Abstract
Objective:
To assess treatment adherence in attention deficit/hyperactivity disorder (ADHD) patients initiated on Lisdexamfetamine (LDX) vs other FDA-approved stimulants and non-stimulant medications.
Methods:
ADHD patients initiated on an ADHD medication (index medication) were selected from a large US administrative claims database. Based on age and previous treatment status, patients were classified into treatment-naïve children and adolescents (6–17 years old), previously treated children and adolescents, treatment-naïve adults (over 18 years old), and previously treated adults. Furthermore, based on their index medication, patients were classified into seven mutually exclusive treatment groups: LDX, atomoxetine (ATX), osmotic release methylphenidate hydrochloride long acting (OROS MPH), other methylphenidate/dexmethylphenidate long acting (MPH LA) and short acting (MPH SA), and amphetamine/dextroamphetamine short acting (AMPH SA) and long acting (AMPH LA). Treatment adherence (proportion of days covered by the index medication ≥0.8) over a 12-month period was compared across treatment groups using multivariate logistic regression models.
Results:
In children and adolescents, LDX patients were more likely to be adherent compared to patients in each of the other treatment groups, except in treatment-naïve patients where LDX patients had a similar likelihood (p = 0.6925) and were less likely (p = 0.0004) to be adherent compared to ATX and OROS MPH patients, respectively. In adults, the LDX treatment group was also more likely to be adherent compared to each of the other treatment groups, except compared to AMPH LA, where statistically insignificant differences were observed (previously treated: p = 0.6471, treatment-naïve: p = 0.0733).
Limitations:
ADHD severity information was not available in the database. Accordingly, this study did not control for ADHD severity.
Conclusion:
Overall, LDX-treated patients demonstrated a better treatment adherence compared to patients initiated on other ADHD medications, except for AMPH LA in adult and OROS MPH and ATX in treatment-naïve children and adolescents.
Background
Attention deficit/hyperactivity disorder (ADHD) is a neurobehavioral disorder characterized by severe inattention, hyperactivity, and impulsivity that often results in substantial impairmentCitation1. It is the most common childhood mental disorderCitation2. Although originally conceptualized as a childhood disorder, between one half and two-thirds of children with ADHD have the disorder persist into adulthoodCitation3. The Center for Disease Control (CDC) analyzed data from the National Survey of Children’s Health (NSCH) and reported that, in 2006, ∼4.4 million (7%) American children aged 3–17 years had ADHDCitation4. More recently, a 2011 study also published by the CDC showed that for, the 2007–2009 period, ∼9% of children aged 5–17 years had ever been diagnosed with ADHD in the US and that the prevalence was increasing over time. The prevalence of ADHD diagnosis in children increased from 7% to 9% from 1998–2000 through 2007–2009Citation5. Another study by Kessler et al.Citation6 that examined the American adult prevalence of ADHD, reported an estimated prevalence of ADHD of 4.4% in adults between 18–44 years old.
Untreated ADHD can cause significant long-term impairment in several aspects of an individual’s life including academic and professional accomplishment as well as self-esteem and social relationships, where treatment can contribute to reducing ADHD-related impairmentCitation7,Citation8. Comorbidities related to ADHD include learning disabilities, mood disorders, language disabilities, anxiety disorders, and disruptive behavior disordersCitation2,Citation9. Although it is unclear whether untreated ADHD causes the emergence of these co-morbidities or vice versa, untreated ADHD is associated with “gradual accumulation of adverse processes and events that increase the risk of serious psychopathology later in life”Citation10.
The burden of ADHD may extend beyond the patient. Studies have shown that having a child diagnosed with ADHD might also have a significant impact on family functioning, relationships among family members, and their health-related quality-of-life. More specifically, families with a child diagnosed with ADHD have been found to have a higher likelihood of parental depression, anxiety and stress, disruption of family activities, and inconsistent and hostile parenting behaviorsCitation11.
Available treatments focus on reducing specific symptoms of ADHD and improving overall functioning and well-being. Treatments include medication, various types of psycho-therapy, as well as education or specific training (for the individual with ADHD and/or caregiver). Some guidelines recommend the combination of different approachesCitation12,Citation13. For many ADHD patients, ADHD medications may significantly reduce hyperactivity and improve the ability to focus and overall functioning. In the US, stimulant medications are the most common pharmacological therapy used to manage ADHD and are generally considered a first-line pharmacological treatment optionCitation7,Citation14–18. Studies have shown that most ADHD patients respond to a stimulant medication shortly after treatment initiationCitation19–21. In patients who do not respond to a stimulant, treatment response to an alternative class of stimulant might be reached in up to 90% of patientsCitation21. However, although stimulants have been shown to be effective therapy in ADHD, adherence to treatment, defined as the extent to which a patient’s actions correspond to the treatment recommendations, is generally lowCitation22, which limits the observed real world efficacy of ADHD treatmentCitation23.
ADHD poses a specific challenge to treatment adherence as some of the core symptoms of the disease, including forgetfulness, impulsivity, and disorganization, may decrease adherence and, thus, impede patients from receiving the full therapeutic benefit of the medicationCitation24. Non-adherence to medication has been reported to be very common in different chronic diseases. In developed countries, adherence for most common chronic diseases has been reported to be as low as 50%Citation25. Medical non-adherence is both a serious and often costly problem for health treatments, particularly the treatment of mental or psychiatric disordersCitation26.
A systematic literature review on ADHD treatment adherence and persistence in children and adults has recently been publishedCitation22 and showed that prevalence of treatment discontinuation was common in ADHD patients, ranging between 13–64% across studies. The 11 reviewed studies were based on a variety of data sources, including parental self-reports (for children), self-reports (for adults), pill counts, and claims data analyses (persistence analyses) where adherence and persistence were defined using a wide range of definitions and follow-up periods across studies (ranging from 1 week to 9 years). The studies also focused on different types of ADHD medications and were also conducted on specific sub-groups of the population. Therefore, because of the lack of consistency of research methods and studied samples in the literature, it is very difficult to draw conclusions on treatment adherence in an overall ADHD population as well as in specific age and previous treatment status sub-groups.
To fill this knowledge gap, this study analyzed adherence to the most common currently prescribed stimulants and non-stimulants in the US, and compared adherence levels between treatment cohorts using a large longitudinal population-level real-world dataset. In order to better understand adherence to these treatments, both treatment-naïve and previously treated ADHD patients across different age groups were examined, using the same methodology across all studied cohorts. The ADHD medications studied included Lisdexamfetamine (LDX), atomoxetine (ATX) (the only non-stimulant approved by the FDA at the time of the study), osmotic release methylphenidate hydrochloride long acting (OROS MPH), other methylphenidate/dexmethylphenidate long acting (MPH LA), methylphenidate/dexmethylphenidate short acting (MPH SA), amphetamine/dextroamphetamine long acting (AMPH LA), and amphetamine/dextroamphetamine short acting (AMPH SA). Findings from this study may provide information on the comparative adherence levels to most commonly prescribed stimulants and non-stimulants in different ADHD populations.
Methods
Data source
This study was conducted using data from the Truven Health Analytics MarketScan® (MarketScan) database from 2006–2009. The MarketScan database is a high-quality compilation of private sector electronic claims databases of large employers in the US collected from ∼100 different insurance companies, Blue Cross Blue Shield plans, and third party administrators. All census regions are represented, although most enrollees are from the South and North Central (Midwest) regions. The database includes enrollment history and claims for medical (provider and institutional) and pharmacy services for insured employees and their dependents, as well as for Medicare-eligible retirees with employer-provided Medicare Supplemental plans. Inpatient services are summarized at the claim and admission level. Data are de-identified and comply with the patient requirements of the Health Insurance Portability and Accountability Act (HIPAA). Therefore, Institutional Review Board approval was not required.
Patient selection and construction of study cohorts
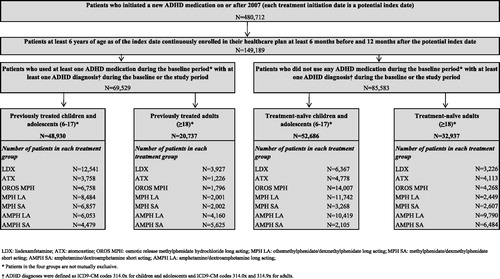
The MarketScan database was used to select ADHD patients who initiated a new treatment with an FDA-approved ADHD medication between 2007–2009. Patients were selected if they had received at least one ADHD diagnosis (International Classification of Diseases, 9th Revision: 314.0× for children and adolescents and 314.0x or 314.9× for adults) and if they were continuously enrolled in their healthcare plan at least 6 months prior to and 12 months after the treatment initiation date (index date). Based on their age, patients were split into two populations: (1) children and adolescents (6–17 years old) and (2) adults (≥18 years old). Depending on previous treatment status (i.e., if patients used another ADHD medication or not during the 6-month period prior to the index date), the two populations were divided into four cohorts: (1) treatment-naïve children and adolescents, (2) previously treated children and adolescents, (3) treatment-naïve adults, and (4) previously treated adults. Furthermore, based on the ADHD medication initiated on the index date, patients were classified into seven mutually exclusive treatment groups: (1) LDX, (2) ATX, (3) OROS MPH, (4) MPH SA, (5) MPH LA, (6) AMPH SA, and (7) AMPH LA.
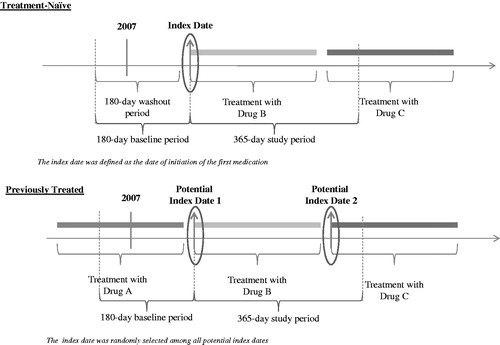
For treatment-naïve patients, the first prescribed medication on/after 2007 and the first fill date were defined as the index medication and the index date, respectively (). For previously treated patients, since patients may have been treated with more than two ADHD medications during the observation period, the index date (and corresponding index drug) was randomly selected among all the new ADHD medications on which a patient was initiated on/after 2007. It is possible that for some patients the newly initiated ADHD medication was added to another ADHD medication (therapy augmentation). In such cases, the newly added medication was defined as the index medication. Patients initiating two new ADHD medications at the same time were excluded.
Figure 1. Study Design. (A) Treatment-naïve: Patients were initiated on an ADHD medication for the first time on or after February 23, 2007, preceded by a 6-month period without ADHD drug prescriptions. The first drug on which a patient was initiated on or after February 23, 2007, and the date of its first fill date, based on pharmacy claims, were referred to as the index drug and the index date, respectively. (B) Previously treated: Patients were treated with an ADHD medication prior to February 23, 2007, and were switched to another medication on or after that date. Each new drug on which a patient was initiated on or after February 23, 2007, and the date of its first fill date, based on pharmacy claims, were referred to as the potential index drug and the potential index date, respectively. As patients may have initiated several ADHD medication over time, one index drug (and corresponding index date) was randomly selected among all potential index drugs (and corresponding potential index dates). The baseline period was defined as the 6-month period preceding the index date, while the study period was defined as the 12-month period following the index date. Treatment discontinuation was defined as an interruption of the index treatment for at least 30 consecutive days, that is, a gap between the end of one prescription and the start of the next prescription for the index drug of at least 30 days.
A retrospective cohort design was used to compare outcomes between the LDX treatment group and each of the other treatment groups. For each cohort, results are presented by comparing (1) LDX and the most commonly prescribed stimulant and non-stimulant (OROS MPH and ATX, respectively) and (2) LDX and the remaining ADHD medication classes (MPH SA, MPH LA, AMPH SA, and AMPH LA).
The baseline period was defined as the 6-month period preceding the index date. The study period spanned from the index date up to 12 months following the index date.
Outcomes and statistical analysis
Baseline characteristics
Baseline characteristics were compared across all treatment groups (i.e., LDX, ATX, OROS MPH, MPH LA, MPH SA, AMPH LA, and AMPH SA) using Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables. The following baseline variables were reported: age, gender, prior ADHD-related psychotherapies, Charlson Comorbidity Index (CCI)Citation27, physical co-morbidities, mental co-morbidities, healthcare resource utilization, and year of medication index date. ADHD medications used during the baseline period were also reported for the previously treated cohorts. Physical and mental comorbidities were assessed based on the presence of claims associated with conditions listed in the Agency for Healthcare Research and Quality Comorbidity SoftwareCitation28 and the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition [DSM-IV]Citation29, respectively. Only physical and mental co-morbidities that demonstrated significant differences across treatment groups and had a prevalence estimate of at least 5% in each treatment group were reported.
Baseline characteristics with p-values comparing the LDX treatment group to each of the other treatment groups individually were also reported as additional information in Appendices 1 and 2.
Treatment adherence to the index medication
Treatment adherence to the index medication was measured over the 12-month study period using the proportion of days covered (PDC), regardless of whether or not the patients discontinued or switched to another ADHD medication. The PDC was calculated as the number of days covered by the prescribed index drug divided by the number of days in the study period (i.e., 365 days)Citation30. The PDC was analyzed as a continuous variable as well as a dichotomous variable. The dichotomous PDC variable was created by categorizing patients into two groups: adherent patients to the index medication (PDC ≥ 0.8) and non-adherent patients to the index medication (PDC < 0.8). This 80% threshold is a common approach used in the literature to define treatment adherenceCitation31. Average PDCs were compared between LDX patients and patients in each of the other treatment groups using generalized linear models, where the dependent variable was the PDC (continuous variable) and the independent variables were the treatment groups (LDX as the reference group). The proportion of adherent and non-adherent patients were compared using chi-square tests, and the probability of being adherent was compared using logistic regression models, where the dependent variable was a dichotomous variable for adherence (PDC ≥ 0.8 = 1; PDC < 0.8 = 0) and the independent variables were the treatment groups (LDX as the reference group). Multivariate regression analyses were also conducted to control for confounding factors. Covariates included in the multivariate regression model were baseline demographics (age and gender), CCI, class and number of ADHD medication classes used at baseline (for previously treated patients), baseline healthcare resource utilization, baseline mental co-morbidities, and other baseline characteristics that showed statistically significant differences between cohorts and with occurrence of at least 5% in each treatment group (including mental and physical co-morbidities, prior ADHD-related psychotherapy, year and month of treatment initiation, and most common non-ADHD medication classes used during the baseline period).
As a sensitivity analysis, adherence to the index medication was also measured using the medication possession ratio (MPR) calculated as the number of days of medication supplied during the study period divided by the number of calendar days in the study periodCitation32. While the MPR double-counts covered days when two prescriptions overlap, the PDC method leads to a more conservative estimate of treatment adherence.
Another sensitivity analysis was conducted where multivariate models were adjusted for all statistically significant differences at baseline between cohorts and with an occurrence of at least 2% in each treatment group.
The Bonferonni-Holm adjustment method was applied to adjust for multiple comparisons as a final sensitivity analysisCitation33.
All statistical analyses were conducted using SAS 9.2 statistical software (SAS Institute, Inc., Cary, NC).
Results
Among the 480,712 patients who initiated a new ADHD treatment on or after 2007, a total of 149,189 patients met all of the selection criteria ().
Baseline characteristics
Overall, except for their ADHD condition, patients had a relatively good health profile (i.e., low CCI and absence of chronic conditions). In terms of demographic characteristics, statistically significant differences were observed among all age and treatment groups as described below.
Children and adolescents
Overall, the child and adolescent population was comprised of 52,686 treatment-naïve and 48,930 previously treated patients (). The average age of patients across treatment groups ranged from 10.1–11.9 years and from 10.9–12.1 years in the treatment-naïve and previously treated cohorts, respectively (). For both cohorts, most of the patients were males with proportions ranging from 65.8–70.7% and from 70.3–73.4%, across treatment groups in the treatment-naïve and previously treated cohorts, respectively. The comorbidity burden based upon CCI was low; the average CCI ranged between 0.061–0.075 and between 0.062–0.067 across treatment groups in treatment-naïve patients and previously treated patients, respectively. A relatively low proportion of patients in the treatment-naïve cohort (from 13.4–17.6% across treatment groups) had at least one ADHD-related psychotherapy visit prior to the index date. In the previously treated cohort, the proportion was around twice as high as the proportion observed among treatment-naïve patients (ranging from 24.6–31.7%). The main differences between treatment groups were observed in the prior treatment used in the previously treated cohort (). Among the previously treated cohort, patients in the LDX (39.3%) and AMPH SA (71.2%) treatment groups were most commonly previously treated with AMPH LA. OROS MPH was previously prescribed in 35.9% of ATX patients, 39.4% of MPH LA patients and 39.3% of the AMPH LA patients, whereas MPH LA was previously prescribed to 38.2% of the OROS MPH and 50.8% of the MPH SA patients. Depressive disorder was the only mental comorbidity statistically significantly different in at least 5% of patients in each treatment group among both cohorts in the child and adolescent population. In addition, adjustment disorder was also found to be statistically significantly different across treatment groups in previously treated patients.
Table 1. Patients baseline demographic and clinical characteristics: Children and adolescents.
Adults
Among adults, a total of 32,937 and 20,737 patients were included in the treatment-naïve and previously treated cohorts, respectively (). The average age across treatment groups ranged from 31.5–35.2 years in the treatment-naïve cohort and from 32.0–34.3 in the previously treated cohort (). In contrast to the population of children and adolescents, most of the treatment groups in the adult population had an ∼1:1 ratio of males-to-females; the proportion ranged from 45.3–50.2% and from 44.0–52.8% in the treatment-naïve and previously treated cohorts, respectively. The comorbidity burden based upon CCI was low, with an average CCI ranging between 0.103–0.172 and between 0.113–0.143 across treatment groups in treatment-naïve and previously treated cohorts, respectively. Similar to the child and adolescent population, the proportion of patients with at least one ADHD-related psychotherapy visit in the previously treated cohort was also more than twice as high as the proportion observed in the treatment-naïve cohort and the proportions ranged from 8.5–16.5% in the treatment-naïve cohort and from 25.0–36.5% in the previously treated cohort.
Table 2. Patients baseline demographic and clinical characteristics: Adults.
Among the previously treated patients, patients in the LDX (53.1%), ATX (44.2%), AMPH SA (73.8%), and OROS MPH (33.6%) treatment groups were most commonly previously treated with AMPH LA. In the MPH LA and AMPH LA groups, patients were most commonly previously treated with the short acting formulation (MPH SA: 37.0%; AMPH SA: 48.5%, respectively). In the MPH SA group, patients were most commonly previously treated with OROS MPH (37.1%) (). Depressive disorders, anxiety disorders, and adjustment disorders were the only three mental comorbidities that presented statistically significant differences and were reported in at least 5% of patients in each treatment group in both cohorts. In addition, hypertension was also found to be significantly different across treatment groups in the previously treated cohort.
Treatment adherence
Overall, patients in the LDX treatment group had a better or comparable treatment adherence profile during the 12-month study period compared to patients in most of the other treatment groups, except in the treatment-naïve children and adolescent cohort. Key findings are summarized in . Consistent results were observed in the sensitivity analysis where adherence was measured using the MPR (results not presented) and in the second sensitivity analysis where multivariate models were adjusted for all covariates that showed statistically significant differences between cohorts and with an occurrence of at least 2% in each treatment group (results not presented). Results were also consistent after Bonferroni-Holm adjustments for multiple comparisons for all four cohorts (results not presented).
Table 3. Summary table: Comparison of adherence across all cohorts.
Children and adolescents
In the child and adolescent population, LDX prescribed patients had a better adherence to their index medication compared to patients in each of the other treatment groups, except when compared to OROS MPH and ATX patients in the treatment-naïve cohort.
LDX patients in the treatment-naïve cohort had a significantly lower level of adherence (PDC = 0.43 ± 0.28) compared to OROS MPH patients (PDC = 0.45 ± 0.29) (adjusted difference = −0.0120, p = 0.0048) and a higher adherence level compared to the ATX treatment group (PCD = 0.43 ± 0.28 vs 0.40 ± 0.30; adjusted difference = 0.0382, p < 0.0001) (). Similarly, a significantly lower proportion of patients were adherent to their treatment among LDX patients when compared to patients in the OROS MPH treatment group (14.9% vs 17.7%, p < 0.0001); however, no statistically significant differences were observed in the proportion of patients being adherent between LDX patients and ATX patients (14.9% vs 15.6%, p = 0.3062) (). After adjustment for confounders, LDX patients were less likely to be adherent when compared to OROS MPH patients (adjusted odds ratio [OR] = 1.16 [1.07; 1.27], p = 0.0004) and were not significantly different when compared to ATX patients (adjusted OR = 0.98, [0.88; 1.09], p = 0.6925).
Table 4. Comparison of treatment adherence and probability of being adherent in patients treated with LDX vs other ADHD medications: Children and adolescents.
In comparison, in the previously treated children and adolescents cohort, the LDX treatment group demonstrated a significantly higher level of adherence (PDC = 0.53 ± 0.31) compared to the OROS MPH (PDC = 0.49 ± 0.31; adjusted difference = 0.0304, p < 0.0001) and ATX treatment groups (PDC =0.42 ± 0.31; adjusted difference = 0.0994, p < 0.0001) (). The proportion of adherent patients in the LDX treatment group was also statistically significantly higher compared to the proportion in the OROS MPH (28.4% vs 24.5%, p < 0.0001) and ATX treatment groups (28.4% vs 20.1%, p < 0.0001). Moreover, after adjusting for confounding factors, patients prescribed LDX had an 8% (1/0.9254) higher probability of being adherent compared to patients who were prescribed OROS MPH (OR = 0.93 [0.86; 1.00], p = 0.0438) and were 57% more likely to be adherent than patients who were prescribed ATX (OR = 0.64 [0.58; 0.70], p < 0.0001).
Overall, in the child and adolescent population, LDX prescribed patients had a significantly greater average PDC relative to patients in each of the other remaining treatment groups (MPH LA, MPH SA, AMPH LA, and AMPH SA) for both the treatment-naïve (PDC = 0.43 vs range between 0.29–0.42, all p < 0.0001) and previously treated cohorts (0.53 vs range between 0.28–0.43, all p < 0.0001) (). The LDX treated patients were also significantly more likely to be adherent to their index medication compared to patients in all other treatment groups (adjusted OR ranged between 0.12–0.83, all p < 0.0001).
Adults
In the adult population, LDX patients demonstrated a better adherence level to their index medication compared to patients in each of the other remaining treatment groups, except when compared to AMPH LA patients, where differences were not statistically significant.
LDX prescribed patients in the treatment-naïve cohort had a significantly higher level of treatment adherence (PDC = 0.49 ± 0.31) compared to OROS MPH treated patients (PDC = 0.38 ± 0.29; adjusted difference = 0.1036, p < 0.0001) and to ATX treated patients (PCD = 0.31 ± 0.28; adjusted difference = 0.1782, p < 0.0001) (). The proportion of adherent patients was also significantly higher in the LDX treatment group compared to the proportion in the OROS MPH (24.6% vs 14.1%, p < 0.0001) and ATX (24.6% vs 10.5%, p < 0.0001) treatment groups. After adjusting for confounding factors, LDX treated patients were ∼2-times more likely of being adherent compared to patients in the OROS MPH treatment group (adjusted OR = 0.51 [0.46; 0.58], p < 0.0001) and almost 3-times more likely to be adherent than patients in the ATX treatment group (adjusted OR = 0.34 [0.30; 0.39], p < 0.0001).
Table 5. Comparison of treatment adherence and probability of being adherent in patients treated with LDX vs other ADHD Medications: Adults.
Previously treated patients who were prescribed LDX also had a significantly higher level of treatment adherence compared to patients in the OROS MPH (PDC = 0.48 ± 0.33 vs 0.36 ± 0.30; adjusted difference = 0.1217, p < 0.0001) and ATX (0.48 ± 0.33 vs 0.31 ± 0.29; adjusted difference = 0.1556, p < 0.0001) treatment groups (). The proportion of LDX treated patients adherent to their treatment was also significantly higher compared to the proportion of adherent patients in the OROS MPH (25.2% vs 15.5%, p < 0.0001) and ATX treatment groups (25.2% vs 11.7%, p < 0.0001). After adjusting for confounding factors, patients prescribed LDX were 81% more likely to be adherent than patients in the OROS MPH treatment group (OR = 0.55 [0.47; 0.64], p < 0.0001) and more than 2-times more likely to be adherent than patients in the ATX treatment group (OR = 0.42 [0.34; 0.51], p < 0.0001).
Overall, in the adult population, when compared to LDX patients, patients in each of the other remaining treatment groups had a significantly lower average PDC for both treatment-naïve (0.49 vs range between 0.31–0.47, all p < 0.0001) and previously treated cohorts (0.48 vs range between 0.30–0.45, all p < 0.001), except when compared to AMPH LA patients (treatment-naïve: 0.49 vs 0.49, p = 0.9716; previously treated: 0.48 vs 0.47, p = 0.2816) (). The LDX patients were also more likely to be adherent to their medication (adjusted OR ranged between 0.23–0.77, all p < 0.0001) compared to patients in each one of the other treatment groups, except for the AMPH LA treatment group (treatment-naïve: OR = 0.92, p = 0.0733; previously treated: OR = 1.02, p = 0.6471), where no significant differences were observed.
Discussion
This study demonstrated that, over a 12-month follow-up period, patients initiated on LDX had an overall better adherence level to their index medication when compared to patients initiated on either OROS MPH or ATX among previously treated children and adolescents and among adults who were either treatment-naïve or previously treated. However, this was not observed for the treatment-naïve children and adolescents where LDX had a significantly lower treatment adherence rate compared to OROS MPH and with a similar treatment adherence compared to ATX. Among all age and treatment groups, the LDX treatment group was also generally found to have a better treatment adherence profile compared to each of the remaining treatment groups (i.e., MPH LA, MPH SA, AMPH LA, and AMPH SA), except when compared to AMPH LA in adults. Sensitivity analyses using MPR to estimate treatment adherence and using multivariate models controlling for different baseline covariates showed results that were consistent with the main analysis (results not presented). In addition, results were also consistent after Bonferroni-Holm adjustments for multiple comparisons (results not presented). The convergence of the results between the main analysis and sensitivity analyses suggests that the study results are robust.
The results of the current study suggest that adherence to the index ADHD medication is consistently low among all treatment groups and age populations, regardless of whether patients were treatment-naïve or previously treated. The average PDC ranged from 0.28–0.53 across the different treatment groups, and the percentage of adherent patients was also very low, ranging from 4.3–28.4% in children and adolescents and from 7.2–25.2% in adults. Although treatment adherence rates vary widely in the published literature, depending on the definition used to classify adherent and non-adherent patients, these findings are consistent with the poor rates of treatment adherence found in previous studies among patients with ADHDCitation22. In particular, a recent study conducted on a Netherlands population that was selected based on relatively similar sample selection criteria (i.e., in children [6–17 years old] newly initiated on ADHD therapy), reported that the proportion of patients adherent (MPR ≥ 0.8) to any of the studied treatments varied from 35–57% among ADHD medications (43% for patients initiated on MPH SA, 50% for MPH LA, 35% for AMPH SA, and 57% for ATX)Citation34. In contrast to the above study which reported a treatment adherence rate to any ADHD medication, our study, which focused on the adherence to the index medication, showed a lower treatment adherence rate in the treatment-naïve children and adolescent cohort: 4.3% for MPH SA, 13.5% for MPH LA, 17.7% for OROS MPH, 5.9% for AMPH SA, and 15.6% for ATX. One hypothesis that could explain the lower treatment adherence level found in our study is the high discontinuation rate of the index medication across patients. Data from the present study and a concurrent study by the authors showed that between 74–96% of the patients discontinued the index medication over the 12-month study period, although they did not discontinue any ADHD medication permanently; between 33–90% of patients switched to/initiated another ADHD medication at some point during the 12-month study periodCitation35.
The problem of low treatment adherence is not specific to the ADHD population. A study published by the World Health Organization (WHO)Citation25 in 2003 suggested that the lack of adherence to treatment is common for most conditions that require medications to be taken on a daily basis over an extended period of time, even in conditions that could be life threatening if not well controlled. The WHO study reported that treatment adherence to long-term therapy among patients with chronic illnesses in developed countries averaged 50%. According to the WHO study, treatment adherence might be compromised by several factors including social and economic factors, characteristics of the disease, the healthcare system, as well as other patient-related factors. More specifically to ADHD patients, the nature of the symptoms, such as disorganization and difficulties with planning, may contribute to the poor treatment adherence level and the fact that some patients may intermittently miss some doses. In depression, the reported non-adherence rates ranged between 20–43% depending on the population and the period of observationCitation25. Recent studies on ADHD patients have found that level of parenting stress as well as level of child impairment might influence treatment adherenceCitation36. In our study, although the reasons for non-adherence were not available in the database, data from the present study and a concurrent study by the authors suggested that the low treatment adherence level was mainly driven by high discontinuation rates of the index medicationCitation35; however, the reasons for discontinuation were not available in the database for further investigation.
For some patients, ADHD is a lifetime disorder. Untreated ADHD can cause significant long-term impairment in several aspects of an individual’s life and treatment can contribute to reduce such impairmentCitation7,Citation8. Pharmacologic treatment is one of the most common options used to reduce and control symptoms in ADHD patientsCitation37. The poor adherence levels to the index medication found in the present study suggest a need for better disease management programs to educate clinicians, patients, and parents, in order to improve treatment adherence and ultimately prevent long-term societal and economic consequences of undertreated/untreated ADHD.
The present study is subject to common limitations that are inherent to retrospective observational studies using claims data. First, the severity of ADHD symptoms varies among individuals and might affect patients’ treatment adherence. Claims databases record diagnostic and procedural codes only and do not indicate disease severity, reason for treatment non-adherence, and other factors that are likely to affect treatment adherence, such as lifestyle measures and education. Despite multivariate regression models used to adjust for observable differences in patient characteristics, dissimilarity in unobserved confounding factors, including differences in disease severity, education or lifestyle, may still exist and impact treatment adherence differences observed between treatment groups. Retrospective databases are also subject to coding errors or data omissions; however, these are expected to affect all treatment cohorts to a similar extent and are unlikely to alter conclusions. Also, treatment adherence was analyzed based on claims for a filled prescription which does not guarantee the actual medication consumption by the patient. Nonetheless, claims data remain a valuable source of information, as they comprise a valid and large sample and have the advantage of reflecting patients’ behavior in a real world setting. In addition to the above limitation this study is also subject to other general limitations. First, symptoms and disease management in ADHD patients may vary among sub-groups of patients with different demographic factors (including gender), insurance status, or severity. Further analyses would be warranted to investigate the impact of comparative adherence level across different sub-groups of patients. Second, this study provides a good understanding of the comparative adherence levels to most commonly prescribed stimulants and non-stimulants observed in real word setting. However, this study was not designed to specifically assess causation relationship. Additional prospective studies using a randomized design would be necessary to evaluate a causation relationship between ADHD treatment and patients’ adherence level to treatment.
To the best of our knowledge, the present study is the only study to date that has comprehensively compared treatment adherence to ADHD index medication in the US across most commonly prescribed stimulant and non-stimulant medications, among all relevant age groups, as well as across all treatment groups (i.e., treatment-naïve and previously treated patients). Therefore, findings from this study provide a comprehensive picture of treatment adherence to most prescribed ADHD medications for both previously treated and treatment-naïve patients across a wide spectrum of ages, in a real-world setting. Further analyses, however, are warranted to better understand the factors that influence treatment adherence in ADHD patients and to assess the direct and indirect costs associated with adherent vs non-adherent ADHD patients.
Conclusions
This study investigated adherence levels to the index ADHD medications in the full spectrum of patients with ADHD, stratified by age and treatment groups, for both treatment naïve and previously treated patients. Across all the studied treatment groups, the proportion of patients who were adherent to their initial ADHD medication over a 1-year period was low. These findings suggest a need for better/new disease management programs that could help educate clinicians, patients, and parents on the importance of treatment adherence to control symptoms efficiently. Despite these low treatment adherence rates, among all the treatment groups, LDX-treated patients showed a better treatment adherence compared to patients initiated on other ADHD medications, except when compared to the OROS MPH and ATX treatment groups in the treatment-naïve children and adolescents and compared to AMPH LA in adult patients.
Transparency
Declaration of funding
This analysis was supported by Shire Development, LLC. Shire develops and markets psychiatric ADHD medications including those to treat ADHD.
Declaration of financial/other relationships
JS, PH, and MHE are employees of Shire Development, LLC and own stock/stock options. AG, GG, MC, and EQW are employees of Analysis Group Inc. which has received consultancy fees from Shire Development, LLC.
Supplementary Material
Download PDF (59.5 KB)Acknowledgments
No additional contributors to acknowledge. No assistance in the preparation of this article is to be declared.
References
- Verma R, Balhara YP, Mathur S. Management of attention-deficit hyperactivity disorder. J Pediatr Neurosci 2011;6:13-18
- Wolraich ML. Attention-deficit hyperactivity disorder. Semin Pediatr Neurol 2006;13:279-85
- Barkley R, Fischer M, Smallish L, et al. The persistence of attention-deficit/hyperactivity disorder into young adulthood as a function of reporting source and definition of disorder. J Abnorm Psychol 2002;111:279-89
- Bloom B, Cohen RA. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2006. National Center for Health Statistics. Vital Health Stat 2007;10:1-79
- Kinbami L, Xiang L, Pastor P, et al. Attention Deficit Hyperactivity Disorder among children aged 5-17 years in the United States, Hyattsville, MD: National Center for Health Statistics. 1998-2009, NCHS Data Brief, No.70. 2011
- Kessler CR, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006;163:716-23
- National Institute for Health and Clinical Excellence. Attention deficit hyperactivity disorder: The NICE Guideline on diagnosis and management of ADHD in children, young people and adults. Leicester (UK): British Psychological Society (UK). 2009, National Clinical Practice Guideline 72
- Shaw M, Hodgkins P, Caci H, et al. A systematic review and analysis of long-term outcomes in ADHD: effects of treatment and non-treatment. BMC Med 2012;10:99
- Spencer TJ. ADHD and comorbidity in childhood. J Clin Psychiatry 2006;67(8 Suppl):27-31
- Goldman L, Genel M, Bezman R, et al. Diagnosis and treatment of Attention-Deficit/Hyperactivity Disorder in children and adolescents. JAMA 1998;279:1100-7
- Cussen A, Sciberras E, Ukoumunne O, et al. Relationship between symptoms of attention-deficit/hyperactivity disorder and family functioning: a community-based study. Eur J Pediatr 2012;171:271-80
- Canadian ADHD Resource Alliance. Canadian ADHD Practice Guidelines (CAP-Guidelines). 3rd edn. Toronto ON; CADDRA. 2011, p 158
- GroupHealth. Attention deficit hyperactivity disorder (ADHD): Adults – Diagnosis and treatment guideline. Group Health Cooperative. Seattle, WA. 2011, p 15
- Olfson M, Gameroff M, Marcus S, et al. National trends in the treatment of attention deficit hyperactivity disorder. Am J Psychiatry 2003;160:1071-7
- Christensen L, Sasané R, Hodgkins P, et al. Pharmacological treatment patterns among patients with attention-deficit/hyperactivity disorder: retrospective claims-based analysis of a managed care population. Curr Med Res Opin 2010;26:977-89
- American Academy of Pediatrics. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 2011;128:2011-654
- Weiss M, Murray C. Assessment and management of attention-deficit hyperactivity disorder in adults. Can Med Ass J 2003;168:715-22
- Seixas M, Weiss M, M�U. Systematic review of national and international guidelines on attention-deficit hyperactivity disorder. J Psychopharmacol. 2012;26:753-65
- Elia J, Ambrosini PJ, Rapoport JL. Treatment of attention-deficit-hyperactivity disorder. N Engl J Med 1999;340:780-8
- Jensen P, Arnold E, Severe J, et al. A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. The MTA Cooperative Group. Multimodal Treatment Study of Children with ADHD. Arch Gen Psychiatry 1999;56:1073-86
- Hodgkins P, Shaw M, Coghill D, et al. Amfetamine and methylphenidate medications for ADHD: complementary treatment options. Eur Child Adol Psych 2012;21:477-92
- Adler LD, Nierenberg AA. Review of medication adherence in children and adults with ADHD. Postgrad Med 2010;122:184-91
- Cascade E, Kalali AH, Weisler RH, et al. Seasonality and the changing adult/child prescription ratios in ADHD Therapy. Psychiatry (Edgmont) 2008;5:23-5
- Safren SA, Duran P, Yovel I, et al. Medication adherence in psychopharmacologically treated adults with ADHD. J Atten Disord 2007;10:257-60
- World Health Organization. Adherence to long-term therapies: evidence for action. Geneva, Switzerland: World Health Organization, 2003. p 19-26
- Elliot RA, Barber N, Horne R. Cost-effectiveness of adherence-enhancing interventions: a quality assessment of the evidence. Ann Pharmacother 2005;39:508-15
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Medical Care 2005;43:1130-9
- Elixhauser A, Steiner C, Kruzikas D. Comorbidity Software Documentation. 2004. HCUP Methods Series Report # 2004-1. ONLINE February 6, 2004. U.S. Agency for Healthcare Research and Quality. Available: http://www.hcupus.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edition [DSM-IV], 1994
- Choudhry NK, Shrank WH, Levin RL, et al. Measuring concurrent adherence to multiple related medications. Am J Managed Care 2009;15:457-64
- Yeaw J, Benner JS, Walt JG, et al. Comparing adherence and persistence across 6 chronic medication classes. J Manag Care Pharm 2009;15:728-40
- Peterson AM, Nau DP, Cramer JA, et al. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3-12
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 1979;6:65-70
- Hodgkins P, Sasané R, Meijer WM. Pharmacologic treatment of Attention-Deficit/Hyperactivity Disorder in children: incidence, prevalence, and treatment patterns in the Netherlands. Clin Therapeut 2011;33:188-203
- Setyawan J, Hodgkins P, Guerin A, et al. Comparison of treatment persistency in patients initiated on of Lisdexamfetamine and other medications for the treatment of attention deficit/hyperactivity disorder: a retrospective analysis (In Process Citation)
- Dreyer S, O’Laughlin L. Parental adherence to clinical recommendations in an ADHD evaluation clinic. J Clin Psychol 2010;66:1101-20
- Faraone S, Biederman J, Mick E. The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow up studies. Psychol Med 2006;36:159-65