Abstract
Background:
Invasive fungal infections (IFIs) present a major issue in clinical practice, due to their high morbidity and mortality rates. In a pivotal multi-centre, randomized clinical trial, posaconazole prophylaxis prevented IFIs more effectively than did either fluconazole or itraconazole, and improved overall survival.
Objective:
The aim of this study was to perform an economic evaluation of the aforementioned therapeutic strategies for IFI prophylaxis in neutropenic patients, in the Greek healthcare setting.
Method:
A decision analytic model was developed, which described the course of neutropenic patients under posaconazole or standard azole (fluconazole or itraconazole) treatment. Effectiveness data for each treatment regimen were derived from published results of a pivotal, multi-centre, randomized clinical trial. Cost and healthcare resources utilization data depict Greek clinical practice and are derived from official Greek sources, from a third party payer perspective.
Results:
Prophylaxis with posaconazole resulted in fewer IFIs (0.05 vs 0.11 per patient) compared to treatment with fluconazole or itraconazole, during the first 100 days from initiation of prophylaxis treatment. The cost per avoided IFI with posaconazole was €6455, while the incremental cost per life year gained (LYG) was estimated at €24,196. Extensive sensitivity analyses corroborated the base-case results. Possible limitations of the study are the exclusion of indirect and outpatient costs from the analysis and the inherent uncertainty with regards to the transferability of the clinical efficacy results of the clinical trial to the Greek healthcare setting.
Conclusions:
The utilization of posaconazole for prophylaxis of IFIs neutropenic patients is a therapeutic strategy that provides superior clinical efficacy, while being cost-effective compared to alternative therapies.
Introduction
Invasive fungal infections (IFIs) remain difficult to diagnose and treat, despite the fact that the number of cases has been increasingCitation1–3. This rise can partly be explained by the improvements in chemotherapy and transplantation regimes and the associated rise of life expectancy indicators among immunocompromised patientsCitation1. Along with the increased morbidity and mortality related to IFIs come the associated management costs and the overall significant economic burden to healthcare systems internationally.
Indicatively, in their 2002 study, Wilson et al.Citation4 identified that the extra cost of treatment during the initial hospitalization of an IFI patient compared to a non-IFI peer, was $(US)47,915. More recent US data by Menzin et al.Citation5 suggest that IFI patients were associated with $55,400 in excess costs compared to their non-IFI peers. The situation is not different in the European setting; in their studies in Germany and Switzerland, Jansen et al.Citation6 and Greiner et al.Citation7 calculated the average cost of IFI treatment to be in the area of €23,000 for a 3-month period and CHF 1428 per treatment day, respectively, whereas a study by Michallet et al.Citation8 in the French healthcare setting estimated direct costs attributable to IFI at €51,033. European studies focusing on IFI prophylaxis report mean values that lie in a range of 4000–8000 Euros per case, approximately. Indicatively, Spanish data (Grau et al.Citation9) report that estimated total costs associated with IFI prophylaxis in the absence of confirmed invasive infection are between €6121–€7928, whereas respective mean per patient prophylaxis costs ranged from €4412–€4595 in the Dutch settingCitation10.
Established risk factors for the development of IFIs are prolonged neutropenia, extended use of immunosuppressants, use of broad-spectrum antibiotics, older age, active haematological malignancy, presence of a central venous catheter and previous fungal infectionsCitation1,Citation7,Citation11. Prophylactic treatment is mainly undertaken using triazoles (posaconazole, fluconazole or itraconazole) or micafungin. The triazoles are available as oral preparations, whereas micafungin only exists in intravenous formulations, limiting its use in prevention regimes. Despite evidence that fluconazole improves morbidity and mortality rates, when used as prophylaxis in haemopoietic stem cell transplantation (HSCT) patients, its preventative use in neutropenic patients has been challenged due to its relative narrow spectrumCitation12. Fluconazole has been shown to have less activity against Aspergillus species when compared to other triazoles. Itraconazole, on the other hand, has a broader spectrum of activity; however, its tolerability is of clinical concernCitation10.
Posaconazole, a broad spectrum second generation triazole, is indicated for the prophylaxis of invasive fungal infections in high-risk patients receiving remission-induction chemotherapy for acute myelogenous leukaemia (AML) or myelodysplastic syndrome (MDS), expected to result in prolonged neutropenia. It is also indicated for haematopoietic stem cell transplant recipients who are receiving high doses of immunosuppressants for graft vs host disease (GVHD)Citation13. The newer triazole demonstrates an improved tolerability profile. Posaconazole was licensed, for prophylactic use against IFI’s, following results of two randomized controlled trialsCitation13,Citation14, which compared its efficacy against other triazoles in neutropenic AML or MDS patients and recipients of allogeneic HSCT.
Despite the fact that the clinical effectiveness of posaconazole has been established and due to the fact that healthcare resources are scarce (today more than ever) and allocation decisions are an absolute necessity, newer products have to be pharmacoeconomically evaluated before being introduced to the clinical practice. In this light, the objective of this study was to investigate the cost-effectiveness of posaconazole prophylaxis vs first generation triazole anti-fungals (fluconazole or itraconazole) taking into account a third party (social security) perspective in Greece.
Methods
Model design
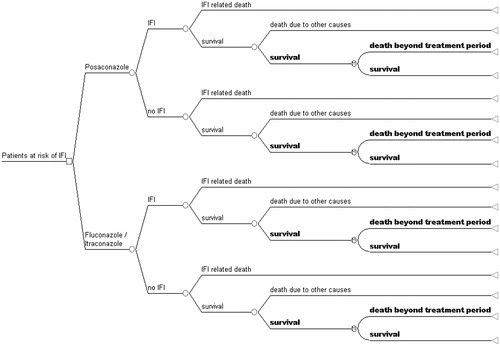
For the purposes of the study, a decision analytic model estimating health outcomes and corresponding costs for immunocompromised patients that received prophylaxis against IFIs was constructed, based on a previously published work by O’Sullivan et al.Citation15, and adapted for the local setting. Patients in the model are assumed to receive either posaconazole or standard treatment (first generation triazole) for the prevention of potential IFIs. According to the administered agent, the model predicts the probabilities of occurrence of adverse treatment outcomes, i.e., an IFI or death.
The model was populated with data from the study by Cornely et al.Citation14, a randomized, open-label, double-blind multi-national/multi-centre study, that compared the efficacy and safety of posaconazole with those of fluconazole or itraconazole as prophylaxis for patients with prolonged neutropenia. The study followed patients that received prophylaxis, either posaconazole (n = 304), fluconazole (n = 240) or itraconazole (n = 58) with each cycle of chemotherapy until recovery from neutropenia and complete remission, until occurrence of an invasive fungal infection or for up to 12 weeks, whichever came first. Total follow-up period was 100 days after randomization. Therefore, and in order to obtain a larger time frame for the economic evaluation, the pharmacoeconomic model consists of two components: (a) the first 100 days and subsequent clinical events were modelled via a typical decision tree according to the outcome probabilities reported by Cornely et al.Citation14; and (b) after the 100-day period survival was modelled through a simple Markov model, consisting of two states (‘survive’ and ‘death beyond 100 days’) extending to a life time horizon, i.e., running until all patients in the model were in the ‘dead’ state. The model is presented in .
Model parameters
As mentioned above, probabilities of an IFI occurrence for the 100-day period post-prophylaxis were derived by the publication by Cornely et al.Citation14. The authors also provided data for the risk of dying because of an IFI or from other (non-IFI related) causes. Given that the reported case fatalities for IFI were not significantly different between treatment arms (0.36 and 0.48 for posaconazole and fluconazole/itraconazole, respectively), this could imply that IFI-related death is not significantly influenced by the prophylactic strategy. Therefore, fatality rates were assumed equal and were pooled for the two treatment groups. This assumption was adapted as the most conservative scenario, as well as in accordance to a number of other similar studies that have followed this approachCitation12,Citation15.
Based on these grounds, the same assumption was made for deaths from other causes. With regards to the transition probabilities after the 100-day period, the occurrence of ‘death’ (having survived or not having suffered an IFI in the first 100 days) was dependent only to the underlying condition, i.e., AML or MDS. Due to lack of Greek-specific data, age adjusted 5-year relative survival rates for AML patients were derived from the National Cancer Institute’s Surveillance, Epidemiology and End Results (SEER) registryCitation16, whereas the corresponding rates for MDS patients were taken from the study of Kantarjian et al.Citation17. The proportion of patients with AML and MDS was used as a weighting coefficient for the relative survival rates, which were then applied to age-specific death rates for the general population of Greece. In the base case scenario and in accordance with the Cornely et al.Citation14 study, patients were assumed to have an average age of 50 years, 14% suffering from MDS and the remaining 86% from AML.
Cost calculations
In absence of Greek data on the cost of IFI treatment, the research team conducted an expert panel survey based on semi-structured, questionnaire-based interviews. For this purpose a team of 10 experts—hospital physicians with experience in oncology/haematology and microbiology—were requested to record all health resource items that are utilized for the treatment of a ‘typical’ neutropenic patient presenting with an infectious complication post-chemotherapy (including possible IFI), admitted to a Greek hospital. The questionnaire was divided into two similar parts associated with the management of two different patient scenarios, depicting the course of chemotherapy for AML or MDS. Thus, the questionnaires examining healthcare resource utilization produced 20 sets of results for the two scenarios under investigation. Each expert was asked to specify empiric treatment regimes and duration. Furthermore, all diagnostics and changes in therapeutic management were recorded before and after culture results. Finally, duration of hospitalization and ICU stay were determined for each of the two patient scenarios. The average inpatient management cost of the 12 scenarios was considered as the mean inpatient direct cost for the management of an IFI. Health resource use for each scenario was multiplied by the official NHS tariffs in order to obtain one cost estimate per scenario per expert. Mean values for each scenario were then produced. Outpatient healthcare resource expenditure was not included in the analysis as such costs are known to have negligible impact on the overall IFI management cost. Indicatively, a review by Lyseng-WilliamsonCitation12 reports that outpatient costs are ∼3% of the total IFI management direct costs.
Pharmaceutical costs for each prophylaxis treatment were produced by multiplying the official pharmaceutical prices for each agent, given by the Greek price bulletinsCitation18, with the formulations, dosages, and durations of treatment reported by the trial. The lowest available prices for fluconazole and itraconazole were selected (prices of generic preparations).
Given that the analysis followed a third party payer perspective, only direct costs were included in the calculations. All costs are reported in year 2011 values (Euros) and are discounted at a 3% rate per annum.
All base case parameters for the model are presented in .
Table 1. Base case parameters.
Sensitivity analyses
To address uncertainty of the model parameters and evaluate the robustness of the results, a series of multi-way probabilistic sensitivity analyses were performed. In order to perform the probabilistic analysis, random values according to a beta distribution were assigned to transition probabilities (probabilities of events) and to a gamma distribution to costs and mean days to exposure in prophylaxis (). The sensitivity analysis results for the incremental cost-effectiveness ratios (ICERs) for treatment relative to no-treatment were produced after a 1000 sample Monte Carlo simulation.
Table 2. Variables varied in the sensitivity analysis and parameters of distributions.
Results
Base case scenario
The mean inpatient management cost for a ‘typical’ IFI patient was estimated at €26,071.16 (SE = 2193.9). Medications accounted for 30.45% of the total costs, diagnostic/laboratory procedures for 4.95% and hospitalizations for 64.6%. The results of the base case scenario, i.e., the costs and effects of posaconazole vs standard azole treatment for a lifetime horizon are reported in .
Table 3. Cost-effectiveness results of posaconazole vs standard azole treatment for a lifetime horizon.
As shown in , posaconazole was more costly as well as more effective than standard azole treatment, in terms of life years saved from each treatment, when the analysis follows a lifetime perspective. This is mainly explained by the lower frequency of IFI events in the posaconazole arm, as reported in the results from the Cornely et al.Citation14 study. With regards to the results for the first 100 days of treatment, as per the duration in the original clinical trial, costs and effects are reported in .
Table 4. Cost-effectiveness results of posaconazole vs standard azole treatment for the 100 first days of treatment.
Sensitivity analysis
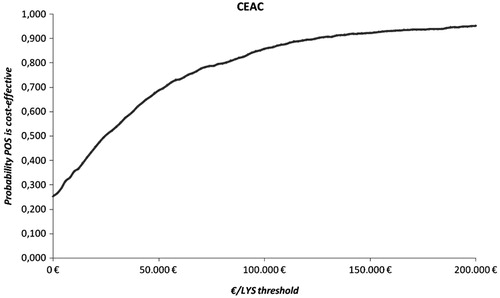
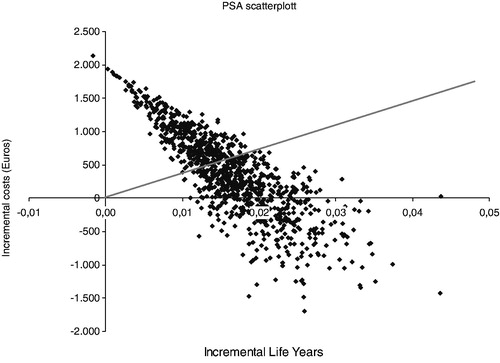
The sensitivity analysis demonstrated that the results were robust for various values of the baseline parameters, randomly chosen according to the predefined distributions. shows the probabilistic sensitivity analysis (PSA) scatterplot, where posaconazole was dominant for 24.1% of the Monte Carlo iterations and cost-effective for 54.1% of the iterations for an implicit €30,000/life year gained threshold. depicts the cost-effectiveness acceptability curve, i.e., the percentage of ICERs produced with the PSA that fall below a specific threshold
Figure 2. Probabilistic sensitivity analysis scatterplot for posaconazole vs standard azole treatment. Trendline represents the €30,000/LYS threshold, points represent ICERs. Points that fall in the SW quadrant represent ‘dominant’ ICERs (posaconazole more effective and less costly than alternatives). Points that fall in the SW quadrant plus those that fall under the trendline represent cost-effective ICERs.
Discussion
The present study aimed at evaluating the costs and outcomes of IFI prophylaxis treatment with a newer (and more costly) agent, i.e., posaconazole, vs the established treatment options in the clinical setting in Greece, itraconazole and fluconazole. The economic evaluation indicated that IFI prophylaxis with oral posaconazole proved to be more costly when compared to standard triazole prophylaxis in high risk neutropenic patients with AML or MDS. However, due to its improved clinical profile in terms of IFI occurrence prophylaxis, those costs were largely offset.
As a result the incremental cost-effectiveness ratio of prophylaxis treatment with posaconazole vs fluconazole/itraconazole was estimated at €24,196 per LY gained, a figure that is well below the generally acknowledged threshold of €30,000/LY, under which interventions are considered cost-effective. The above-mentioned results performed well under varying assumptions in the PSA and indicated that posaconazole was dominant against comparators for 24.1% of the iterations and cost-effective for 54.1%, implying that the outcomes were robust to different scenarios of treatment and cost inputs.
The results of the analysis are in line with previous findings reported in the literature for various healthcare settings. Indicatively, posaconazole was found to be cost-effective when compared to standard azole treatment for IFI prophylaxis in patients in GermanyCitation19 and BelgiumCitation20, with ICERs ranging from €1173–€6820 per LY gained or as high as €42,112/LY gained in the Czech RepublicCitation21. However, many studies do report a dominance of posaconazole vs alternatives, i.e. that posaconazole was both a less costly and more effective treatment when compared to fluconazole or itraconazoleCitation7–11,Citation15.
As with any study of this kind, the present one has some limitations that should be acknowledged. For one, the model and subsequent analyses were based on the results of the clinical trial of Cornely et al.Citation14 and were then extrapolated for a lifetime horizon using simulation techniques. Although this is a commonly used methodology in economic evaluations of this sort, there exists some uncertainty as to whether these data would actually reflect a real-life situation in Greece. In the absence of primary Greek-specific data and in order to account for the above-mentioned uncertainty the base case parameters were extensively evaluated in the sensitivity analysis, which returned robust results. Moreover, it should be noted that the present analysis, being undertaken from an insurance (third party payer) perspective, does not include indirect costs of treatment and follow-up. Although one would expect indirect costs from productivity losses to be low in those patients, the same thing cannot be said for some aspects of treatment like atypical care. Should those costs have been included, the reduction in the burden of morbidity with posaconazole would also ‘translate’ to less costs for atypical treatment and, thus, to a more favourable ICER. Additionally, outpatient costs were also not included in the analysis, although its inclusion would have a minimal impact, as stated earlier in the text. Finally another limitation of this study lies within the methodology regarding the estimation of the direct IFI treatment costs, i.e. the expert panel approach, in the absence of relevant data within the Greek NHS. The estimated mean cost of IFI treatment (€26,071.16) is placed in the lower range of corresponding values reported from other similar studiesCitation4–7,Citation15,Citation22. Needless to say, a possible under-estimation of IFI treatment costs would have been associated with less favourable results for posaconazole.
Conclusions
Notwithstanding its methodological limitations, the results of this study indicate that posaconazole could be a cost-effective intervention when compared to standard triazole IFI prophylaxis with fluconazole or itraconazole, in a high-risk neutropenic population with haematological malignancies in the Greek healthcare setting. In the context of scarce healthcare resources, evidence from cost-effectiveness analyses can be valuable inputs in the rational allocation of health budgets.
Transparency
Declaration of funding
The present study was financially supported by MSD Hellas. The sponsor of the study had no involvement in the collection, analysis, interpretation of data or writing of the manuscript.
Declaration of financial/other interests
The authors have no relevant financial relationships to disclose. KA and IP were responsible for the analyses, calculations and interpretation of the results presented in the manuscript. KA and IP also drafted the initial versions of the manuscript, which was critically reviewed by JK. JME peer reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Malani AN, Kauffman CA. Changing epidemiology of rare mould infections: implications for therapy. Drugs 2007;67:1803–12
- Richardson MD. Changing patterns and trends in systemic fungal infections. J antimicrob Chemother 2005;56(1 Suppl):i5–11
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm 2009;66:1711–7
- Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health 2002;5:26–34
- Menzin J, Meyers JL, Friedman M, et al. The economic costs to United States hospitals of invasive fungal infections in transplant patients. Am J Infect Control 2011;39:e15–20
- Jansen JP, Kern WV, Cornely OA, et al. Economic evaluation of voriconazole versus conventional amphotericin B in the treatment of invasive aspergillosis in Germany. Value Health 2006;9:12–23
- Greiner RA, Meier Y, Papadopoulos G, et al. Cost-effectiveness of posaconazole compared with standard azole therapy for prevention of invasive fungal infections in patients at high risk in Switzerland. Oncology 2010;78:172–80
- Michallet M, Gangneux JP, Lafuma A, et al. Cost effectiveness of posaconazole in the prophylaxis of invasive fungal infections in acute leukaemia patients for the French healthcare system. J Med Econ 2011;14:28–35
- Grau S, de la Camara R, SanzM, et al. Cost-effectiveness of posaconazole versus standard azole treatment (fluconazole or itraconazole) in the prevention of invasive fungal infections among high-risk neutropenic patients in Spain. Abstract presented at the 18th European Congress of Clinical Microbiology and Infectious Diseases; 2008 Apr 19–22; Barcelona, Spain
- Stam WB, O’Sullivan AK, Rijnders B, et al. Economic evaluation of posaconazole vs. standard azole prophylaxis in high risk neutropenic patients in the Netherlands. Eur J Haematol 2008;81:467–74
- Dranitsaris G, Khoury H. Posaconazole versus fluconazole or itraconazole for prevention of invasive fungal infections in patients undergoing intensive cytotoxic therapy for acute myeloid leukemia or myelodysplasia: a cost effectiveness analysis. Support Care Cancer 2011;19:1807–13
- Lyseng-Williamson K. Posaconazole. A pharmacoeconomic review of its use in the prophylaxis of invasive fungal disease in immunocompromised hosts. Pharmacoeconomics 2011;29:251–68
- Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007;356:335–47
- Cornely OA, Maertens J, Winston DJ, et al. Posaconazole versus fluconazole ore itraconazole prophylaxis in patients with neutropenia. N Engl J Med 2007;356:348–59
- O'Sullivan AK, Pandya A, Papadopoulos G, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health 2009;12:666–73
- National Cancer Institute. SEER Cancer Statistics Review 1975–2003. Age-adjusted SEER incidence and US death rates and 5-year relative survival rates. 2006. Available at http://seer.cancer.gov/csr/1975_2003/results_single/sect_01_table.04_2pgs.pdf; Accessed July 12 2012
- Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer 2006;106:1099–109
- Ministry of Health. Official price bulletins. http://www.yyka.gov.gr/articles/times-farmakwn/deltia-timwn. Accessed July 2012
- Thalheimer M, Cornely OA, Hoppe-Tichy T, et al. Pharmaco- economic analysis of posaconazole versus standard azole prophylaxis in high-risk neutropenic AML/MDS patients in Germany. Abstract presented at the 18th European Congress of Clinical Microbiology and Infectious Diseases; 2008 Apr 19-22; Barcelona, Spain
- Maertens M, Aoun M, Bron D, et al. Cost-effectiveness of posaconazole (Noxafil) versus standard azole therapy in the prevention of invasive fungal infections among high-risk neutropenic patients in Belgium. Abstract presented at the 10th International Symposium on Febrile Neutropenia; 2008 Feb 8–9; Brussels, Belgium
- Suchankova E, Dolezal T, Simonova J. Cost-effectiveness of antifungal prophylaxis with posaconazole versus standard azole therapy in the prevention of invasive fungal infections among high risk haematology patients in Czech Republic. Value Health 2009;12:A380
- Jansen JP, Kern WV, Cornely OA, et al. Economic evaluation of voriconazole versus conventional amphotericin B in the treatment of invasive aspergillosis in Germany. Value Health 2006;9:12–23