposity in U.S. adults: prevalence and trends, 1960-2000. Prev Med 2004;39:197-206
- Golden SH, Robinson KA, Saldanha I, et al. Clinical review: prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab 2009;94:1853-78
- Thomson Reuters MarketScan® Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits Databases. Ann Arbor, MI: Thomson Reuters (Healthcare) Inc.; 2010
- The International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM). Hyattsville, MD (NCHS) and Baltimore, MD (CMS): The National Center for Health Statistics (NCHS) and the Centers for Medicare and Medicaid Services, 2010
- Fredrickson DS, Lees RS. A system for phenotyping hyperlipoproteinemia. Circulation 1965;31:321-7
- Rice J. Mathematical statistics and data analysis. 2nd edn. Belmont, CA: Duxbury Press, 1995. p 492-4
- Siegel S. Non-parametric statistics for the behavioral sciences. New York: McGraw-Hill, 1956. p 75-83
- Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 2008;371:143-52
- SAS Institute Inc. SAS® software, Version 9.2 for Microsoft® Windows XP. Copyright©. Cary, NC: SAS Institute Inc., 2008
- Balu S, Thomas J, 3rd. Incremental expenditure of treating hypertension in the United States. Am J Hypertens 2006;19:810-6; discussion 817
- American Diabetes Association. Economic costs of diabetes in the U.S. In 2007. Diabetes Care 2008;31:596-615
- Gaudet D, de Wal J, Tremblay K, et al. Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl 2010;11:55-60
- Frey CF, Zhou H, Harvey DJ, et al. The incidence and case-fatality rates of acute biliary, alcoholic, and idiopathic pancreatitis in California, 1994-2001. Pancreas 2006;33:336-44
- Christian JB, Arondekar B, Buysman EK, et al. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol 2012;6:450-61
- Fagenholz PJ, Fernandez-del Castillo C, Harris NS, et al. Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas 2007;35:302-7
- Gaudet D, Gagne C. La morbidité économique des dyslipidémies familiales au Québec. Les actualités du cœur 1997;2:12